Patient Satisfaction with Aesthetic Outcomes Following OnabotulinumtoxinA Treatment for Chronic Migraine: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Patient Population
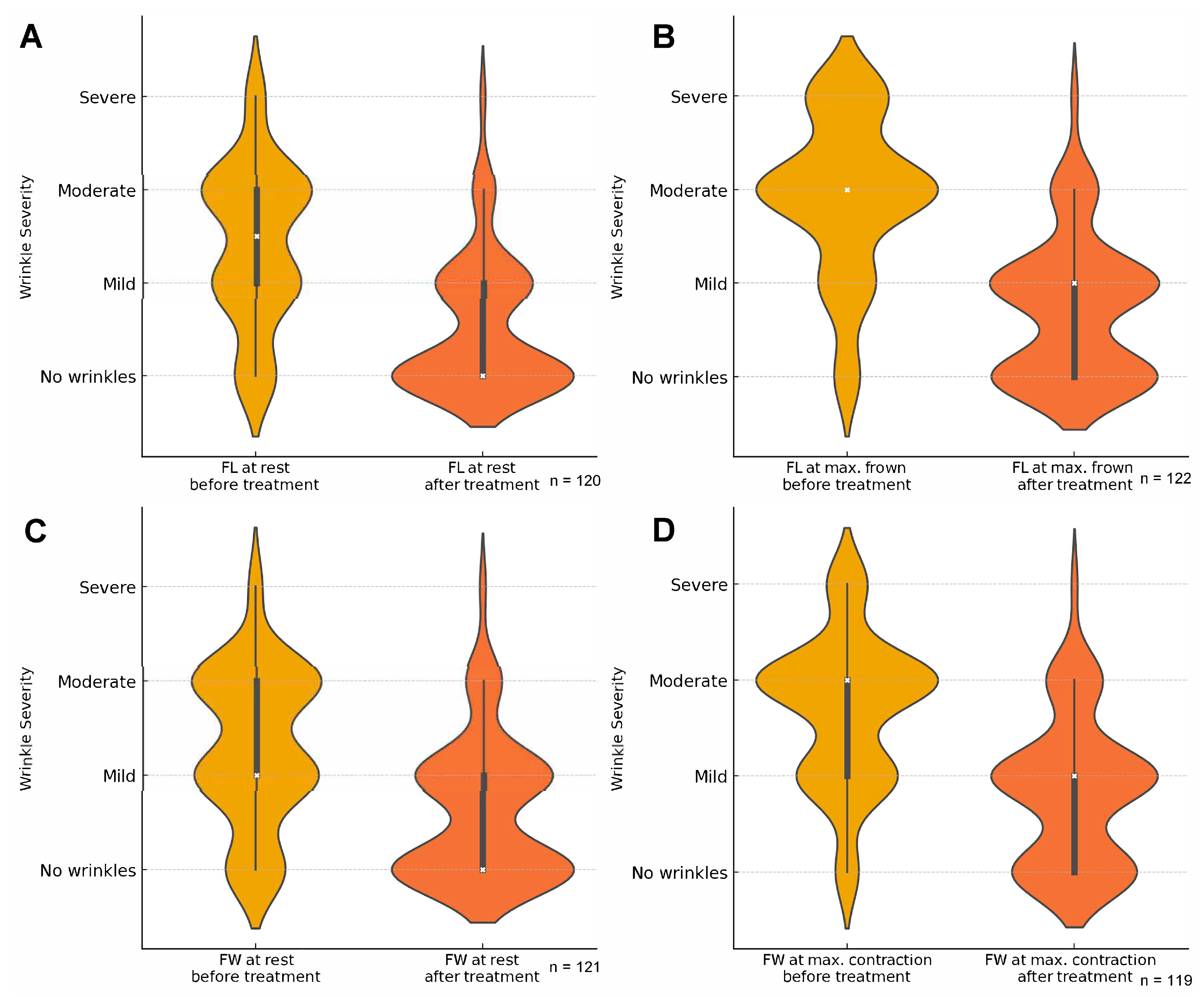
2.2. Primary Outcome
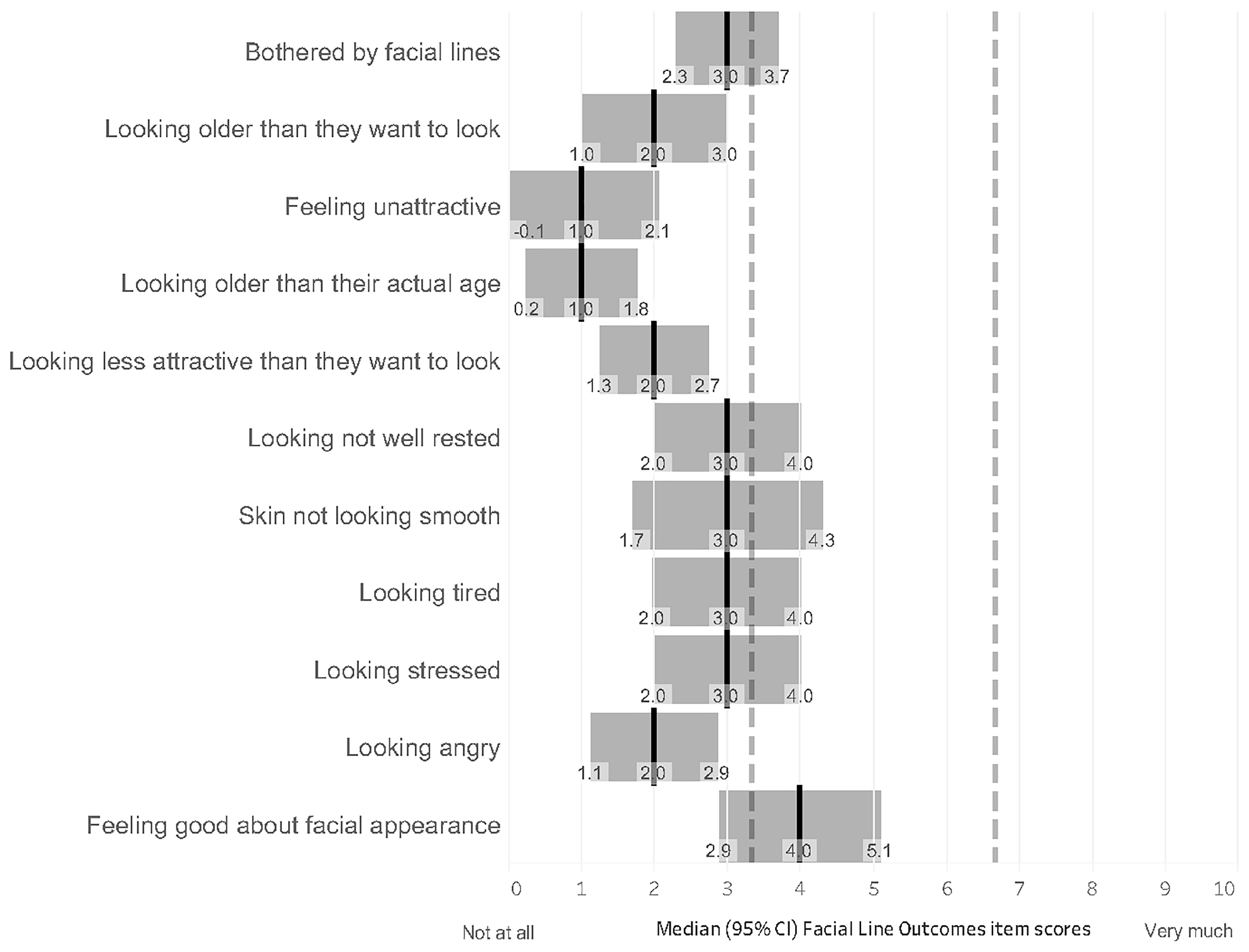
2.3. Secondary Outcomes
2.4. Association Between the Effect of Treatment on Chronic Migraine and Satisfaction with Facial Appearance
2.5. Safety of Chronic Migraine Treatment with OnabotulinumtoxinA
3. Discussion
Limitations of the Study
4. Conclusions
5. Materials and Methods
5.1. Patients
5.2. Outcomes
5.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| BoNT-A | Botulinum toxin A |
| CI | Confidence interval |
| CM | Chronic migraine |
| GAIS | Global Aesthetic Improvement Scale |
| FL | Frown line |
| FLO-11 | Facial Line Outcomes questionnaire |
| FLSQ | Facial Line Satisfaction Questionnaire |
| FDA | Food and Drug Administration |
| FW | Forehead wrinkles |
| ICHD-3 | International Classification of Headache Disorders-3 |
| onaBoNT-A | OnabotulinumtoxinA |
| PREEMPT | Phase III REsearch Evaluating Migraine Prophylaxis Therapy |
| PRO | Patient-reported outcome |
References
- Onan, D.; Farham, F.; Martelletti, P. Clinical Conditions Targeted by OnabotulinumtoxinA in Different Ways in Medicine. Toxins 2024, 16, 309. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Manack Adams, A.; Brin, M.F. Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef]
- Patel, K.R.; Rastogi, S.; Prather, H.B. A comprehensive review on the history, uses, and safety of onabotulinum toxin type A (Botox). Dermatol. Rev. 2022, 3, 180–196. [Google Scholar] [CrossRef]
- Binder, W.; Brin, M.; Blitzer, A.; Schoenrock, L. Botulinum toxin A (Botox) for treatment in migraine headache: An open label assessment. Headache 1999, 39, 344. [Google Scholar]
- Binder, W.J.; Brin, M.F.; Blitzer, A.; Schoenrock, L.D.; Pogoda, J.M. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: An open-label study. Otolaryngol. Head Neck Surg. 2000, 123, 669–676. [Google Scholar] [CrossRef]
- Aurora, S.K.; Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Boczarska-Jedynak, M.; Blumenfeld, A.M. Injection technique of the upper face with onabotulinumtoxinA in chronic migraine. Headache 2023, 63, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Corasaniti, M.T.; Bagetta, G.; Nicotera, P.; Tarsitano, A.; Tonin, P.; Sandrini, G.; Lawrence, G.W.; Scuteri, D. Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Toxins 2023, 15, 332. [Google Scholar] [CrossRef]
- Boczarska-Jedynak, M. Experience of treatment of chronic migraine with botulinum toxin type A among aesthetic medicine professionals in Poland. Neurol. Neurochir. Pol. 2023, 57, 363–370. [Google Scholar] [CrossRef]
- Carruthers, J.A.; Lowe, N.J.; Menter, M.A.; Gibson, J.; Nordquist, M.; Mordaunt, J.; Walker, P.; Eadie, N. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J. Am. Acad. Dermatol. 2002, 46, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, J.D.; Lowe, N.J.; Menter, M.A.; Gibson, J.; Eadie, N. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast. Reconstr. Surg. 2003, 112, 1089–1098. [Google Scholar] [CrossRef]
- Fagien, S.; Cohen, J.L.; Coleman, W.; Monheit, G.; Carruthers, J.; Street, J.; Larsen, K.E.; Yushmanova, I.; Lei, X.; Lee, E.; et al. Forehead Line Treatment with OnabotulinumtoxinA in Subjects with Forehead and Glabellar Facial Rhytids: A Phase 3 Study. Dermatol. Surg. 2017, 43 (Suppl. S3), S274–S284. [Google Scholar] [CrossRef]
- De Boulle, K.; Werschler, W.P.; Gold, M.H.; Bruce, S.; Sattler, G.; Ogilvie, P.; Street, J.; Larsen, K.E.; Yushmanova, I.; Lei, X.; et al. Phase 3 Study of OnabotulinumtoxinA Distributed Between Frontalis, Glabellar Complex, and Lateral Canthal Areas for Treatment of Upper Facial Lines. Dermatol. Surg. 2018, 44, 1437–1448. [Google Scholar] [CrossRef]
- Rivers, J.K.; Bertucci, V.; McGillivray, W.; Muhn, C.; Rosen, N.; Solish, N.; Weichman, B.M.; Wheeler, S.; Daniels, S.R.; Gallagher, C.J. Subject satisfaction with onabotulinumtoxinA treatment of glabellar and lateral canthal lines using a new patient-reported outcome measure. Dermatol. Surg. 2015, 41, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Dayan, S.; Yoelin, S.G.; De Boulle, K.; Garcia, J.K. The Psychological Impacts of Upper Facial Lines: A Qualitative, Patient-Centered Study. Aesthet. Surg. J. Open Forum 2019, 1, ojz015. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, P.; Rivkin, A.Z.; Dayan, S.; Yoelin, S.G.; Weichman, B.M.; Garcia, J.K. OnabotulinumtoxinA for Treatment of Forehead and Glabellar Lines: Subject-Reported Satisfaction and Impact From a Phase 3 Double-Blind Study. Dermatol. Surg. 2019, 45, 689–699. [Google Scholar] [CrossRef]
- Rivkin, A.Z.; Ogilvie, P.; Dayan, S.; Yoelin, S.G.; Weichman, B.M.; Garcia, J.K. OnabotulinumtoxinA for Simultaneous Treatment of Upper Facial Lines: Subject-Reported Satisfaction and Impact From a Phase 3 Study. Dermatol. Surg. 2020, 46, 50–60. [Google Scholar] [CrossRef]
- Dayan, S.; Coleman, W.P., 3rd; Dover, J.S.; De Boulle, K.; Street, J.; Romagnano, L.; Daniels, S.; Kowalski, J.W.; Lei, X.; Lee, E. Effects of OnabotulinumtoxinA treatment for crow’s feet lines on patient-reported outcomes. Dermatol. Surg. 2015, 41 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef]
- Puledda, F.; Sacco, S.; Diener, H.C.; Ashina, M.; Al-Khazali, H.M.; Ashina, S.; Burstein, R.; Liebler, E.; Cipriani, A.; Chu, M.K.; et al. International Headache Society Global Practice Recommendations for Preventive Pharmacological Treatment of Migraine. Cephalalgia 2024, 44, 3331024241269735. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Aurora, S.K.; Diener, H.C.; DeGryse, R.E.; Lipton, R.B.; Turkel, C.C. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J. Neurol. Neurosurg. Psychiatry 2015, 86, 996–1001. [Google Scholar] [CrossRef]
- Herd, C.P.; Tomlinson, C.L.; Rick, C.; Scotton, W.J.; Edwards, J.; Ives, N.; Clarke, C.E.; Sinclair, A. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst. Rev. 2018, 6, Cd011616. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.; Silberstein, S.D.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; Binder, W.J. Method of injection of onabotulinumtoxinA for chronic migraine: A safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache 2010, 50, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Keen, M.; Blitzer, A.; Aviv, J.; Binder, W.; Prystowsky, J.; Smith, H.; Brin, M. Botulinum toxin A for hyperkinetic facial lines: Results of a double-blind, placebo-controlled study. Plast. Reconstr. Surg. 1994, 94, 94–99. [Google Scholar] [CrossRef]
- Klassen, A.F.; Cano, S.J.; Scott, A.; Snell, L.; Pusic, A.L. Measuring patient-reported outcomes in facial aesthetic patients: Development of the FACE-Q. Facial Plast. Surg. 2010, 26, 303–309. [Google Scholar] [CrossRef]
- Yaworsky, A.; Daniels, S.; Tully, S.; Beddingfield, F., 3rd; Kowalski, J.; Fitzgerald, K.; Somogyi, C.; Burgess, S.M. The impact of upper facial lines and psychological impact of crow’s feet lines: Content validation of the Facial Line Outcomes (FLO-11) Questionnaire. J. Cosmet. Dermatol. 2014, 13, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.E.; Finn, J.C.; Stetler, L.; Mackowiak, J.; Kowalski, J.W. Development of the Facial Lines Treatment Satisfaction Questionnaire and initial results for botulinum toxin type A-treated patients. Dermatol. Surg. 2003, 29, 444–449; discussion 449. [Google Scholar] [CrossRef]
- Pompilus, F.; Burgess, S.; Hudgens, S.; Banderas, B.; Daniels, S. Development and validation of a novel patient-reported treatment satisfaction measure for hyperfunctional facial lines: Facial line satisfaction questionnaire. J. Cosmet. Dermatol. 2015, 14, 274–285. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; Food and Drug Administration: Silver Spring, MD, USA, 2009. [Google Scholar]
- Patel, V.; Lee, E.; Silberberg, M.B. Facial Line Outcomes (FLO-11) and Facial Line Satisfaction Questionnaire (FLSQ) Meet FDA Patient-Reported Outcome Guidance. Aesthet. Surg. J. 2020, 40, Np710–Np711. [Google Scholar] [CrossRef]


| Characteristic | N = 124 |
|---|---|
| Sex, n (%) | |
| Female | 115 (92.7) |
| Male | 9 (7.3) |
| Median age (range), years | 42.5 (21.0–72.0) |
| Median body mass index (range) | 23.6 (16.8–35.4) |
| Overweight (BMI > 30), n (%) | 34 (27.4) |
| Obesity (BMI > 30), n (%) | 9 (7.3) |
| Median age at first migraine episode (range), years | 16.5 (6.0–45.0) |
| Median disease duration (range), years | 23.0 (6.0–62.0) |
| Median number of onaBoNT-A treatment cycles (range) | 3 (3–10) |
| Any comorbidity, n (%) | 103 (83.1) |
| Tension-type headache | 50 (40.3) |
| Anxiety | 20 (16.1) |
| Bruxism | 20 (16.1) |
| Hypothyroidism | 20 (16.1) |
| Depression | 19 (15.3) |
| Insomnia | 14 (11.3) |
| Adverse Events, n (%) | N = 124 |
|---|---|
| Forehead movement discrepancies | 36 (29.0) |
| Eyebrow asymmetry | 9 (7.3) |
| Eyelid ptosis | 7 (5.6) |
| Brow ptosis | 5 (4.0) |
| Mefisto sign | 3 (2.4) |
| Crow’s feet discrepancies | 2 (1.6) |
| Forehead wrinkles | 1 (0.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boczarska-Jedynak, M.; Bott-Karoń, M.; Marschollek, K.; Antolak, M.; Świat, M.; Waliszewska-Prosół, M. Patient Satisfaction with Aesthetic Outcomes Following OnabotulinumtoxinA Treatment for Chronic Migraine: A Cross-Sectional Study. Toxins 2025, 17, 292. https://doi.org/10.3390/toxins17060292
Boczarska-Jedynak M, Bott-Karoń M, Marschollek K, Antolak M, Świat M, Waliszewska-Prosół M. Patient Satisfaction with Aesthetic Outcomes Following OnabotulinumtoxinA Treatment for Chronic Migraine: A Cross-Sectional Study. Toxins. 2025; 17(6):292. https://doi.org/10.3390/toxins17060292
Chicago/Turabian StyleBoczarska-Jedynak, Magdalena, Marta Bott-Karoń, Karol Marschollek, Mariola Antolak, Maciej Świat, and Marta Waliszewska-Prosół. 2025. "Patient Satisfaction with Aesthetic Outcomes Following OnabotulinumtoxinA Treatment for Chronic Migraine: A Cross-Sectional Study" Toxins 17, no. 6: 292. https://doi.org/10.3390/toxins17060292
APA StyleBoczarska-Jedynak, M., Bott-Karoń, M., Marschollek, K., Antolak, M., Świat, M., & Waliszewska-Prosół, M. (2025). Patient Satisfaction with Aesthetic Outcomes Following OnabotulinumtoxinA Treatment for Chronic Migraine: A Cross-Sectional Study. Toxins, 17(6), 292. https://doi.org/10.3390/toxins17060292





