Cyanobacterial Blooms and the Presence of Cyanotoxins in the Brazilian Amazon
Abstract
1. Introduction
2. Results
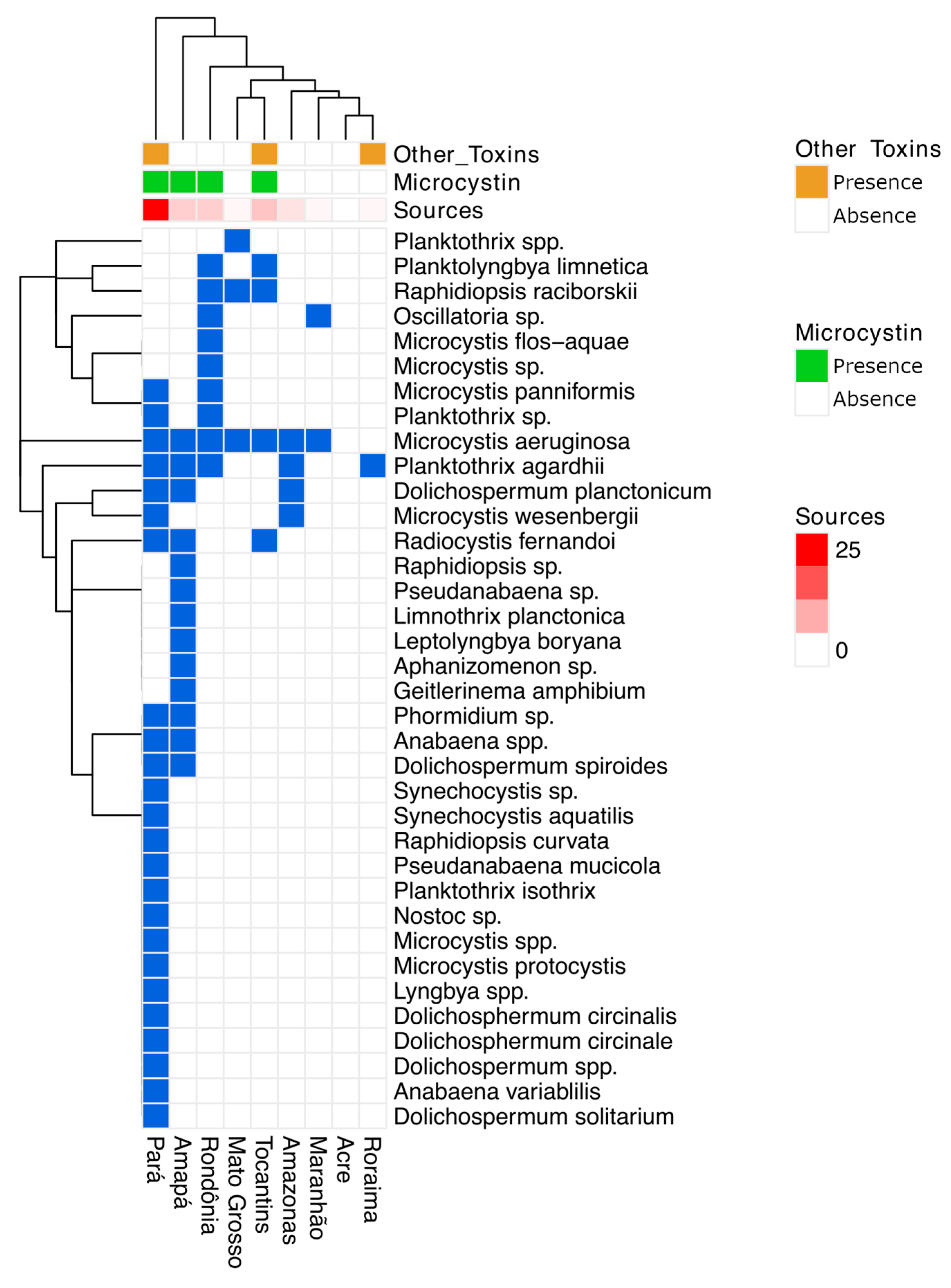
2.1. Literature Review
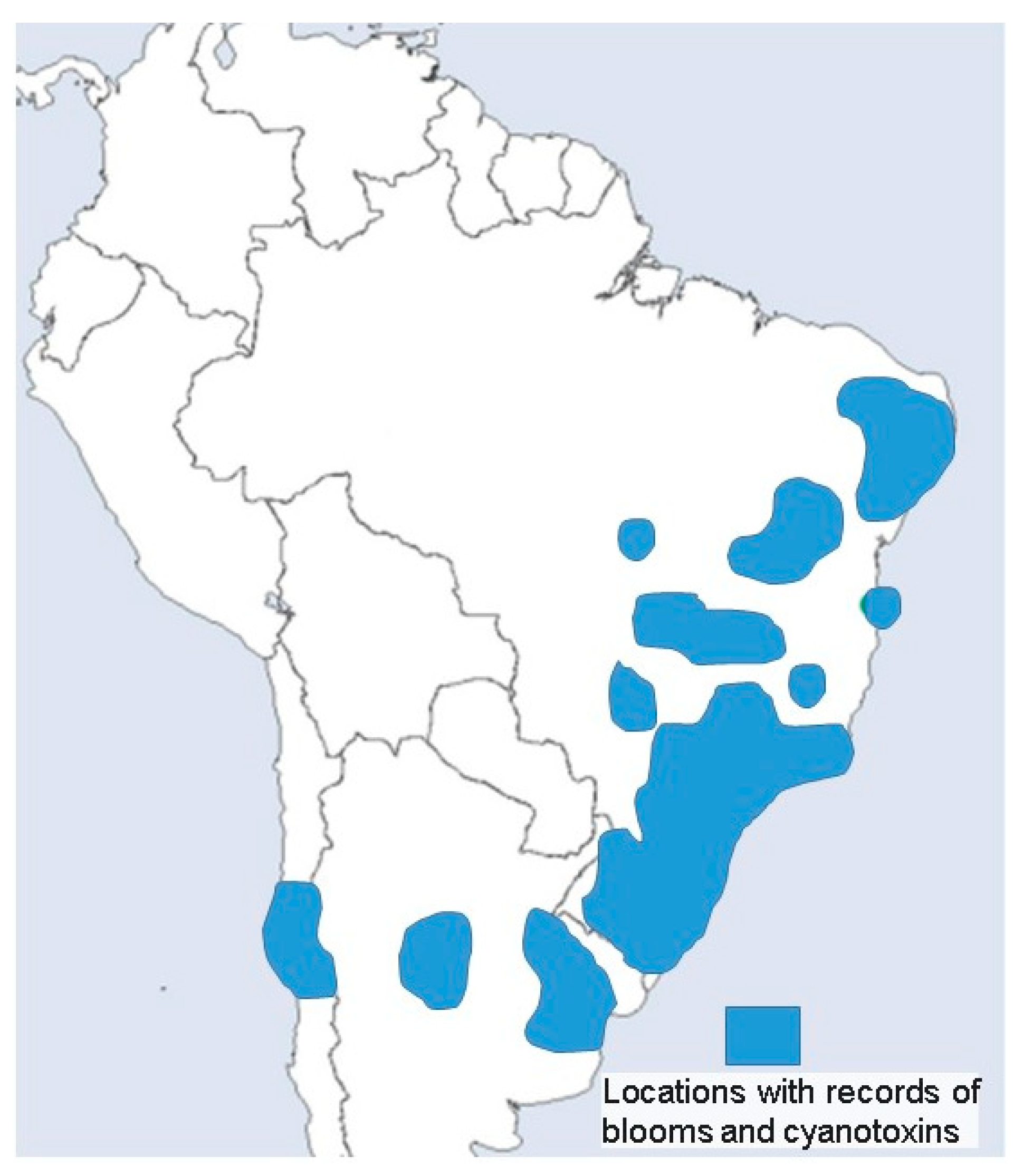
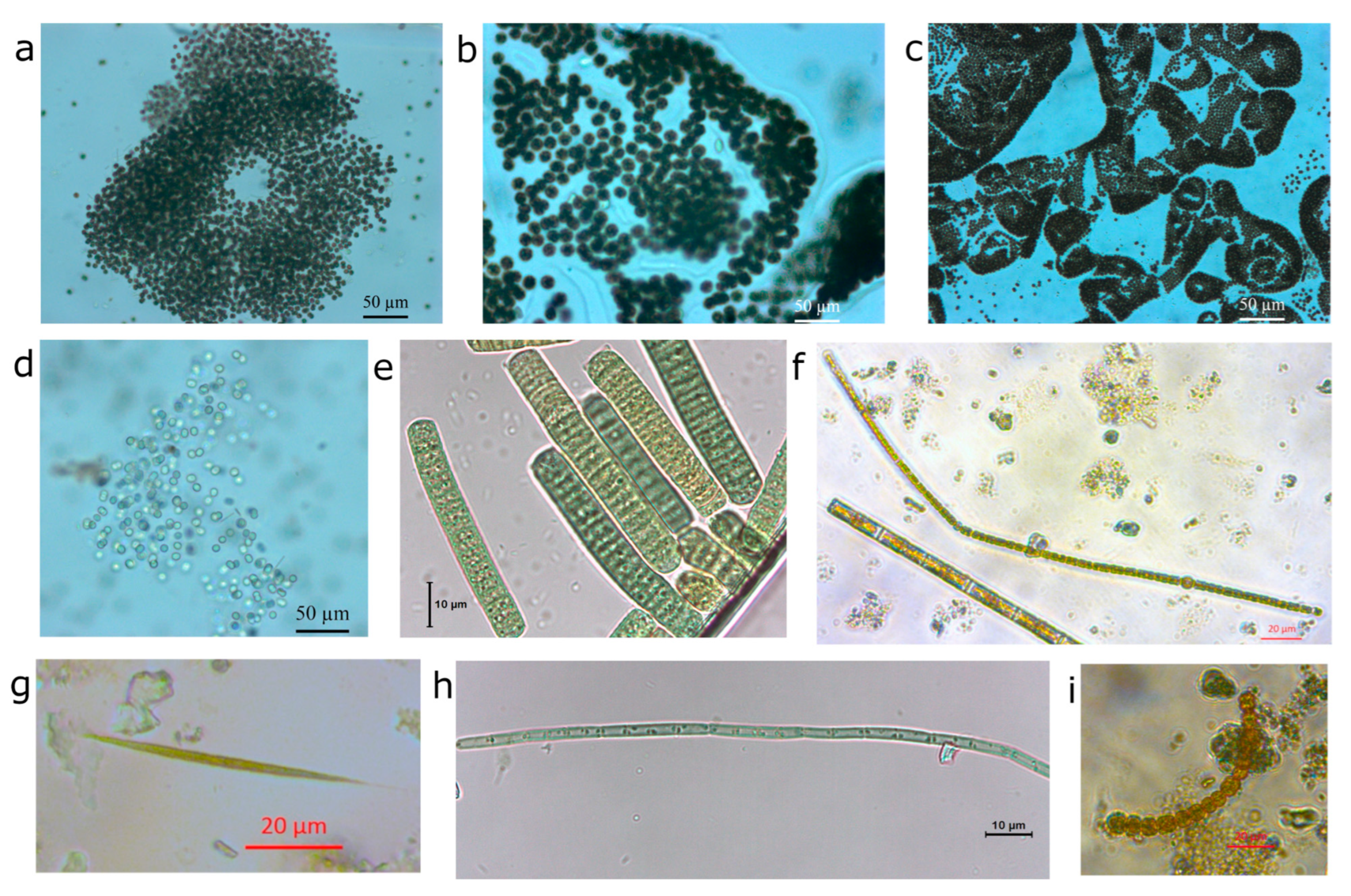
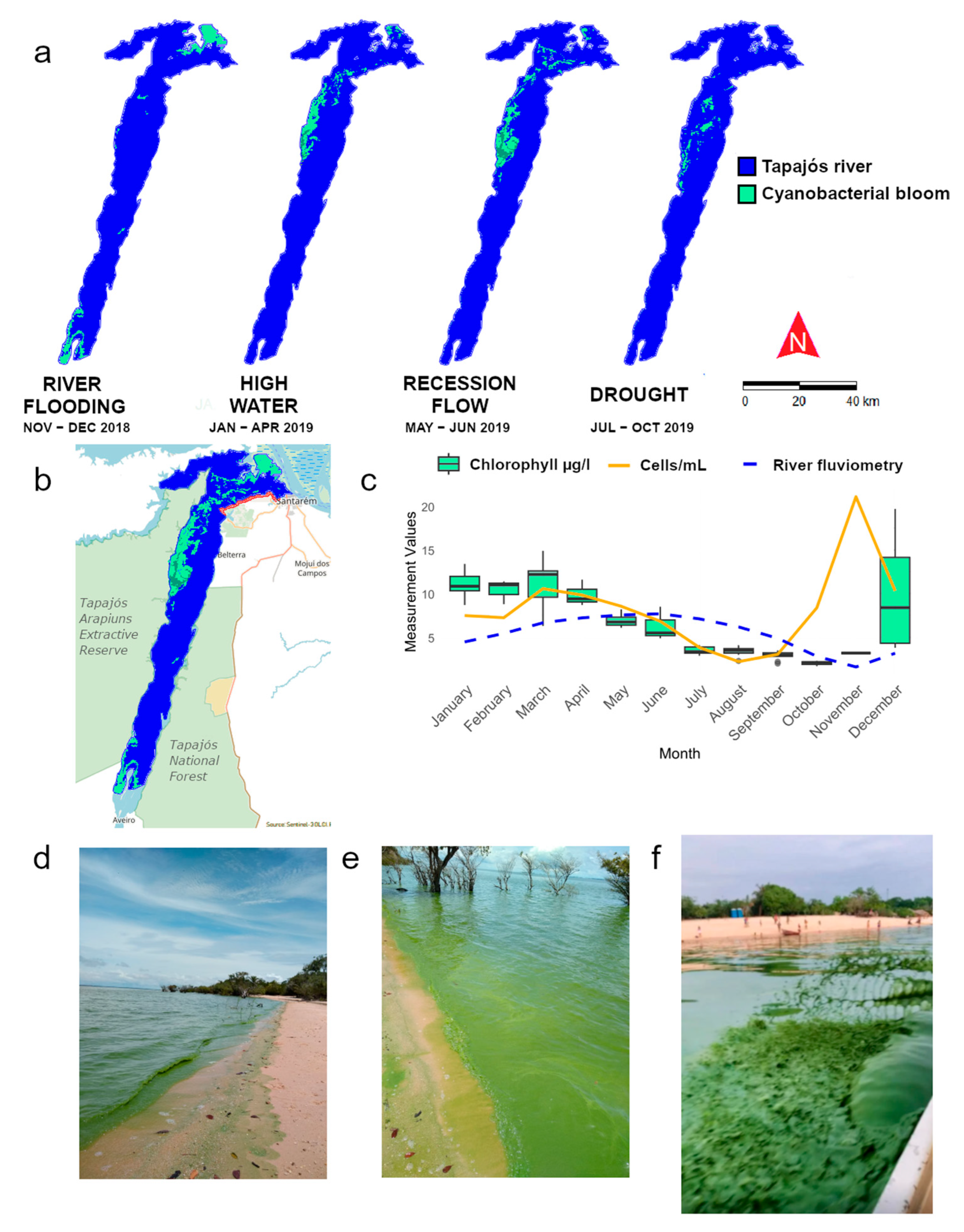
2.2. Blooms
2.3. Cyanotoxins
3. Discussion
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANA | National Water and Sanitation Agency |
| chlo-a | Chlorophyll-a |
| ELISA | Enzyme-linked immunosorbent assay |
| HPLC | High-performance liquid chromatography |
| IBGE | Brazilian Institute of Geography and Statistics |
| L | Liter |
| M | Meters |
| MC | Total Microcystins |
| MC-LR | Microcystin variant with leucine in position 2 and arginine in position 4 |
| mL | Milliliter |
| cells.mL−1 | Density of cyanobacteria cells |
| pH | Potential hydrogen |
| STX | Saxitoxin |
| WHO | World Health Organization |
| μg∙mL | Microgram per liter |
References
- IBGE. Amazônia Legal. Instituto Brasileiro de Geografia e Estatística. 2025. Available online: https://www.ibge.gov.br/geociencias/cartas-e-mapas/mapas-regionais/15819-amazonia-legal.html (accessed on 15 January 2025).
- Brazil. Ministério do Meio Ambiente. In Dados sobre a Amazônia Legal; Ministério do Meio Ambiente: Brasília, Brazil, 2025. [Google Scholar]
- Agência Nacional de Águas e Saneamento Básico (ANA). Conjuntura dos Recursos Hídricos no Brasil 2024: Informe Anual; ANA: Brasília, Brazil, 2024. [Google Scholar]
- Neto, L.C.F.; Diniz, C.G.; Maretto, R.V.; Persello, C.; Pinheiro, M.L.S.; Castro, M.C.; Sadeck, L.W.R.; Filho, A.F.; Cansado, J.; Souza, A.A.D.A.; et al. Uncontrolled Illegal Mining and Garimpo in the Brazilian Amazon. Nat. Commun. 2024, 15, 9847. [Google Scholar] [CrossRef] [PubMed]
- Trata Brazil. Saneamento é Saúde: Ranking do Saneamento 2024 (SNIS 2022); GO Associados: São Paulo, Brazil, 2024; Available online: https://tratabrasil.org.br/ranking-do-saneamento-2024/ (accessed on 9 January 2025).
- Genuário, D.B.; Vaz, M.; Melo, I. Phylogenetic Insights into Homocytous Cyanobacteria from Amazonian Rivers. Mol. Phylogenet. Evol. 2017, 116, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; da Silva, P.H.; Carneiro, F.M.; Silva, D.P. Distribution Models for Cyanobacteria Blooms in Brazil. Biota Neotrop. 2020, 20, e20190756. [Google Scholar] [CrossRef]
- Nascimento, E.L.D.; Koschek, P.R.; dos Santos, M.E.V.; Pacheco, A.B.F.; Gomes, A.M.D.A.; de Souza, C.M.M.; Bastos, W.R.; Azevedo, S.M.F.D.O. Influence of Iron on Microcystin in Microcystis panniformis. Curr. Microbiol. 2021, 78, 2345–2354. [Google Scholar] [CrossRef]
- Brazil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Monitoramento de Cianobactérias nos Mananciais de Abastecimento de Água para Consumo Humano no Brasil, 2012. Bol. Epidemiológico 2014, 45, 1–15. [Google Scholar]
- Brazilian Ministry of Health Database SISAGUA. Available online: http://sisagua.saude.gov.br/sisagua/paginaExterna.jsf (accessed on 15 May 2025).
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Cyanotoxin Distribution and Poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Dias, M.B. Fitoplâncton na Reserva Biológica do Lago Piratuba. Master’s Thesis, INPA, Amazonas, Brazil, 2007. [Google Scholar]
- Barbosa, L.P.J.D.L. Toxicidade de Cianobactérias e Microcistinas em Peixes. Master’s Thesis, Universidade Federal do Amapá, Macapá, Amapá, Brazil, 2015. Available online: https://www2.unifap.br/ppcs/files/2015/04/Disserta%C3%A7%C3%A3o_Mestrado_Larissa_Barbosa_PPGCS_2015-I.pdf (accessed on 9 January 2025).
- Oliveira, E.D.C. Cianobactérias no Rio Amazonas: Análises Moleculares e Potencial Tóxico. Ph.D. Thesis, Federal University of Amapá, Macapá, Brazil, 2018. [Google Scholar]
- Oliveira, E.D.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First Detection of Microcystin-LR in the Amazon River. Toxins 2019, 11, 669. [Google Scholar] [CrossRef]
- Cavalcante, E.C.; Faustino, S.M.M.; Silva, L.M.A.; Cunha, A.C.; Oliveira, E.D.C. Monitoring of phytoplankton in the raw water of ETA Macapá and inferences about COVID-19. RICA 2021, 12, 664–678. Available online: https://sustenere.inf.br/index.php/rica/article/view/CBPC2179-6858.2021.003.0053 (accessed on 14 May 2025). [CrossRef]
- Pascoaloto, D.; Gomes, N.A.; Cunha, H.B. Variação diurna de algumas variáveis ambientais em uma lagoa eutrofizada e com floração de cianobactérias no município de Manaus/AM. In Simpósio Brasileiro de Recursos Hídricos; ABRH: Belo Horizonte, Brazil, 2021. [Google Scholar]
- Arcos, A.; Amaral, A.; Santos, M.; De, C.; Silva, M.; Kochhann, D.; Tadei, W. Water Quality of Urban Lakes in the Central-Southern Region of Manaus, Amazon. Sci. Amazon. 2018, 7, CAm1–CAm11. Available online: http://www.scientia-amazonia.org (accessed on 14 May 2025).
- Melo, S.; Ribeiro, L.D.B.; Pereira, A.C.; Werner, V.R. Planktonic Cyanobacteria from Urban Lakes in Manaus. Rodriguesia 2024, 75, e00182023. [Google Scholar] [CrossRef]
- Cutrim, M.V.J.; Ferreira, F.S.; dos Santos, A.K.D.; Cavalcanti, L.F.; de Oliveira Araújo, B.; Gomes de Azevedo-Cutrim, A.C.; Lima Oliveira, A.L. Trophic State of an Urban Coastal Lagoon (Northern Brazil), Seasonal Variation of the Phytoplankton Community and Environmental Variables. Estuar. Coast. Shelf Sci. 2018, 216, 98–109. [Google Scholar] [CrossRef]
- Da Costa, R.L.; Todeschini, T.; Ribeiro, M.J.P.; Teixeira-Oliveira, M. Florações de cianobactérias potencialmente tóxicas em tanques de pisciculturas da região centro-sul do estado de Mato Grosso. Biodiversidade 2017, 16, 33–45. [Google Scholar]
- Schmidt, G.W. Primary Production of Phytoplankton in Three Types of Amazonian Waters: Investigation on Phytoplankton and Productivity in the Lower Rio Tapajós. Amazoniana 1982, 7, 335–348. [Google Scholar]
- Vieira, J.M.D.S.; Azevedo, M.T.D.P.; Azevedo, S.M.F.D.O.; Honda, R.Y.; Corrêa, B. Toxic cyanobacteria and microcystin concentrations in a public water reservoir in the Brazilian Amazon. Toxicon 2005, 45, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Sá, L.L.C.; Vieira, J.M.D.S.; Mendes, R.D.A.; Pinheiro, S.C.C.; Vale, E.R.; Alves, F.A.D.S.; Jesus, I.M.D.; Santos, E.C.D.O.; Costa, V.B.D. Ocorrência de floração de cianobactérias tóxicas na margem direita do Rio Tapajós. Rev. Pan Amaz. Saude 2010, 1, 159–166. [Google Scholar] [CrossRef]
- Brandão, I.L.D.S.; Mannaerts, C.M.; Verhoef, W.; Saraiva, A.C.F.; Paiva, R.S.; Da Silva, E.V. Using limnology and satellite imagery to identify algal blooms in an Amazonian reservoir. Sustainability 2017, 9, 2194. [Google Scholar] [CrossRef]
- Lobo, F.D.L.; Barbosa, C.C.F.; Yunes, J.S.; Junior, E.D.P.; Guimarães, P.S.; Novo, E.M.L.D.M.; de Carvalho, L.S.; Jorge, D.S.F. Estudo espaço-temporal de florações de cianobactérias no Rio Tapajós. In Simpósio Brasileiro de Sensoriamento Remoto; INPE: Santos, Brazil, 2017; Available online: https://proceedings.science/sbsr/trabalhos/estudo-espaco-temporal-de-floracoes (accessed on 15 May 2025).
- Lobo, M.T.M.P.S.; de Souza Nogueira, I.; Sgarbi, L.F.; Kraus, C.N.; de Oliveira Bomfim, E.; Garnier, J.; da Motta Marques, D.; Bonnet, M.-P. Functional groups for characterizing phytoplankton in Amazon floodplain lakes. Ecol. Indic. 2018, 95, 579–588. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.-P.; de Souza Nogueira, I.; Lobo, M.T.M.P.S.; da Motta Marques, D.; Garnier, J.; Vieira, L.G.G. Unraveling Flooding Dynamics and Nutrients’ Controls upon Phytoplankton Functional Dynamics in Amazonian Floodplain Lakes. Water 2019, 11, 154. [Google Scholar] [CrossRef]
- de Sá, L.L.C.; Vieira, J.M.D.S.; Mendes, R.D.A.; Pinheiro, S.C.C.; Vale, E.R.; Alves, F.A.D.S.; de Jesus, I.M.; Santos, E.C.D.O.; da Costa, V.B. Flutuação temporal de cianotoxinas no Rio Tapajós. Sci. Plena 2019, 15, 082402. [Google Scholar] [CrossRef]
- Torres, K.M.A.; Lopes, R.B.; Passos, C.J.S.; Pereira, A.C.; de Moura, L.S. Dominance of toxic cyanobacteria on the Santarém waterfront. Rev. Ibero Am. Cienc. Ambient. 2020, 11, 298–314. [Google Scholar]
- Gomes, A.L.; Cunha, C.J.; Lima, M.O.; DE Sousa, E.B.; Costa-Tavares, V.B.; Martinelli-Lemos, J.M. Biodiversity and interannual variation of cyanobacteria density in an estuary of the brazilian Amazon. An. Acad. Bras. Cienc. 2021, 93, e20191452. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.M.; Lopes, J.; Lima, J.S.; Reis, A.R. Percepção sobre o “limo” na comunidade Parauá. Contrib. Cienc. Sociais 2024, 17, 875–887. [Google Scholar]
- Maciel, D.A.; Kraus, C.N.; Novo, E.; Bonnet, M.-P.; Barbosa, C.; de Carvalho, L.S.; Ciotti, Á.M.; Begliomini, F.N. A New Remote Sensing Algorithm for Unveiling the Amazon Floodplain Lakes’ Phytoplankton Biodiversity from Space. Available online: https://ssrn.com/abstract=4792005 (accessed on 23 November 2024).
- Silva, G.M.; Silva, B.J.S.; Oliveira, D.S.C.; Rodrigues, A.G.C.; Lopes, L.F.; Rodrigues, L.P.M.; Rodrigues, F.A.S. Bacia Hidrográfica do Rio Pará e o Monitoramento das Cianobactérias no Corpo Hídrico da Praia de Beja. Ciênc. Saúde Interdiscip. 2024, 28, 131. [Google Scholar] [CrossRef]
- Leal, A.C.; Soares, M.C.P.; Silva, C.A.M.; Alves, M.M.; Cartágenes, P.R.B.; Bensabath, G. Níveis da Hepatotoxina Microcistina em Ambientes Aquáticos de Área Rural. Ilha de Marajó, Pará, Brasil. Rev. Soc. Bras. Med. Trop. 1999, 32, 448. Available online: https://patua.iec.gov.br/handle/iec/1375 (accessed on 15 October 2024).
- Braun, R. Limnologische Untersuchungen an einigen Seen im Amazonagebiet. Schweiz. Z. Für Hydrol. 1952, 14, 1–128. [Google Scholar]
- Sioli, H. The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and Its Basin; Dr. W. Junk Publishers: Hague, The Netherlands, 1984; p. 761. [Google Scholar]
- Vieira, J.M.S. Toxicidade de Cianobactérias e Concentração de Microcistinas em uma Represa de Abastecimento Público da Região Amazônica do Brasil. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2002. [Google Scholar]
- Silva, S.C.F. Variação Espaço-Temporal de Cianobactérias no Baixo Rio Tapajós, Pará, Brasil. Ph.D. Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2020. [Google Scholar]
- Alves, C.P.P. The Dynamics of Phytoplankton in an Amazon Floodplain—Seasonal and Nycthemeral Variations (Lago Grande de Curuai—Pará, Brazil). Master’s Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2011. [Google Scholar]
- Sousa, E.B. Fatores Ambientais Reguladores da Dinâmica do Fitoplâncton e das Cianobactérias dos Manan-ciais de Abastecimento da Região Metropolitana de Belém, Pará, Brasil. Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Silva, S.C.F. Ecotoxicologia de Cianobactérias no Canal Principal do Baixo Rio Tapajós, Santarém, Pará, Amazônia, Brasil. Master’s Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2012. [Google Scholar]
- Araújo, J.L. Monitoramento por Sensoriamento Remoto da Concentração de Clorofila-a e das Florações de Cianobactérias no Baixo Tapajós: Audiovisual Praias do Tapajós para Gerações Presentes e Futuras. Master’s Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2020. [Google Scholar]
- Oliveira, F.A. Quantificação de Microcistinas em Águas do Rio Tapajós, Amazônia, Brasil. Master’s Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2022. [Google Scholar]
- Souza, D.A. Efeitos da Hidrodinâmica sobre a Comunidade Fitoplanctônica e Grupos Funcionais no Lago Grande do Curuai, PA. Master’s Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2015. [Google Scholar]
- Instituto Evandro Chagas (IEC). IEC Acompanha a Presença de Cianobactérias com Potencial Tóxico à Saúde Humana em Barcarena. Gov.br. 2024. Available online: https://www.gov.br/iec/pt-br/assuntos/noticias/iec-acompanha-a-presenca-de-cianobacterias-com-potencial-toxico-a-saude-humana-em-barcarena (accessed on 13 February 2025).
- Rebello, J.F.S.; Lima, M.O.; Silva, O.A. Relatório de Pesquisa: A Mortandade de Peixes no Rio Iriri. 2003. Available online: https://acervo.socioambiental.org/acervo/documentos/mortandade-de-peixes-no-rio-iriri-relatorio-de-pesquisa (accessed on 13 February 2025).
- Oliveira, F.A.; Guerrero-Moreno, M.A.; Silva, E.C.; Cunha, K.M.L.C.; Santos, M.A.; Dunck, B.; Pires, P.; Junior, J.M.B.O.; Talgatti, D.M. Technical Report Análise de Microcistinas nas Águas das Praias de Ponta de Pedras; Alter do Chão: Santarém, Brazil; e Pindobal: Belterra, PA, USA, 2025. [Google Scholar] [CrossRef]
- Pinheiro, M.M.D.L.; Santos, B.L.T.; Filho, J.V.D.; Pedroti, V.P.; Cavali, J.; dos Santos, R.B.; Nishiyama, A.C.O.C.; Guedes, E.A.C.; Schons, S.D.V. First monitoring of cyanobacteria and cyanotoxins in freshwater from fish farms in Rondonia State, Brazil. Heliyon 2023, 9, e18518. [Google Scholar] [CrossRef]
- Nascimento, E.L. Fatores Ambientais Reguladores da Dinâmica de Cianobactérias no Reservatório da Usina Hidrelétrica de Samuel-Rondônia (Amazônia Ocidental, Brasil). Ph.D. Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Hurtado, F.B.; da Costa, R.L.; Figueiredo, F.M.; de Queiroz, C.B.; Bay, M. Diversidade Fitoplanctônica da Piscicultura Santa Helena, Alvorada D’Oeste, RO: Ocorrência de Floração de Cianobactérias. 2014. Available online: https://www.researchgate.net/profile/fernanda-bay-hurtado/publication/269097868_diversidade-fitoplanctonica_da_piscicultura_santa_helena_alvorada-d’oeste_ro_ocorrencia_de_floracao_de_cianobacterias.pdf (accessed on 10 January 2025).
- Bandeira, I.M.S. Ocorrência de Florações de Cianobactérias no Rio Urupá (Rondônia): Uma Abordagem Ambiental e de Saúde Pública. Master’s Thesis, Federal University of Rondônia, Ji-Paraná, Brazil, 2021. [Google Scholar]
- Kozerski, G.R. Qualidade Hídrica do Rio Jamari na Região de Ariquemes-RO: Ênfase no Estudo de Cianobactérias. Master’s Thesis, Federal University of Rondônia, Ji-Paraná, Brazil, 2023. [Google Scholar]
- Furtado, E.F. Bioensaio Toxicológico com Cianobactérias do Efluente da Lagoa de Estabilização e do Igarapé Grande. Master’s Thesis, Federal University of Roraima, Boa Vista, Brazil, 2011. [Google Scholar]
- Silva, J.R.L.; Jardim, F.A.; Cunha, I.L.; Sousa, F.P.; Wettman, A. Diagnóstico Preliminar da Ocorrência de Cianobactérias Tóxicas e Potencialmente Tóxicas em Estações de Tratamento de Água e na Praia da Graciosa em Palmas—TO. In 55ª Reunião da SBPC; UFPE: Recife, Brazil, 2003. [Google Scholar]
- Silva, J.R.L.; Nogueira, I.S.; Silva, N.M.; Marques, J.A.V. Ocorrência de Floração de Cylindrospermopsis raciborskii em Águas do Rio Tocantins. Estudo de Caso: ETA Tocantínia. In 23º Congresso Brasileiro de Engenharia Sanitária e Ambiental; ABES: Campo Grande, Brazil, 2005. [Google Scholar]
- Pereira, V.L.R. Limnologia e o Gerenciamento Integrado do Reservatório da Usina Hidroelétrica Luís Eduardo Magalhães—UHE—Lajeado/Tocantins. Ph.D. Thesis, Universidade de São Paulo, São Carlos, Brazil, 2002. [Google Scholar]
- Silva, J.R.L. Cianobactérias e Cianotoxinas no Reservatório da UHE Lajeado, Palmas-TO: Fatores Condicionantes ao Surgimento de Floração e Avaliação da Remoção por Meio de uma Instalação Piloto de Dupla Filtração. Ph.D. Thesis, Universidade de São Paulo, São Carlos, Brazil, 2014. [Google Scholar]
- Silva, J.R.L. Dinâmica de Cianobactérias e Cianotoxinas em um Braço do Reservatório da Usina Hidroelétrica Luiz Eduardo Magalhães e Suas Implicações para o Abastecimento Público de Palmas-TO. Master’s Thesis, Universidade Federal do Rio Grande do Sul, Rio Grande do Sul, Brazil, 2009. [Google Scholar]
- Brazil. Ministério da Saúde. Instituto Evandro Chagas Identifica a Presença de Cianobactérias com Potencial Tóxico à Saúde Humana em Barcarena. 2024. Available online: https://www.gov.br/iec/pt-br/assuntos/noticias/instituto-evandro-chagas-identifica-a-presenca-de-cianobacterias-com-potencial-toxico-a-saude-humana-em-barcarena (accessed on 9 October 2024).
- Aguilera, A.; Almanza, V.; Haakonsson, S.; Palacio, H.; Rodas, G.; Barros, M.; Capelo-Neto, J.; Urrutia, R.; Aubriot, L.; Bonilla, S. Cyanobacterial bloom monitoring and assessment in Latin America. Harmful Algae 2023, 125, 102429. [Google Scholar] [CrossRef]
- Frau, D. Towards a Quantitative Definition of Cyanobacteria Blooms. Environ. Rev. 2023, 31, 643–651. [Google Scholar] [CrossRef]
- PORTARIA GM/MS No 888; Ministry of Health. Office of the Minister: Brasília, Brazil, 2021.
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 295–304. [Google Scholar]
- Miranda, R.G.; Pereira, S.F.P.; Alves, D.T.V.; Oliveira, G.R.F. Qualidade dos recursos hídricos da Amazônia—Rio Tapajós: Avaliação de caso em relação aos elementos químicos e parâmetros físico-químicos. Ambi Agua 2009, 4, 75–92. [Google Scholar] [CrossRef]
- Novo, E.M.L.M.; Barbosa, C.C.F.; Freitas, R.M.; Melack, J.; Shimabukuro, Y.E.; Filho, W.P. Distribuição sazonal de fitoplâncton no Lago Grande de Curuai em resposta ao pulso de inundação do Rio Amazonas a partir da análise de imagens MODIS. In Anais do XII Simpósio Brasileiro de Sensoriamento Remoto; INPE: Goiânia, Brasil, 2005; pp. 3175–3182. [Google Scholar]
- Sioli, H. Das Wasser in Amazonasgebiet. Forsch. Fortschr. 1950, 26, 274–280. [Google Scholar]
- Monte, C.N.; Rodrigues, A.P.C.; Macedo, S.; Régis, C.; Saldanha, E.C.; Ribeiro, A.C.; Machado, W. A Influência Antrópica na Qualidade da Água do Rio Tapajós, na Cidade de Santarém-PA. Rev. Bras. Geogr. Fís. 2021, 14, 3695–3710. Available online: https://periodicos.ufpe.br/revistas/index.php/rbgfe/article/view/249613/40213 (accessed on 6 January 2025). [CrossRef]
- Bomfim, E.O.; Kraus, C.N.; Lobo, M.T.M.P.S.; Nogueira, I.S.; Peres, L.G.M.; Boaventura, G.R.; Laques, A.-E.; Garnier, J.; Seyler, P.; Marques, D.M.; et al. Trophic state index validation based on the phytoplankton functional group approach in Amazon floodplain lakes. Inland Waters 2019, 9, 309–319. [Google Scholar] [CrossRef]
- Prestes, Y.O.; Rollnic, M.; Silva, M.S.; Rosario, R.P. Volume Transport in the Tidal Limit of the Pará River, Brazil. In Proceedings of the 17th Physics of Estuaries and Coastal Seas (PECS) Conference, Porto Galinhas, Pernambuco, Brazil, 19–24 October 2014. [Google Scholar]
- Ferreira, D.P.M.; Carneiro, B.S.; Marques, L.C.A.; El-Robrini, M. Qualidade das Águas Estuarinas do Rio Pará na Zona Portuária de Vila do Conde (Município de Barcarena/Pará); Geografia: Rio Claro, Brazil, 2022; Volume 47. [Google Scholar]
- Gomes, L.C.S.B.; Vasconcelos Júnior, N.T.; Faial, K.C.F.; Neto, N.N.; Medeiros, A.C.; Carneiro, B.S. Avaliação da Qualidade da Água Superficial do Rio Pará nas Proximidades da Região Portuária de Vila do Conde, Barcarena-PA. Rev. Foco 2024, 17, e4142. [Google Scholar] [CrossRef]
- Prestes, Y.O. Interações Físicas Entre o Estuário do Rio Pará e a Plataforma Continental no Norte do Brasil. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2016. [Google Scholar]
- Pereira Neta, O.S. Processos Antropogênicos e Seus Impactos Para a Saúde em uma Área Industrial Amazônica: Um Olhar Histórico Epidemiológico e a Evolução da DPOC (1980–2020). Master’s Thesis, Instituto Evandro Chagas, Ananindeua, Brazil, 2023; p. 171. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Academic Google. Available online: https://scholar.google.com.br/ (accessed on 12 May 2025).
- Scielo Database. Available online: https://www.scielo.br/ (accessed on 12 May 2025).
- Portal Periódicos Database. Available online: https://www.periodicos.capes.gov.br/ (accessed on 17 May 2025).
- Repository from the National Institute for Amazon Research (INPA). Available online: https://repositorio.inpa.gov.br/ (accessed on 17 May 2025).
- Repository of the National Institute for Space Research. Available online: http://bibdigital.sid.inpe.br/ (accessed on 17 May 2025).
- Repository of the Federal University of Amapá. Available online: http://repositorio.unifap.br/simple-search (accessed on 17 May 2025).
- Repository of the Federal University of Pará. Available online: https://www.repositorio.ufpa.br/ (accessed on 17 May 2025).
- Repository of the Federal University of West Pará. Available online: https://repositorio.ufopa.edu.br (accessed on 17 May 2025).
- Repository of the Federal University of Amazonas. Available online: https://riu.ufam.edu.br/ (accessed on 17 May 2025).
- Repository of the Federal University of Rondônia. Available online: https://ri.unir.br/jspui/handle/123456789/4701 (accessed on 17 May 2025).
- Oasisbr. Available online: https://oasisbr.ibict.br/ (accessed on 17 May 2025).
- Limnologische Untersuchungen an Einigen Seen im Amazonasgebiet. Available online: https://www.research-collection.ethz.ch/handle/20.500.11850/132746 (accessed on 17 May 2025).
- Cyano Lakes. Available online: www.cyanolakes.com (accessed on 15 October 2024).


| Cyanobacteria Genera | Acre | Amapá | Amazonas | Maranhão | Mato Grosso | Pará | Rondônia | Roraima | Tocantins |
|---|---|---|---|---|---|---|---|---|---|
| Anabaena | X | X | |||||||
| Aphanizomenon | X | ||||||||
| Dolichospermum | X | X | X | ||||||
| Lyngbya | X | ||||||||
| Limnothrix | X | ||||||||
| Microcystis | X | X | X | X | X | X | X | ||
| Nostoc | X | ||||||||
| Phormidium | X | ||||||||
| Planktolyngbya | X | X | X | X | |||||
| Planktothrix | X | X | X | X | X | X | |||
| Radiocystis | X | X | |||||||
| Raphidiopsis | X | X | X | X | X | ||||
| Sinechocystis | X |
| Brazil State | Toxin | Maximum Concentration of Toxins | Method | Estimation | Dominant Toxin-Producing Genera | Reference |
|---|---|---|---|---|---|---|
| Pará | STX | 1.5 µg∙mL−1 | HPLC | Visual detection of bloom | Microcystis sp. Raphidiopsis sp. | [47] |
| Pará | MC | 1.25 µg∙mL−1 | ELISA | 20,000 cells∙mL−1 Visual detection of bloom | Microcystis viridis Radiocystis fernandoii | [23,38] |
| Tocantins | MC, STX, CYN | 0.2 µg∙mL−1; 0.07 µg∙mL−1; 1.1 µg∙mL−1 | ELISA | 2486 cells.mL−1 | Raphidiopsis raciborskii Planktolyngbya limnetica | [59] |
| Pará | MC-LR | 12.39 µg∙mL−1 | HPLC | Visual detection of bloom | Dolichospermum sp. | [24] |
| Roraima | - | - | Toxicological test on mice | 164.22 µg∙mL−1 chlo-a Visual detection of bloom | Planktothrix agardhii | [54] |
| Rondônia * | MC | 0.75 µg∙mL−1 (1.26 µg∙mL−1) | ELISA | 3.2 µg∙mL−1 chlo-a 1030 cells.mL−1 | Microcystis panniformes | [50] |
| Pará | MC-LR | 0.1 µg∙mL−1 | ELISA | 2169 cells.mL−1 2.06 µg∙mL−1 chlo-a | Microcystis 933 cells.mL−1 | [29,42] |
| Amapá | MC | 1.73 µg∙mL−1 | ELISA | 146.82 µg∙mL−1 chlo-a | Microcystis aeruginosa | [13] |
| Pará | MC | 0.17 µg∙mL−1 | ELISA | - | - | [35] |
| Amapá * | MC-LR | 2.1 µg∙mL−1 | ELISA | 1090 cells.mL−1, 0.02 mm3∙mL−1 biovolume | Limnothrix planctonica 898.4 cells.mL−1; 0.02 mm3∙mL−1 | [14,15] |
| Pará | MC-LR | 1.35 µg∙mL−1 | HPLC | 37,889 cells.mL−1 | Dolichospermum sp. | [14] |
| Pará | MC | 11.95 µg∙mL−1 | HPLC | 3209 ind.mL−1 | Dolichospermum sp. | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, M.P.C.; Cunha, E.; Silva, L.; Leão, J.; Tavares, V.C.; Brabo de Sousa, E.; Faustino, S. Cyanobacterial Blooms and the Presence of Cyanotoxins in the Brazilian Amazon. Toxins 2025, 17, 296. https://doi.org/10.3390/toxins17060296
Schneider MPC, Cunha E, Silva L, Leão J, Tavares VC, Brabo de Sousa E, Faustino S. Cyanobacterial Blooms and the Presence of Cyanotoxins in the Brazilian Amazon. Toxins. 2025; 17(6):296. https://doi.org/10.3390/toxins17060296
Chicago/Turabian StyleSchneider, Maria Paula Cruz, Elane Cunha, Lucas Silva, James Leão, Vanessa Costa Tavares, Eliane Brabo de Sousa, and Silvia Faustino. 2025. "Cyanobacterial Blooms and the Presence of Cyanotoxins in the Brazilian Amazon" Toxins 17, no. 6: 296. https://doi.org/10.3390/toxins17060296
APA StyleSchneider, M. P. C., Cunha, E., Silva, L., Leão, J., Tavares, V. C., Brabo de Sousa, E., & Faustino, S. (2025). Cyanobacterial Blooms and the Presence of Cyanotoxins in the Brazilian Amazon. Toxins, 17(6), 296. https://doi.org/10.3390/toxins17060296








