Validation of a Methodology for the Quantification of DON in Feces and Feedstuffs by UPLC as Possible Strategy to Evaluate the Detoxifying Efficacy of a Mycotoxin Adsorbent In Vivo
Abstract
1. Introduction
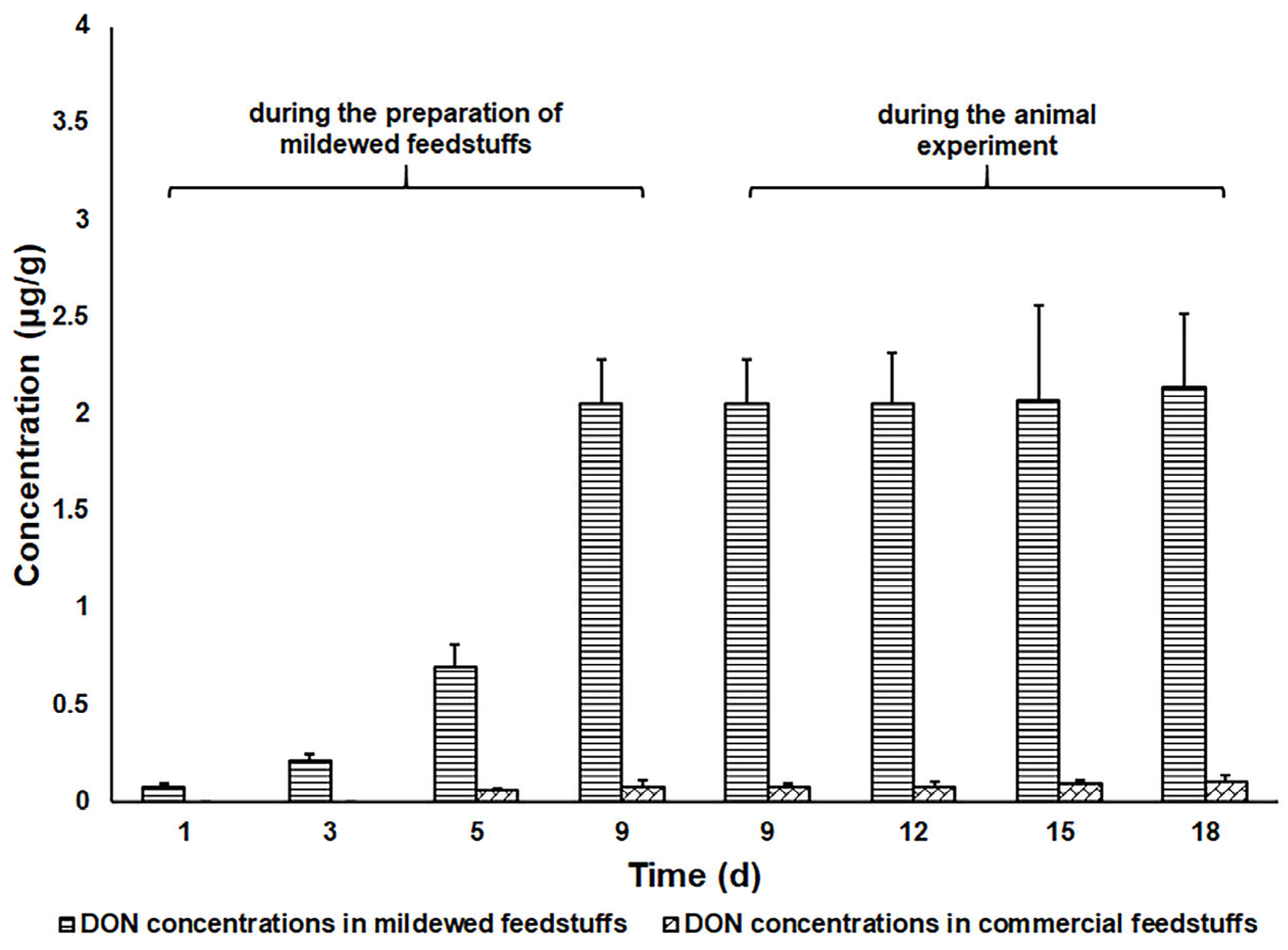
2. Results
2.1. Performance of the UPLC Method
2.2. Detoxification Efficacies of Tree Commercial Adsorbents
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Instruments and Materials
5.3. Animals
5.4. In Vivo Evaluation of the Detoxification Efficacies of Three Commercial Mycotoxin Adsorbents
5.4.1. Analytical Method
5.4.2. Experimental Design
5.4.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DON | Deoxynivalenol |
| EFSA | European Food Safety Authority |
| UPLC | Ultra-performance liquid chromatography |
| LC-MS | Liquid chromatography- mass spectrometry |
| QC | Quality control |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| RSD | Relative standard deviation |
| SD | Standard deviation |
References
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Goncalves, R.A.; Naehrer, K.; Santos, G.A. Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: A real risk to aquaculture? Rev. Aquac. 2018, 10, 263–280. [Google Scholar] [CrossRef]
- Koletsi, P.; Schrama, J.W.; Graat, E.A.M.; Wiegertjes, G.F.; Lyons, P.; Pietsch, C. The occurrence of mycotoxins in raw materials and fish feeds in europe and the potential effects of deoxynivalenol (DON) on the health and growth of farmed fish species—A review. Toxins 2021, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Halawa, A.; Dänicke, S.; Kersten, S.; Breves, G. Intestinal transport of deoxynivalenol across porcine small intestines. Arch. Anim. Nutr. 2013, 67, 134–146. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Kihal, A.; Rodríguez-Prado, M.; Calsamiglia, S. The efficacy of mycotoxin binders to control mycotoxins in feeds and the potential risk of interactions with nutrient: A review. J. Anim. Sci. 2022, 100, skac328. [Google Scholar] [CrossRef]
- Ying, Z.; Zhao, D.; Li, H.; Liu, X.; Zhang, J. Efficient Adsorption of Deoxynivalenol by Porous Carbon Prepared from Soybean Dreg. Toxins 2021, 13, 500. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.; van der Doelen, M.A.; Fink-Gremmels, J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicoses. Mycopathologia 2007, 163, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, Y.; Zhai, W.; Wang, M. A core-shell AuNRs@BUT-16 nanocomposite for enhancement SERS detection and efficient removal of deoxynivalenol. J. Adv. Res. 2025, 67, 15–23. [Google Scholar] [CrossRef]
- Kong, C.; Shin, S.Y.; Kim, B.G. Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: An in vitro approach. Springerplus 2014, 3, 346. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Piva, A.; Chies, L.; Galvano, M. Activated carbons: In vitro affinity for ochratoxin A and deoxynivalenol and relation of adsorption ability to physicochemical parameters. J. Food Prot. 1998, 61, 469–475. [Google Scholar] [CrossRef]
- Pirouz, A.A.; Selamat, J.; Iqbal, S.Z.; Mirhosseini, H.; Karjiban, R.A.; Bakar, F.A. The use of innovative and efficient nanocomposite (magnetic graphene oxide) for the reduction on of Fusarium mycotoxins in palm kernel cake. Sci. Rep. 2017, 7, 12453. [Google Scholar] [CrossRef]
- Tso, K.H.; Ju, J.C.; Fan, Y.K.; Chiang, H.I. Enzyme Degradation Reagents Effectively Remove Mycotoxins Deoxynivalenol and Zearalenone from Pig and Poultry Artificial Digestive Juices. Toxins 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; D’Ascanio, V.; Abbasciano, M.; Santovito, E.; Garbetta, A.; Logrieco, A.F.; Avantaggiato, G. Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose. Toxins 2022, 14, 393. [Google Scholar] [CrossRef]
- Horky, P.; Venusova, E.; Aulichova, T.; Ridoskova, A.; Skladanka, J.; Skalickova, S. Usability of graphene oxide as a mycotoxin binder: In vitro study. PLoS ONE 2020, 15, e0239479. [Google Scholar] [CrossRef]
- Abbasi Pirouz, A.; Selamat, J.; Zafar Iqbal, S.; Iskandar Putra Samsudin, N. Efficient and Simultaneous Chitosan-Mediated Removal of 11 Mycotoxins from Palm Kernel Cake. Toxins 2020, 12, 115. [Google Scholar] [CrossRef]
- Xu, R.; Yiannikouris, A.; Shandilya, U.K.; Karrow, N.A. Comparative Assessment of Different Yeast Cell Wall-Based Mycotoxin Adsorbents Using a Model- and Bioassay-Based In Vitro Approach. Toxins 2023, 15, 104. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Greco, D.; D’Ascanio, V.; Visessanguan, W.; Avantaggiato, G. The Effectiveness of Durian Peel as a Multi-Mycotoxin Adsorbent. Toxins 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Fiorbelli, E.; Lapris, M.; Errico, M.; Della Badia, A.; Riahi, I.; Rocchetti, G.; Gallo, A. Mycotoxin Challenge in Dairy Cows: Assessment of the Efficacy of an Anti-Mycotoxin Agent by Adopting an In Vitro Rumen Simulation Method. Toxins 2024, 16, 490. [Google Scholar] [CrossRef]
- Bruinenberg, P.G.; Castex, M. Evaluation of a Yeast Hydrolysate from a Novel Strain of Saccharomyces cerevisiae for Mycotoxin Mitigation using In Vitro and In Vivo Models. Toxins 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Meneely, J.; Greer, B.; Chevallier, O.; Jones, D.S.; Connolly, L.; Elliott, C. Comparative In Vitro Assessment of a Range of Commercial Feed Additives with Multiple Mycotoxin Binding Claims. Toxins 2019, 11, 659. [Google Scholar] [CrossRef]
- Debevere, S.; Schatzmayr, D.; Reisinger, N.; Aleschko, M.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. Evaluation of the Efficacy of Mycotoxin Modifiers and Mycotoxin Binders by Using an In Vitro Rumen Model as a First Screening Tool. Toxins 2020, 12, 405. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, Y.I.; Parnsen, W.; Kim, S.W. Phytobiotics with Adsorbent to Mitigate Toxicity of Multiple Mycotoxins on Health and Growth of Pigs. Toxins 2021, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougoulias, C.; Devreese, M. Biomarkers for Exposure as A Tool for Efficacy Testing of A Mycotoxin Detoxifier in Broiler Chickens and Pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA J. 2010, 8, 1963. [Google Scholar]
- Worrell, N.R.; Mallett, A.K.; Cook, W.M.; Baldwin, N.C.; Shepherd, M.J. The role of gut micro-organisms in the metabolism of deoxynivalenol administered to rats. Xenobiotica 1989, 19, 25–32. [Google Scholar] [CrossRef]
- Valenta, H.; Dänicke, S.; Döll, S. Analysis of deoxynivalenol and de-epoxy-deoxynivalenol in animal tissues by liquid chromatography after clean-up with an immunoaffinity column. Mycotoxin. Res. 2003, 19, 51–55. [Google Scholar] [CrossRef]
- Kang, R.; Qu, H.; Guo, Y.; Ji, C.; Cheng, J.; Wang, Y.; Huang, S.; Zhao, L.; Ji, C.; Ma, Q. Toxicokinetics of Deoxynivalenol in Dezhou Male Donkeys after Oral Administration. Toxins 2023, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Voyksner, R.D.; Hagler, W.M., Jr.; Swanson, S.P. Analysis of some metabolites of T-2 toxin, diacetoxyscirpenol and deoxynivalenol by thermospray high-performance liquid chromatography-mass spectrometry. J. Chromatogr. 1987, 394, 183–199. [Google Scholar] [CrossRef]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef]
- Wan, D.; Huang, L.; Pan, Y.; Wu, Q.; Chen, D.; Tao, Y.; Wang, X.; Liu, Z.; Li, J.; Wang, L.; et al. Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap time-of-flight mass spectrometry, and online radiometric detection. J. Agric. Food Chem. 2014, 62, 288–296. [Google Scholar] [CrossRef]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Zimmermann, H.E.; Fruhmann, P.; Dänicke, S.; Wiesenberger, G.; Caha, S.; Weber, J.; Berthiller, F. Metabolism of deoxynivalenol and deepoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins 2015, 7, 4706–4729. [Google Scholar] [CrossRef] [PubMed]
- Miró-Abella, E.; Torrell, H.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Monitoring and evaluation of the interaction between deoxynivalenol and gut microbiota in Wistar rats by mass spectrometry-based metabolomics and next-generation sequencing. Food. Chem. Toxicol. 2018, 121, 124–130. [Google Scholar] [CrossRef]
- Miró-Abella, E.; Herrero, P.; Canela, N.; Arola, L.; Ras, R.; Borrull, F.; Fontanals, N. Optimised extraction methods for the determination of trichothecenes in rat faeces followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1105, 47–53. [Google Scholar] [CrossRef]
- European commission decision 2002/657/EC. Off. J. Eur. Communities 2002, 221, 8–36.

| Matrix | Spiked Concentration (μg/g) | Recovery (%) a | Intra-Day RSD (%) | Inter-Day RSD (%) |
|---|---|---|---|---|
| Pig feces | 0.1 | 86.4 ± 0.4 | 0.4 | 0.8 |
| 87.0 ± 0.5 | 0.6 | |||
| 86.8 ± 0.4 | 0.5 | |||
| 86.7 ± 0.5 | 0.6 | |||
| 86.3 ± 1.1 | 1.3 | |||
| 1 | 98.8 ± 1.4 | 1.4 | 1.2 | |
| 97.5 ± 0.3 | 0.3 | |||
| 97.7 ± 1.3 | 1.3 | |||
| 97.2 ± 0.4 | 0.5 | |||
| 98.1 ± 1.1 | 1.1 | |||
| 10 | 104.1 ± 1.3 | 1.2 | 0.3 | |
| 105.3 ± 0.4 | 0.4 | |||
| 104.5 ± 0.9 | 0.9 | |||
| 104.1 ± 1.7 | 1.7 | |||
| 105.1 ± 1.5 | 1.4 | |||
| Pig feedstuffs | 0.01 | 105.3 ± 15.4 | 14.6 | 11.7 |
| 108.6 ± 8.5 | 7.8 | |||
| 105.3 ± 12.6 | 12.0 | |||
| 105.5 ± 6.8 | 6.4 | |||
| 106.6 ± 15.0 | 14.0 | |||
| 0.1 | 93.9 ± 6.1 | 6.5 | 5.6 | |
| 94.6 ± 6.0 | 6.3 | |||
| 96.3 ± 1.4 | 1.5 | |||
| 92.6 ± 3.8 | 4.1 | |||
| 91.8 ± 5.4 | 5.9 | |||
| 1 | 97.7 ± 1.7 | 1.7 | 2.0 | |
| 97.3 ± 2.6 | 2.7 | |||
| 97.3 ± 2.4 | 2.5 | |||
| 96.9 ± 0.8 | 0.8 | |||
| 96.9 ± 1.6 | 1.6 |
| Matrix | Spiked Concentration (μg/g) | Condition | Recovery (%) a | RSD (%) |
|---|---|---|---|---|
| Pig feces | 0.1 | −20 °C, 7 d | 92.4 ± 5.2 | 5.6 |
| 60 °C, 2 d | 93.8 ± 8.4 | 9.0 | ||
| 1 | −20 °C, 7 d | 95.6 ± 7.6 | 7.9 | |
| 60 °C, 2 d | 98.7 ± 4.4 | 4.5 | ||
| 10 | −20 °C, 7 d | 100.3 ± 5.1 | 5.1 | |
| 60 °C, 2 d | 96.9 ± 5.5 | 5.7 | ||
| Pig feedstuffs | 0.01 | −20 °C, 7 d | 100.2 ± 3.7 | 3.7 |
| 60 °C, 2 d | 95.8 ± 7.4 | 7.7 | ||
| 0.1 | −20 °C, 7 d | 91.6 ± 5.2 | 5.7 | |
| 60 °C, 2 d | 90.7 ± 5.8 | 6.4 | ||
| 1 | −20 °C, 7 d | 94.3 ± 2.5 | 2.7 | |
| 60 °C, 2 d | 88.9 ± 4.0 | 4.6 |
| Time (d) | Sampling Time | Group A a | Group B a | Group C a | Group D a | Group E a |
|---|---|---|---|---|---|---|
| 1 | 8 a.m. | ND | 0.06 ± 0.13 | 8.68 ± 2.56 | 5.54 ± 3.21 | ND |
| 6 p.m. | ND | 0.06 ± 0.16 | 0.5 ± 1.16 | ND | 1.52 ± 1.01 | |
| 2 | 8 a.m. | 0.06 ± 0.12 | 0.19 ± 1.3 | 0.28 ± 0.14 | 0.60 ± 1.13 | 0.52 ± 0.42 |
| 6 p.m. | ND | ND | 2.26 ± 1.77 | ND | ND | |
| 3 | 8 a.m. | ND | 1.00 ± 1.23 | ND | 0.36 ± 0.19 | ND |
| 6 p.m. | ND | 0.10± 0.12 | ND | ND | ND | |
| 4 | 8 a.m. | ND | 0.26 ± 0.14 | 0.4 ± 1.08 | 0.14 ± 0.15 | 0.38 ± 0.16 |
| 6 p.m. | ND | ND | 0.52 ± 0.19 | ND | ND | |
| 5 | 8 a.m. | 0.14 ± 0.12 | 0.26 ± 0.16 | 66.28 ± 68.34 | ND | ND |
| 6 p.m. | 0.38 ± 0.20 | 0.14 ± 0.12 | 71.06 ± 67.77 | ND | 0.48 ± 0.26 | |
| 6 | 8 a.m. | 0.9 ± 0.14 | 0.62 ± 0.18 | 0.34 ± 0.12 | ND | 6.5 ± 5.73 |
| 6 p.m. | 1.1 ± 0.16 | ND | 91.92 ± 89.95 | 1.92 ± 1.02 | 6.38 ± 4.35 | |
| 7 | 8 a.m. | ND | 0.32 ± 0.40 | 0.72 ± 0.18 | ND | ND |
| 6 p.m. | 0.06 ± 0.11 | 0.04 ± 0.11 | 23.22 ± 18.45 | 0.66 ± 1.21 | 1.3 ± 1.52 | |
| 8 | 8 a.m. | 1.38 ± 0.16 | ND | 0.7 ± 0.16 | 0.14 ± 0.12 | 0.86 ± 1.22 |
| 6 p.m. | 1.3 ± 1.8 | 0.26 ± 0.11 | 26.24 ± 26.75 | 0.82 ± 1.24 | 0.04 ± 0.11 | |
| 9 | 8 a.m. | 0.2 ± 0.11 | 0.68 ± 1.9 | 30.22 ± 27.38 | ND | 0.20 ±0.16 |
| 6 p.m. | 0.34 ± 1.1 | 0.46 ± 1.7 | ND | 0.26 ± 0.13 | 0.48 ± 0.11 | |
| 10 | 8 a.m. | ND | 0.19 ± 0.12 | 0.22 ± 0.12 | ND | ND |
| 6 p.m. | ND | 0.22 ± 0.11 | 0.12 ± 0.12 | 0.16 ± 0.13 | ND |
| Sample No. | Pig Farm 1 | Pig Farm 2 | Pig Farm 3 | Pig Farm 4 | Pig Farm 5 | Pig Farm 6 |
|---|---|---|---|---|---|---|
| 1 | ND | 0.05 | 0.07 | 0.17 | 0.08 | 0.04 |
| 2 | 0.03 | 0.07 | 0.05 | 0.03 | 0.13 | 0.22 |
| 3 | 27.72 | 0.38 | 20.17 | 0.37 | 0.31 | 1.44 |
| 4 | 0.09 | 1.87 | 12.10 | 0.21 | 0.44 | 0.07 |
| 5 | 0.09 | 0.83 | 0.46 | 0.44 | 0.74 | 0.47 |
| 6 | - | - | 7.96 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Deng, H.; Jia, Y.; Li, D.; Chen, R.; Chen, R.; Zhang, J.; Zhong, Y.; Yi, L.; Wang, F.; et al. Validation of a Methodology for the Quantification of DON in Feces and Feedstuffs by UPLC as Possible Strategy to Evaluate the Detoxifying Efficacy of a Mycotoxin Adsorbent In Vivo. Toxins 2025, 17, 322. https://doi.org/10.3390/toxins17070322
Yang B, Deng H, Jia Y, Li D, Chen R, Chen R, Zhang J, Zhong Y, Yi L, Wang F, et al. Validation of a Methodology for the Quantification of DON in Feces and Feedstuffs by UPLC as Possible Strategy to Evaluate the Detoxifying Efficacy of a Mycotoxin Adsorbent In Vivo. Toxins. 2025; 17(7):322. https://doi.org/10.3390/toxins17070322
Chicago/Turabian StyleYang, Bo, Hui Deng, Yiwei Jia, Dong Li, Rudeng Chen, Ruiqing Chen, Jing Zhang, Yan Zhong, Lingxian Yi, Fuhao Wang, and et al. 2025. "Validation of a Methodology for the Quantification of DON in Feces and Feedstuffs by UPLC as Possible Strategy to Evaluate the Detoxifying Efficacy of a Mycotoxin Adsorbent In Vivo" Toxins 17, no. 7: 322. https://doi.org/10.3390/toxins17070322
APA StyleYang, B., Deng, H., Jia, Y., Li, D., Chen, R., Chen, R., Zhang, J., Zhong, Y., Yi, L., Wang, F., Cui, H., & Yu, D. (2025). Validation of a Methodology for the Quantification of DON in Feces and Feedstuffs by UPLC as Possible Strategy to Evaluate the Detoxifying Efficacy of a Mycotoxin Adsorbent In Vivo. Toxins, 17(7), 322. https://doi.org/10.3390/toxins17070322





