The Biological Role of Conoporins, Actinoporin-like Pore-Forming Toxins from Cone Snails
Abstract
1. Introduction
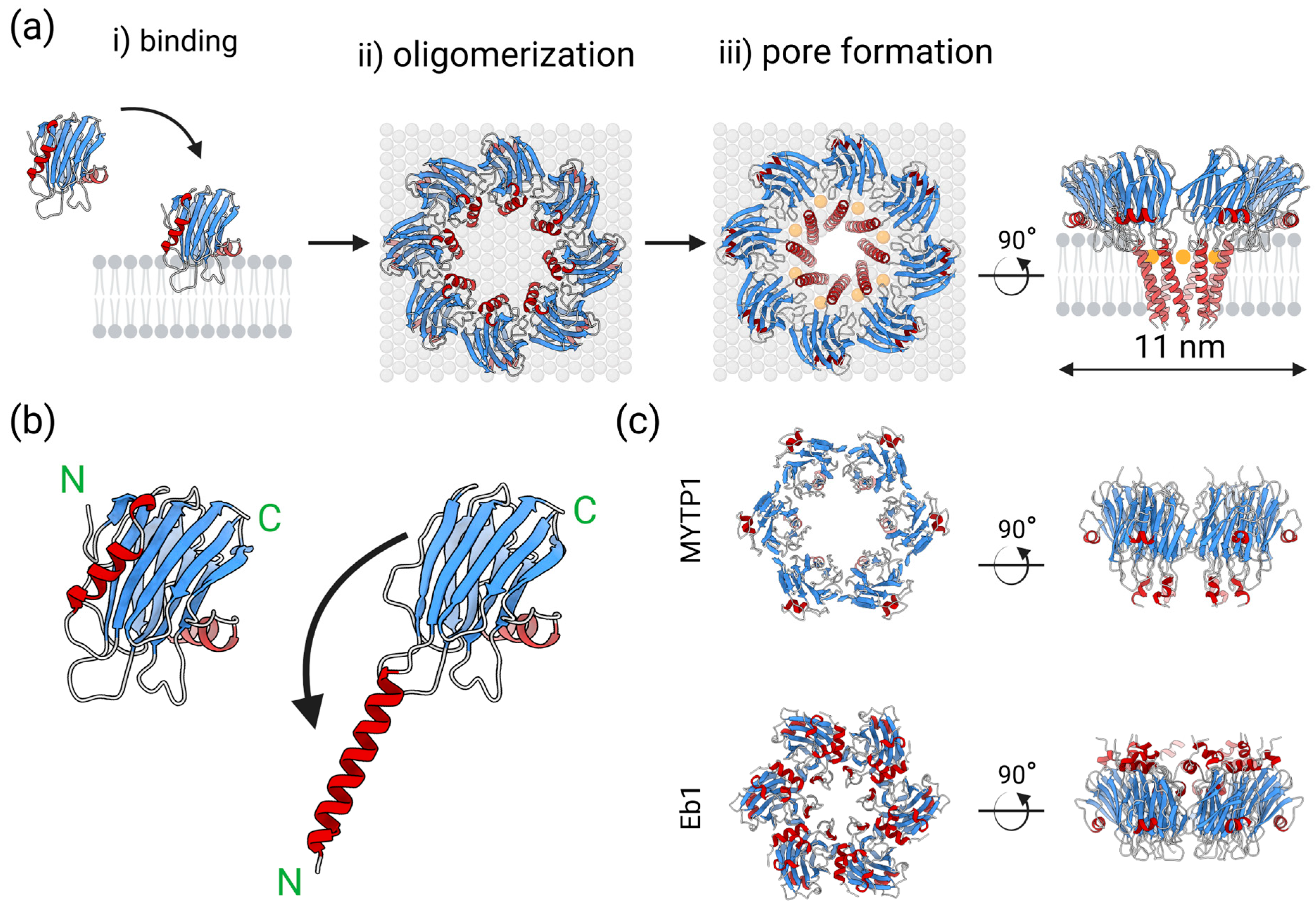
2. Actinoporins
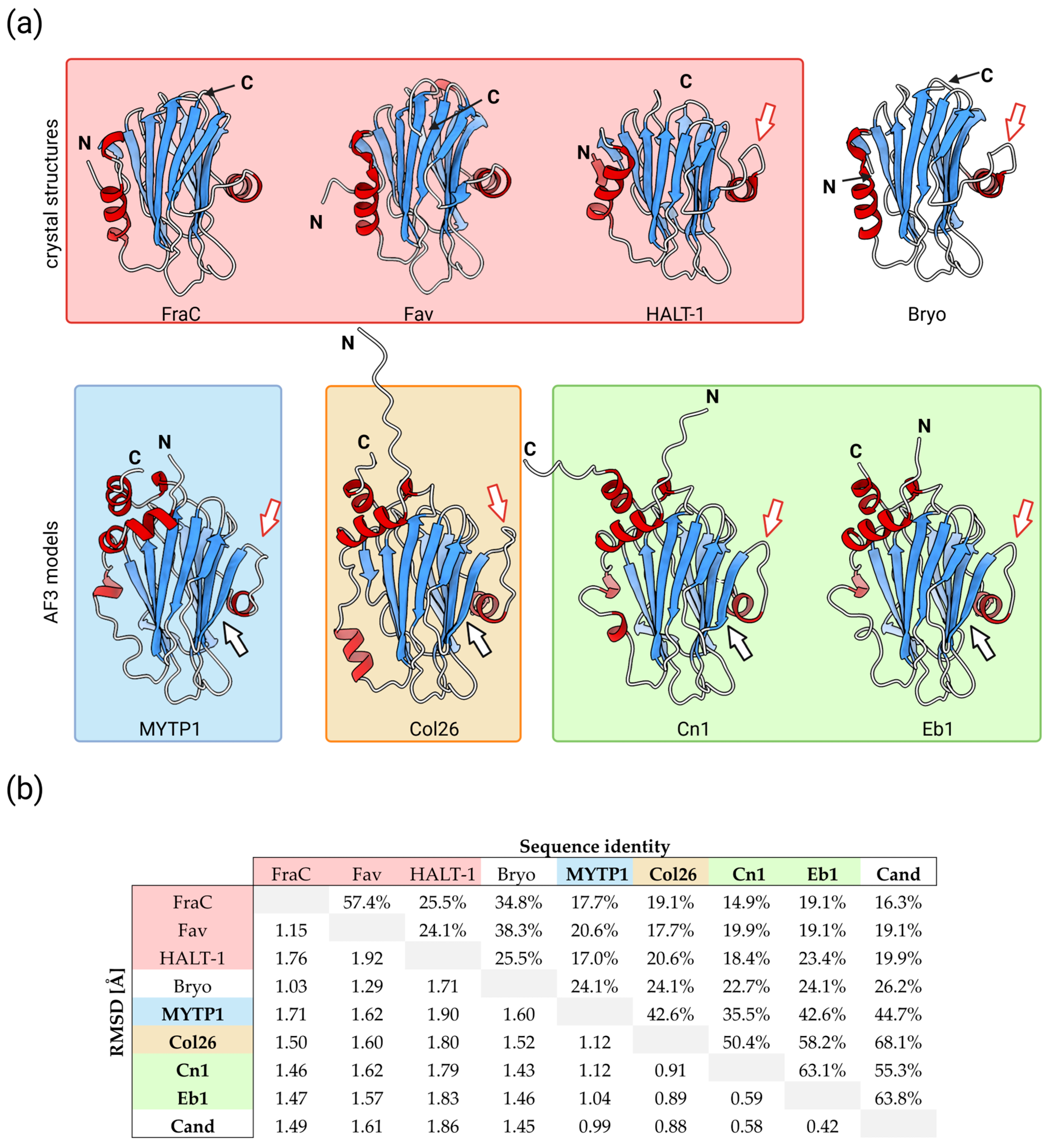
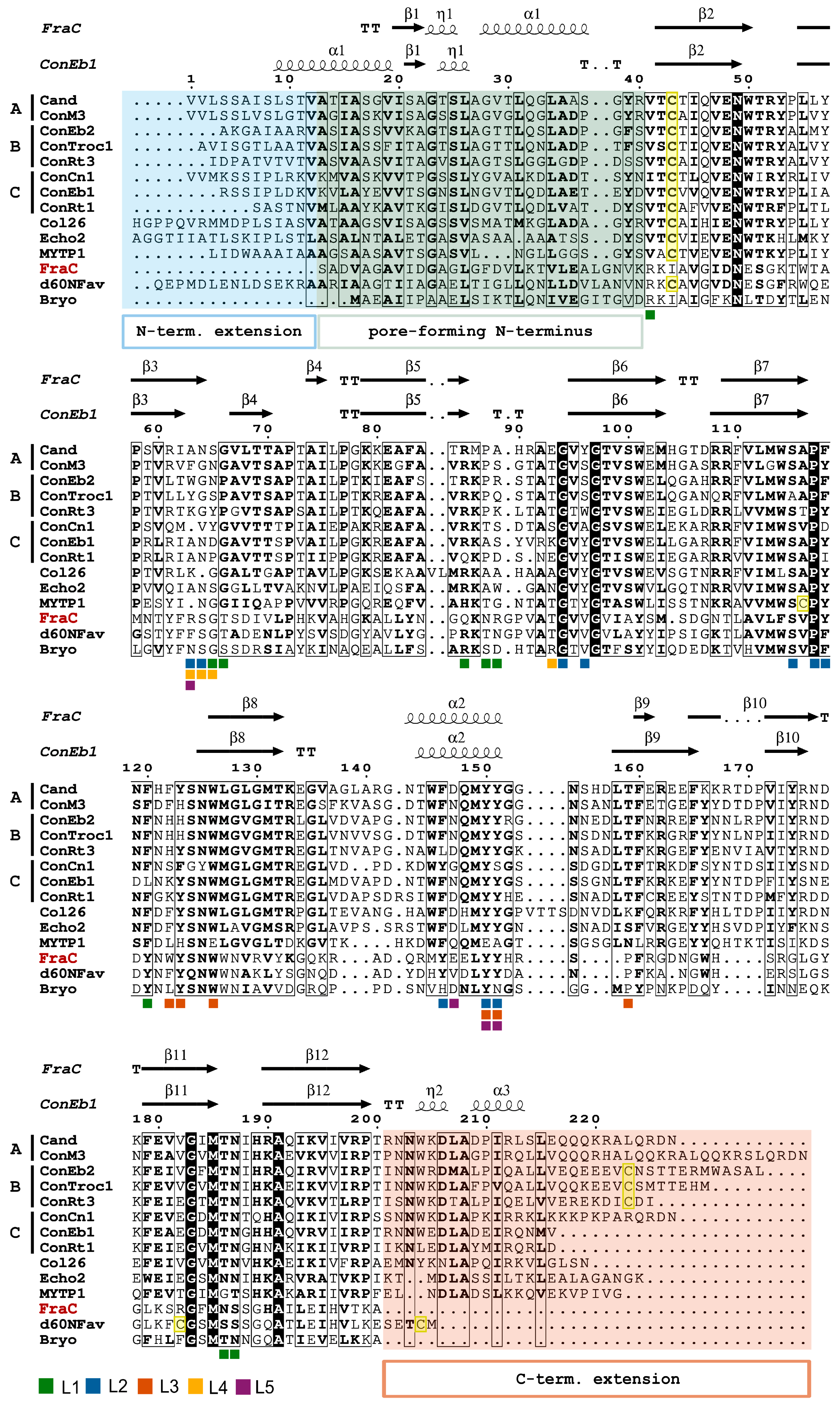
3. Actinoporin-like Proteins in Molluscs
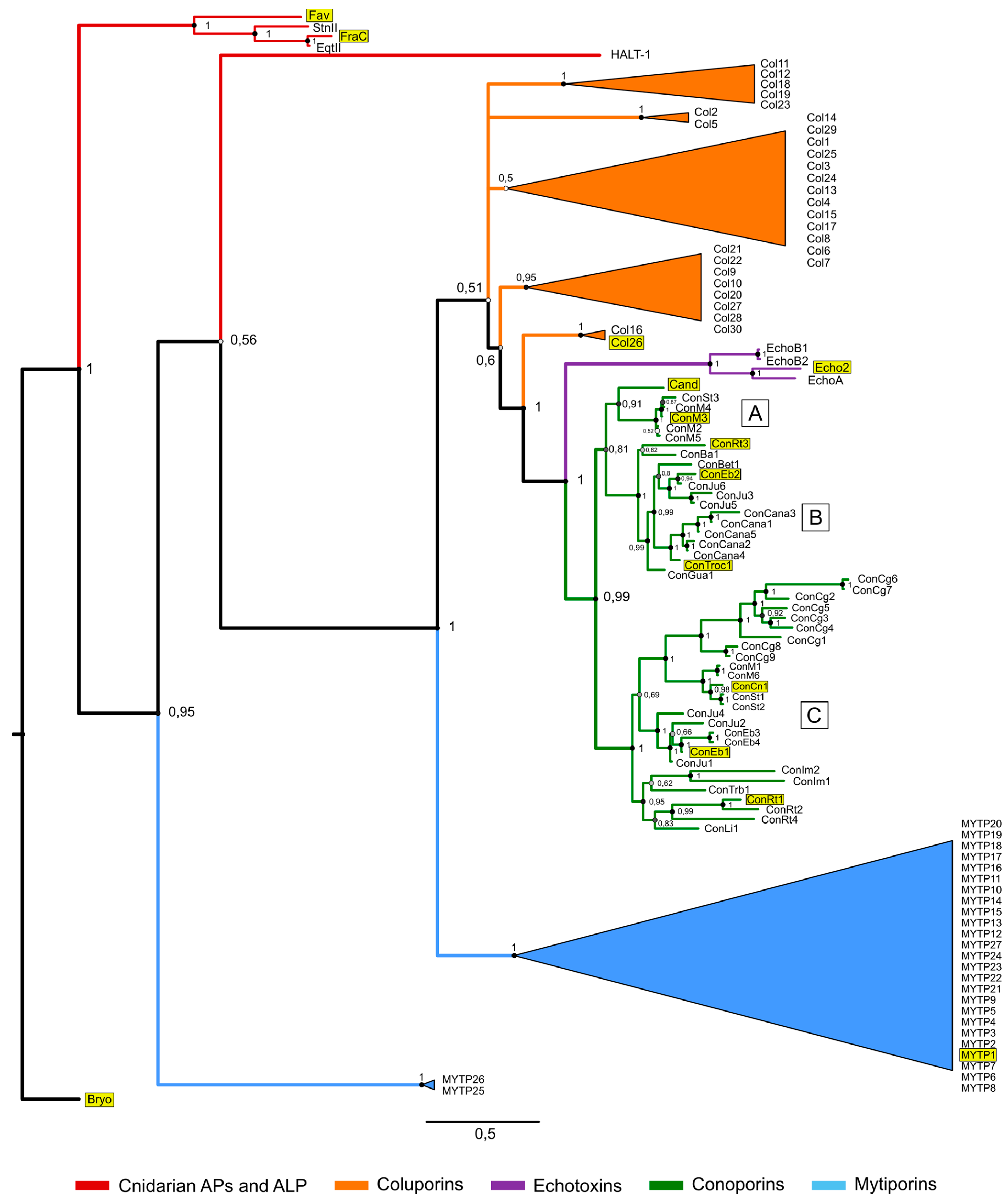
4. Conoporins
5. Biological Role of Conoporins
5.1. Disruption of Cells and Epithelia
5.2. Entry of Small Conotoxins into Cells Through Pores
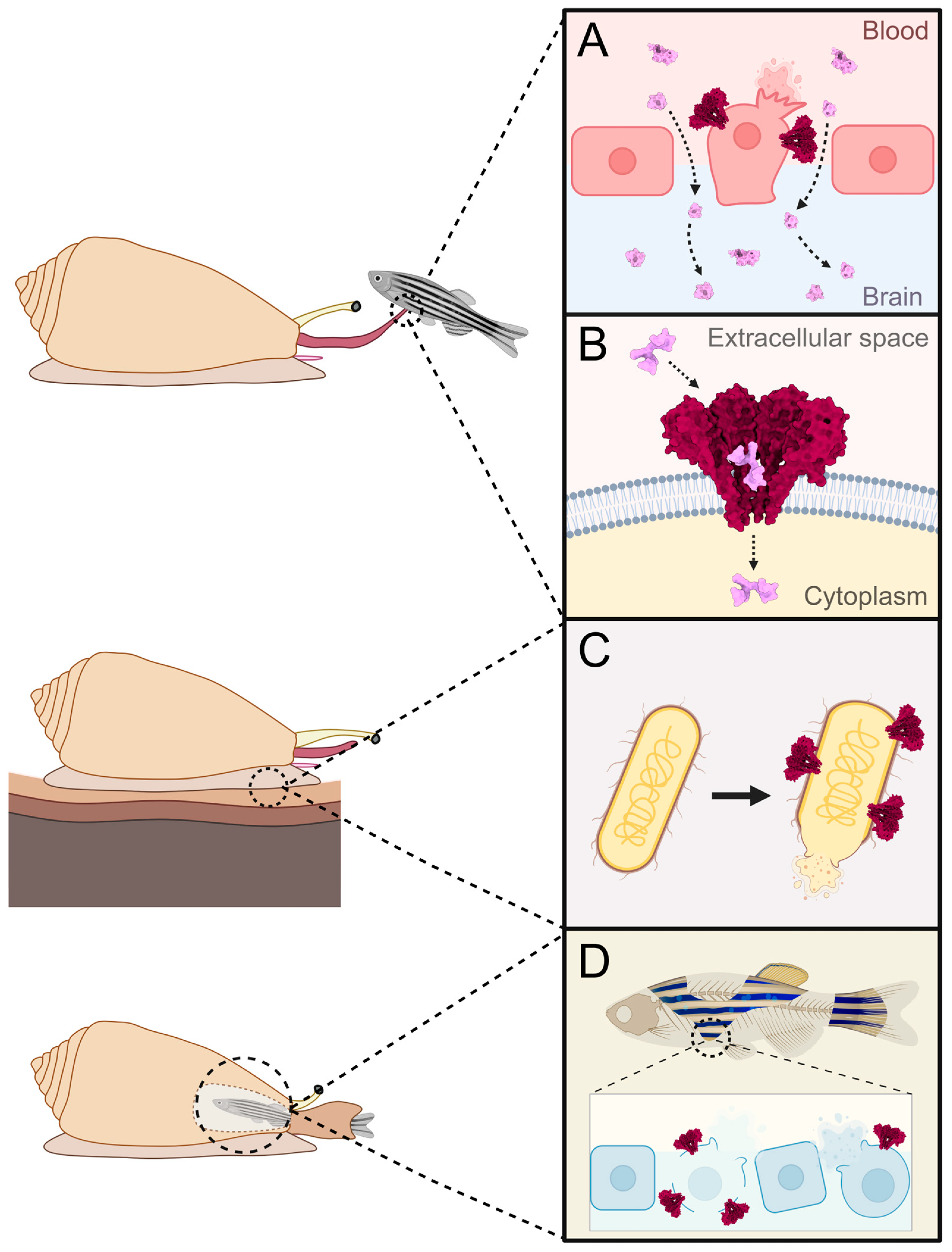
5.3. Part of the Immune System
5.4. Digestion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tucker, J.K.; Tenorio, M.J. Illustrated Catalog of the Living Cone Shells; MdM Publishing: Wellington, FL, USA, 2013; ISBN 978-0-9847140-2-5. [Google Scholar]
- WoRMS. Editorial Board World Register of Marine Species. Available online: https://www.marinespecies.org/imis.php?dasid=1447&doiid=170 (accessed on 28 October 2024).
- Puillandre, N.; Duda, T.F.; Meyer, C.; Olivera, B.M.; Bouchet, P. One, Four or 100 Genera? A New Classification of the Cone Snails. J. Molluscan Stud. 2014, 81, 1. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.F.; Kohn, A.J.; Palumbi, S.R. Origins of Diverse Feeding Ecologies within Conus, a Genus of Venomous Marine Gastropods. Biol. J. Linn. Soc. 2001, 73, 391–409. [Google Scholar] [CrossRef]
- Olivera, B.M.; Seger, J.; Horvath, M.P.; Fedosov, A.E. Prey-Capture Strategies of Fish-Hunting Cone Snails: Behavior, Neurobiology and Evolution. Brain Behav. Evol. 2015, 86, 58–74. [Google Scholar] [CrossRef]
- Olivera, B.M. Conus Venom Peptides: Reflections from the Biology of Clades and Species. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 25–47. [Google Scholar] [CrossRef]
- Davis, J.; Jones, A.; Lewis, R.J. Remarkable Inter- and Intra-Species Complexity of Conotoxins Revealed by LC/MS. Peptides 2009, 30, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Biass, D.; Stöcklin, R.; Favreau, P. Dramatic Intraspecimen Variations within the Injected Venom of Conus Consors: An Unsuspected Contribution to Venom Diversity. Toxicon 2010, 55, 1453–1462. [Google Scholar] [CrossRef]
- Jakubowski, J.A.; Kelley, W.P.; Sweedler, J.V.; Gilly, W.F.; Schulz, J.R. Intraspecific Variation of Venom Injected by Fish-Hunting Conus Snails. J. Exp. Biol. 2005, 208, 2873–2883. [Google Scholar] [CrossRef]
- Terlau, H.; Olivera, B.M. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef]
- Terrat, Y.; Biass, D.; Dutertre, S.; Favreau, P.; Remm, M.; Stöcklin, R.; Piquemal, D.; Ducancel, F. High-Resolution Picture of a Venom Gland Transcriptome: Case Study with the Marine Snail Conus consors. Toxicon 2012, 59, 34–46. [Google Scholar] [CrossRef]
- Puillandre, N.; Koua, D.; Favreau, P.; Olivera, B.M.; Stöcklin, R. Molecular Phylogeny, Classification and Evolution of Conopeptides. J. Mol. Evol. 2012, 74, 297–309. [Google Scholar] [CrossRef]
- Jin, A.-H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hasan, M.M.; Wang, D. In Silico Conotoxin Studies: Progress and Prospects. Molecules 2024, 29, 6061. [Google Scholar] [CrossRef] [PubMed]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, Synthesis, and Structure–Activity Relationships of Conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus Venom Peptide Pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef]
- Norton, R.S.; Olivera, B.M. Conotoxins down Under. Toxicon 2006, 48, 780–798. [Google Scholar] [CrossRef]
- Robinson, S.D.; Norton, R.S. Conotoxin Gene Superfamilies. Mar. Drugs 2014, 12, 6058–6101. [Google Scholar] [CrossRef] [PubMed]
- Milne, T.J.; Abbenante, G.; Tyndall, J.D.A.; Halliday, J.; Lewis, R.J. Isolation and Characterization of a Cone Snail Protease with Homology to CRISP Proteins of the Pathogenesis-Related Protein Superfamily*. J. Biol. Chem. 2003, 278, 31105–31110. [Google Scholar] [CrossRef]
- Qian, J.; Guo, Z.; Chi, C. Cloning and Isolation of a Conus Cysteine-Rich Protein Homologous to Tex31 but without Proteolytic Activity. Acta Biochim. Biophys. Sin. 2008, 40, 174–181. [Google Scholar] [CrossRef]
- Hansson, K.; Thämlitz, A.-M.; Furie, B.; Furie, B.C.; Stenflo, J. A Single γ-Carboxyglutamic Acid Residue in a Novel Cysteine-Rich Secretory Protein without Propeptide. Biochemistry 2006, 45, 12828–12839. [Google Scholar] [CrossRef]
- Leonardi, A.; Biass, D.; Kordiš, D.; Stöcklin, R.; Favreau, P.; Križaj, I. Conus Consors Snail Venom Proteomics Proposes Functions, Pathways, and Novel Families Involved in Its Venomic System. J. Proteome Res. 2012, 11, 5046–5058. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Ghomashchi, F.; Gelb, M.H.; Dooley, D.J.; Stoehr, S.J.; Giordani, A.B.; Naisbitt, S.R.; Olivera, B.M. Conodipine-M, a Novel Phospholipase A2 Isolated from the Venom of the Marine Snail Conus Magus(∗). J. Biol. Chem. 1995, 270, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Violette, A.; Leonardi, A.; Piquemal, D.; Terrat, Y.; Biass, D.; Dutertre, S.; Noguier, F.; Ducancel, F.; Stöcklin, R.; Križaj, I.; et al. Recruitment of Glycosyl Hydrolase Proteins in a Cone Snail Venomous Arsenal: Further Insights into Biomolecular Features of Conus Venoms. Mar. Drugs 2012, 10, 258–280. [Google Scholar] [CrossRef] [PubMed]
- Rojko, N.; Dalla Serra, M.; Maček, P.; Anderluh, G. Pore Formation by Actinoporins, Cytolysins from Sea Anemones. Biochim. Biophys. Acta BBA-Biomembr. 2016, 1858, 446–456. [Google Scholar] [CrossRef]
- Peraro, M.D.; van der Goot, F.G. Pore-Forming Toxins: Ancient, but Never Really out of Fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Podobnik, M.; Anderluh, G. Pore-Forming Toxins in Cnidaria. Semin. Cell Dev. Biol. 2017, 72, 133–141. [Google Scholar] [CrossRef]
- Anderluh, G.; Maček, P. Cytolytic Peptide and Protein Toxins from Sea Anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef]
- Athanasiadis, A.; Anderluh, G.; Maček, P.; Turk, D. Crystal Structure of the Soluble Form of Equinatoxin II, a Pore-Forming Toxin from the Sea Anemone Actinia equina. Structure 2001, 9, 341–346. [Google Scholar] [CrossRef]
- Hinds, M.G.; Zhang, W.; Anderluh, G.; Hansen, P.E.; Norton, R.S. Solution Structure of the Eukaryotic Pore-Forming Cytolysin Equinatoxin II: Implications for Pore Formation. J. Mol. Biol. 2002, 315, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.E.; Bellomio, A.; Gil-Cartón, D.; Morante, K.; Valle, M.; González-Mañas, J.M.; Guérin, D.M.A. Structural Insights into the Oligomerization and Architecture of Eukaryotic Membrane Pore-Forming Toxins. Structure 2011, 19, 181–191. [Google Scholar] [CrossRef]
- Morante, K.; Bellomio, A.; Viguera, A.R.; González-Mañas, J.M.; Tsumoto, K.; Caaveiro, J.M.M. The Isolation of New Pore-Forming Toxins from the Sea Anemone Actinia Fragacea Provides Insights into the Mechanisms of Actinoporin Evolution. Toxins 2019, 11, 401. [Google Scholar] [CrossRef]
- Castrillo, I.; Alegre-Cebollada, J.; Martínez del Pozo, Á.; Gavilanes, J.G.; Santoro, J.; Bruix, M. 1H, 13C, and 15N NMR Assignments of the Actinoporin Sticholysin I. Biomol. NMR Assign. 2009, 3, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mancheño, J.M.; Martín-Benito, J.; Martínez-Ripoll, M.; Gavilanes, J.G.; Hermoso, J.A. Crystal and Electron Microscopy Structures of Sticholysin II Actinoporin Reveal Insights into the Mechanism of Membrane Pore Formation. Structure 2003, 11, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Bakrač, B.; Gutiérrez-Aguirre, I.; Podlesek, Z.; Sonnen, A.F.-P.; Gilbert, R.J.C.; Maček, P.; Lakey, J.H.; Anderluh, G. Molecular Determinants of Sphingomyelin Specificity of a Eukaryotic Pore-Forming Toxin*. J. Biol. Chem. 2008, 283, 18665–18677. [Google Scholar] [CrossRef] [PubMed]
- Šolinc, G.; Srnko, M.; Merzel, F.; Crnković, A.; Kozorog, M.; Podobnik, M.; Anderluh, G. Cryo-EM Structures of a Protein Pore Reveal a Cluster of Cholesterol Molecules and Diverse Roles of Membrane Lipids. Nat. Commun. 2025, 16, 2972. [Google Scholar] [CrossRef]
- Tanaka, K.; Caaveiro, J.M.M.; Morante, K.; González-Manãs, J.M.; Tsumoto, K. Structural Basis for Self-Assembly of a Cytolytic Pore Lined by Protein and Lipid. Nat. Commun. 2015, 6, 6337. [Google Scholar] [CrossRef]
- Šolinc, G.; Švigelj, T.; Omersa, N.; Snoj, T.; Pirc, K.; Žnidaršič, N.; Yamaji-Hasegawa, A.; Kobayashi, T.; Anderluh, G.; Podobnik, M. Pore-Forming Moss Protein Bryoporin Is Structurally and Mechanistically Related to Actinoporins from Evolutionarily Distant Cnidarians. J. Biol. Chem. 2022, 298, 102455. [Google Scholar] [CrossRef]
- Morante, K.; Bellomio, A.; Gil-Cartón, D.; Redondo-Morata, L.; Sot, J.; Scheuring, S.; Valle, M.; González-Mañas, J.M.; Tsumoto, K.; Caaveiro, J.M.M. Identification of a Membrane-Bound Prepore Species Clarifies the Lytic Mechanism of Actinoporins*♦. J. Biol. Chem. 2016, 291, 19210–19219. [Google Scholar] [CrossRef]
- Koritnik, N.; Gerdol, M.; Šolinc, G.; Švigelj, T.; Caserman, S.; Merzel, F.; Holden, E.; Benesch, J.L.P.; Trenti, F.; Guella, G.; et al. Expansion and Neofunctionalization of Actinoporin-like Genes in Mediterranean Mussel (Mytilus Galloprovincialis). Genome Biol. Evol. 2022, 14, evac151. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Gutiérrez-Aguirre, I.; Trontelj, P.; Maček, P.; Lakey, J.H.; Anderluh, G. Membrane Binding of Zebrafish Actinoporin-like Protein: AF Domains, a Novel Superfamily of Cell Membrane Binding Domains. Biochem. J. 2006, 398, 381. [Google Scholar] [CrossRef]
- Črnigoj Kristan, K.; Viero, G.; Dalla Serra, M.; Maček, P.; Anderluh, G. Molecular Mechanism of Pore Formation by Actinoporins. Toxicon 2009, 54, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, C.; Luberacki, B.; Küfner, I.; Koch, W.; Brunner, F.; Weyand, M.; Mattinen, L.; Pirhonen, M.; Anderluh, G.; Seitz, H.U.; et al. A Common Toxin Fold Mediates Microbial Attack and Plant Defense. Proc. Natl. Acad. Sci. USA 2009, 106, 10359–10364. [Google Scholar] [CrossRef] [PubMed]
- Birck, C.; Damian, L.; Marty-Detraves, C.; Lougarre, A.; Schulze-Briese, C.; Koehl, P.; Fournier, D.; Paquereau, L.; Samama, J.-P. A New Lectin Family with Structure Similarity to Actinoporins Revealed by the Crystal Structure of Xerocomus Chrysenteron Lectin XCL. J. Mol. Biol. 2004, 344, 1409–1420. [Google Scholar] [CrossRef]
- Ameirika; Sha, H.X.; Hwang, J.S. Identification of a Target Protein of Hydra Actinoporin-like Toxin-1 (HALT-1) Using GST Affinity Purification and SILAC-Based Quantitative Proteomics. Toxicon 2017, 133, 153–161. [Google Scholar] [CrossRef]
- Yap, W.Y.; Tan, K.J.S.X.; Hwang, J.S. Expansion of Hydra Actinoporin-like Toxin (HALT) Gene Family: Expression Divergence and Functional Convergence Evolved through Gene Duplication. Toxicon 2019, 170, 10–20. [Google Scholar] [CrossRef]
- Sher, D.; Knebel, A.; Bsor, T.; Nesher, N.; Tal, T.; Morgenstern, D.; Cohen, E.; Fishman, Y.; Zlotkin, E. Toxic Polypeptides of the Hydra—A Bioinformatic Approach to Cnidarian Allomones. Toxicon 2005, 45, 865–879. [Google Scholar] [CrossRef]
- Glasser, E.; Rachamim, T.; Aharonovich, D.; Sher, D. Hydra Actinoporin-like Toxin-1, an Unusual Hemolysin from the Nematocyst Venom of Hydra Magnipapillata Which Belongs to an Extended Gene Family. Toxicon 2014, 91, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Liew, Y.J.M.; Soh, W.T.; Jiemy, W.F.; Hwang, J.S. Mutagenesis and Functional Analysis of the Pore-Forming Toxin HALT-1 from Hydra magnipapillata. Toxins 2015, 7, 407–422. [Google Scholar] [CrossRef]
- Cuesta Arenas, Y.; Kalkman, E.R.I.C.; Schouten, A.; Dieho, M.; Vredenbregt, P.; Uwumukiza, B.; Osés Ruiz, M.; van Kan, J.A.L. Functional Analysis and Mode of Action of Phytotoxic Nep1-like Proteins of Botrytis cinerea. Physiol. Mol. Plant Pathol. 2010, 74, 376–386. [Google Scholar] [CrossRef]
- Kelker, M.S.; Berry, C.; Evans, S.L.; Pai, R.; McCaskill, D.G.; Wang, N.X.; Russell, J.C.; Baker, M.D.; Yang, C.; Pflugrath, J.W.; et al. Structural and Biophysical Characterization of Bacillus Thuringiensis Insecticidal Proteins Cry34Ab1 and Cry35Ab1. PLoS ONE 2014, 9, e112555. [Google Scholar] [CrossRef]
- Verma, P.; Chattopadhyay, K. Current Perspective on the Membrane-Damaging Action of Thermostable Direct Hemolysin, an Atypical Bacterial Pore-Forming Toxin. Front. Mol. Biosci. 2021, 8, 717147. [Google Scholar] [CrossRef]
- Mishra, S.; Kundu, N.; Pramanick, I.; Kumar, A.; Chattopadhyay, K.; Dutta, S. Structural Insights into Thermostable Direct Hemolysin of Vibrio Parahaemolyticus Using Single-Particle Cryo-EM. Proteins Struct. Funct. Bioinforma. 2023, 91, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Protein Structure Comparison Service PDBeFold at European Bioinformatics Institute. Available online: http://www.ebi.ac.uk/msd-srv/ssm (accessed on 25 April 2025).
- Kawashima, Y.; Nagai, H.; Ishida, M.; Nagashima, Y.; Shiomi, K. Primary Structure of Echotoxin 2, an Actinoporin-like Hemolytic Toxin from the Salivary Gland of the Marine Gastropod Monoplex echo. Toxicon 2003, 42, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Kawashima, Y.; Mizukami, M.; Nagashima, Y. Properties of Proteinaceous Toxins in the Salivary Gland of the Marine Gastropod (Monoplex Echo). Toxicon 2002, 40, 563–571. [Google Scholar] [CrossRef]
- Bose, U.; Wang, T.; Zhao, M.; Motti, C.A.; Hall, M.R.; Cummins, S.F. Multiomics Analysis of the Giant Triton Snail Salivary Gland, a Crown-of-Thorns Starfish Predator. Sci. Rep. 2017, 7, 6000. [Google Scholar] [CrossRef]
- Lu, A.; Watkins, M.; Li, Q.; Robinson, S.D.; Concepcion, G.P.; Yandell, M.; Weng, Z.; Olivera, B.M.; Safavi-Hemami, H.; Fedosov, A.E. Transcriptomic Profiling Reveals Extraordinary Diversity of Venom Peptides in Unexplored Predatory Gastropods of the Genus Clavus. Genome Biol. Evol. 2020, 12, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Gorson, J.; Ramrattan, G.; Verdes, A.; Wright, E.M.; Kantor, Y.; Rajaram Srinivasan, R.; Musunuri, R.; Packer, D.; Albano, G.; Qiu, W.-G.; et al. Molecular Diversity and Gene Evolution of the Venom Arsenal of Terebridae Predatory Marine Snails. Genome Biol. Evol. 2015, 7, 1761–1778. [Google Scholar] [CrossRef]
- Takara, T.; Nakagawa, T.; Isobe, M.; Okino, N.; Ichinose, S.; Omori, A.; Ito, M. Purification, Molecular Cloning, and Application of a Novel Sphingomyelin-Binding Protein (Clamlysin) from the Brackishwater Clam, Corbicula japonica. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2011, 1811, 323–332. [Google Scholar] [CrossRef]
- Gorbushin, A.; Ruparčič, M.; Anderluh, G. Littoporins: Novel Actinoporin-like Proteins in Caenogastropod Genus Littorina. Fish Shellfish Immunol. 2024, 151, 109698. [Google Scholar] [CrossRef]
- Gerdol, M.; Cervelli, M.; Oliverio, M.; Modica, M.V. Piercing Fishes: Porin Expansion and Adaptation to Hematophagy in the Vampire Snail Cumia reticulata. Mol. Biol. Evol. 2018, 35, 2654–2668. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, Scalable Generation of High-quality Protein Multiple Sequence Alignments Using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Violette, A.; Biass, D.; Dutertre, S.; Koua, D.; Piquemal, D.; Pierrat, F.; Stöcklin, R.; Favreau, P. Large-Scale Discovery of Conopeptides and Conoproteins in the Injectable Venom of a Fish-Hunting Cone Snail Using a Combined Proteomic and Transcriptomic Approach. J. Proteom. 2012, 75, 5215–5225. [Google Scholar] [CrossRef]
- Pardos-Blas, J.R.; Tenorio, M.J.; Galindo, J.C.G.; Zardoya, R. Comparative Venomics of the Cryptic Cone Snail Species Virroconus ebraeus and Virroconus judaeus. Mar. Drugs 2022, 20, 149. [Google Scholar] [CrossRef]
- Kohn, A.J. The Feeding Process in Conus victoriae. In The Marine Flora and Fauna of Dampier, Western Australia; Wells, F.E., Walker, D.I., Jones, D.S., Eds.; Western Australian Museum: Perth, WA, Australia, 2003; pp. 101–108. [Google Scholar]
- Prator, C.A.; Murayama, K.M.; Schulz, J.R. Venom Variation during Prey Capture by the Cone Snail, Conus Textile. PLoS ONE 2014, 9, e98991. [Google Scholar] [CrossRef]
- Phuong, M.A.; Mahardika, G.N.; Alfaro, M.E. Dietary Breadth Is Positively Correlated with Venom Complexity in Cone Snails. BMC Genom. 2016, 17, 401. [Google Scholar] [CrossRef]
- Koch, T.L.; Robinson, S.D.; Salcedo, P.F.; Chase, K.; Biggs, J.; Fedosov, A.E.; Yandell, M.; Olivera, B.M.; Safavi-Hemami, H. Prey Shifts Drive Venom Evolution in Cone Snails. Mol. Biol. Evol. 2024, 41, msae120. [Google Scholar] [CrossRef]
- Galinier, R.; Portela, J.; Moné, Y.; Allienne, J.F.; Henri, H.; Delbecq, S.; Mitta, G.; Gourbal, B.; Duval, D. Biomphalysin, a New β Pore-Forming Toxin Involved in Biomphalaria Glabrata Immune Defense against Schistosoma mansoni. PLOS Pathog. 2013, 9, e1003216. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, D.; Tetreau, G.; Pinaud, S.; Galinier, R.; Crickmore, N.; Gourbal, B.; Duval, D. Glabralysins, Potential New β-Pore-Forming Toxin Family Members from the Schistosomiasis Vector Snail Biomphalaria glabrata. Genes 2020, 11, 65. [Google Scholar] [CrossRef]
- Jiang, K.; Yin, Z.; Zhang, Y.; Xu, Q.; Yu, Y.; Cong, W.; Yan, X.; Nie, H. Genome-Wide Investigation and Expression Analysis of MACPF Gene Family Reveals Its Immune Role in Response to Bacterial Challenge of Manila Clam. Genomics 2021, 113, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Gorbushin, A.M. Membrane Attack Complex/Perforin Domain-Containing Proteins in a Dual-Species Transcriptome of Caenogastropoda Littorina Littorea and Its Trematode Parasite Himasthla elongata. Fish Shellfish Immunol. 2016, 54, 254–256. [Google Scholar] [CrossRef]
- Schultz, J.H.; Bu, L.; Adema, C.M. Comparative Immunological Study of the Snail Physella Acuta (Hygrophila, Pulmonata) Reveals Shared and Unique Aspects of Gastropod Immunobiology. Mol. Immunol. 2018, 101, 108–119. [Google Scholar] [CrossRef]
- Weese, D.A.; Duda, T.F. Data from: “Transcriptomic Resources for Three Populations of Conus Miliaris (Mollusca: Conidae) from Easter Island, American Samoa and Guam” in Genomic Resources Notes Accepted 1 August 2014–30 September 2014. Available online: https://datadryad.org/stash/dataset/doi:10.5061/dryad.t74q4 (accessed on 4 November 2024).
- Abalde, S.; Tenorio, M.J.; Afonso, C.M.L.; Zardoya, R. Comparative Transcriptomics of the Venoms of Continental and Insular Radiations of West African Cones. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200794. [Google Scholar] [CrossRef]
- Pardos-Blas, J.R.; Irisarri, I.; Abalde, S.; Afonso, C.M.L.; Tenorio, M.J.; Zardoya, R. The Genome of the Venomous Snail Lautoconus Ventricosus Sheds Light on the Origin of Conotoxin Diversity. GigaScience 2021, 10, giab037. [Google Scholar] [CrossRef] [PubMed]
- Conoporin [Conus Monile] (GenBank: ANC48005.1). Available online: https://www.ncbi.nlm.nih.gov/protein/ANC48005.1 (accessed on 4 November 2024).
- Herráez-Pérez, A.; Pardos-Blas, J.R.; Afonso, C.M.L.; Tenorio, M.J.; Zardoya, R. Chromosome-Level Genome of the Venomous Snail Kalloconus Canariensis: A Valuable Model for Venomics and Comparative Genomics. GigaScience 2023, 12, giad075. [Google Scholar] [CrossRef]
- Gao, B.; Peng, C.; Zhu, Y.; Sun, Y.; Zhao, T.; Huang, Y.; Shi, Q. High Throughput Identification of Novel Conotoxins from the Vermivorous Oak Cone Snail (Conus Quercinus) by Transcriptome Sequencing. Int. J. Mol. Sci. 2018, 19, 3901. [Google Scholar] [CrossRef]
- Barghi, N.; Concepcion, G.P.; Olivera, B.M.; Lluisma, A.O. Comparison of the Venom Peptides and Their Expression in Closely Related Conus Species: Insights into Adaptive Post-Speciation Evolution of Conus Exogenomes. Genome Biol. Evol. 2015, 7, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barghi, N.; Lu, A.; Fedosov, A.E.; Bandyopadhyay, P.K.; Lluisma, A.O.; Concepcion, G.P.; Yandell, M.; Olivera, B.M.; Safavi-Hemami, H. Divergence of the Venom Exogene Repertoire in Two Sister Species of Turriconus. Genome Biol. Evol. 2017, 9, 2211–2225. [Google Scholar] [CrossRef]
- Peng, C.; Yao, G.; Gao, B.-M.; Fan, C.-X.; Bian, C.; Wang, J.; Cao, Y.; Wen, B.; Zhu, Y.; Ruan, Z.; et al. High-Throughput Identification of Novel Conotoxins from the Chinese Tubular Cone Snail (Conus betulinus) by Multi-Transcriptome Sequencing. GigaScience 2016, 5, s13742-016-0122-0129. [Google Scholar] [CrossRef]
- Pardos-Blas, J.R.; Irisarri, I.; Abalde, S.; Tenorio, M.J.; Zardoya, R. Conotoxin Diversity in the Venom Gland Transcriptome of the Magician’s Cone, Pionoconus magus. Mar. Drugs 2019, 17, 553. [Google Scholar] [CrossRef]
- Rogalski, A.; Himaya, S.W.A.; Lewis, R.J. Coordinated Adaptations Define the Ontogenetic Shift from Worm- to Fish-Hunting in a Venomous Cone Snail. Nat. Commun. 2023, 14, 3287. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, C.; Zhu, Y.; Fu, J.; Ruan, Z.; Shi, Q.; Gao, B. High Conopeptide Diversity in Conus Striatus: Revealed by Integration of Two Transcriptome Sequencing Platforms. Front. Mar. Sci. 2022, 9, 1060432. [Google Scholar] [CrossRef]
- Safavi-Hemami, H.; Hu, H.; Gorasia, D.G.; Bandyopadhyay, P.K.; Veith, P.D.; Young, N.D.; Reynolds, E.C.; Yandell, M.; Olivera, B.M.; Purcell, A.W. Combined Proteomic and Transcriptomic Interrogation of the Venom Gland of Conus geographus Uncovers Novel Components and Functional Compartmentalization. Mol. Cell. Proteom. MCP 2014, 13, 938–953. [Google Scholar] [CrossRef]
- Abalde, S.; Tenorio, M.J.; Afonso, C.M.L.; Zardoya, R. Conotoxin Diversity in Chelyconus Ermineus (Born, 1778) and the Convergent Origin of Piscivory in the Atlantic and Indo-Pacific Cones. Genome Biol. Evol. 2018, 10, 2643–2662. [Google Scholar] [CrossRef] [PubMed]
- Cajnko, M.M.; Marušić, M.; Kisovec, M.; Rojko, N.; Benčina, M.; Caserman, S.; Anderluh, G. Listeriolysin O Affects the Permeability of Caco-2 Monolayer in a Pore-Dependent and Ca2+-Independent Manner. PLoS ONE 2015, 10, e0130471. [Google Scholar] [CrossRef]
- Bishop, B.L.; Lodolce, J.P.; Kolodziej, L.E.; Boone, D.L.; Tang, W.J. The Role of Anthrolysin O in Gut Epithelial Barrier Disruption during Bacillus Anthracis Infection. Biochem. Biophys. Res. Commun. 2010, 394, 254–259. [Google Scholar] [CrossRef]
- Bücker, R.; Krug, S.M.; Rosenthal, R.; Günzel, D.; Fromm, A.; Zeitz, M.; Chakraborty, T.; Fromm, M.; Epple, H.-J.; Schulzke, J.-D. Aerolysin from Aeromonas hydrophila Perturbs Tight Junction Integrity and Cell Lesion Repair in Intestinal Epithelial HT-29/B6 Cells. J. Infect. Dis. 2011, 204, 1283–1292. [Google Scholar] [CrossRef]
- Nagahama, M.; Sakurai, J. Distribution of Labeled Clostridium perfringens Epsilon Toxin in Mice. Toxicon 1991, 29, 211–217. [Google Scholar] [CrossRef]
- Finnie, J.; Hajduk, P. An Immunohistochemical Study of Plasma Albumin Extravasation in the Brain of Mice after the Administration of Clostridium Perfringens Type D Epsilon Toxin. Aust. Vet. J. 1992, 69, 261–262. [Google Scholar] [CrossRef]
- Popoff, M.R. Epsilon Toxin: A Fascinating Pore-Forming Toxin. FEBS J. 2011, 278, 4602–4615. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.; Linden, J.R.; Shetty, S.V.; Ma, Y.; Bokori-Brown, M.; Titball, R.W.; Vartanian, T. Clostridium perfringens Epsilon Toxin Compromises the Blood-Brain Barrier in a Humanized Zebrafish Model. iScience 2019, 15, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.W.; Mülders, M.S. Effect of Clostridium perfringens Epsilon Toxin on the Blood Brain Barrier of Mice. Onderstepoort J. Vet. Res. 1975, 42, 25–27. [Google Scholar]
- Jiang, J.; Pentelute, B.L.; Collier, R.J.; Zhou, Z.H. Atomic Structure of Anthrax Protective Antigen Pore Elucidates Toxin Translocation. Nature 2015, 521, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Sterling, H.J.; Tang, I.I.; Williams, E.R.; Krantz, B.A. Anthrax Toxin Receptor Drives Protective Antigen Oligomerization and Stabilizes the Heptameric and Octameric Oligomer by a Similar Mechanism. PLoS ONE 2010, 5, e13888. [Google Scholar] [CrossRef]
- Geny, B.; Popoff, M.R. Bacterial Protein Toxins and Lipids: Pore Formation or Toxin Entry into Cells. Biol. Cell 2006, 98, 667–678. [Google Scholar] [CrossRef]
- Madden, J.C.; Ruiz, N.; Caparon, M. Cytolysin-Mediated Translocation (CMT): A Functional Equivalent of Type III Secretion in Gram-Positive Bacteria. Cell 2001, 104, 143–152. [Google Scholar] [CrossRef]
- Jackson, K.E.; Spielmann, T.; Hanssen, E.; Adisa, A.; Separovic, F.; Dixon, M.W.A.; Trenholme, K.R.; Hawthorne, P.L.; Gardiner, D.L.; Gilberger, T.; et al. Selective Permeabilization of the Host Cell Membrane of Plasmodium falciparum-Infected Red Blood Cells with Streptolysin O and Equinatoxin II. Biochem. J. 2007, 403, 167–175. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and Granzymes: Function, Dysfunction and Human Pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Nüssing, S.; Sutton, V.R.; Trapani, J.A.; Parish, I.A. Beyond Target Cell Death—Granzyme Serine Proteases in Health and Disease. Mol. Aspects Med. 2022, 88, 101152. [Google Scholar] [CrossRef]
- Bruhn, H.; Winkelmann, J.; Andersen, C.; Andrä, J.; Leippe, M. Dissection of the Mechanisms of Cytolytic and Antibacterial Activity of Lysenin, a Defence Protein of the Annelid Eisenia fetida. Dev. Comp. Immunol. 2006, 30, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Shogomori, H.; Kobayashi, T. Lysenin: A Sphingomyelin Specific Pore-Forming Toxin. Biochim. Biophys. Acta BBA-Gen. Subj. 2008, 1780, 612–618. [Google Scholar] [CrossRef]
- De Colibus, L.; Sonnen, A.F.-P.; Morris, K.J.; Siebert, C.A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; et al. Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins and Its Mode of Sphingomyelin Recognition. Structure 2012, 20, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Kisovec, M.; Kraševec, N.; Gilbert, R.J.C. Distribution of MACPF/CDC Proteins. In MACPF/CDC Proteins-Agents of Defence, Attack and Invasion; Anderluh, G., Gilbert, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 7–30. ISBN 978-94-017-8881-6. [Google Scholar]
- Sun, J.; Wang, L.; Song, L. The Primitive Complement System in Molluscs. Dev. Comp. Immunol. 2023, 139, 104565. [Google Scholar] [CrossRef]
- Estévez-Calvar, N.; Romero, A.; Figueras, A.; Novoa, B. Involvement of Pore-Forming Molecules in Immune Defense and Development of the Mediterranean Mussel (Mytilus galloprovincialis). Dev. Comp. Immunol. 2011, 35, 1017–1031. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.; Yu, Z. An Mpeg (Macrophage Expressed Gene) from the Pacific Oyster Crassostrea Gigas: Molecular Characterization and Gene Expression. Fish Shellfish Immunol. 2011, 30, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Giglio, M.L.; Ituarte, S.; Milesi, V.; Dreon, M.S.; Brola, T.R.; Caramelo, J.; Ip, J.C.H.; Maté, S.; Qiu, J.W.; Otero, L.H.; et al. Exaptation of Two Ancient Immune Proteins into a New Dimeric Pore-Forming Toxin in Snails. J. Struct. Biol. 2020, 211, 107531. [Google Scholar] [CrossRef]
- Fujii, Y.; Gerdol, M.; Hasan, I.; Koide, Y.; Matsuzaki, R.; Ikeda, M.; Rajia, S.; Ogawa, Y.; Kawsar, S.M.A.; Ozeki, Y. Phylogeny and Properties of a Novel Lectin Family with β-Trefoil Folding in Mussels. Trends Glycosci. Glycotechnol. 2018, 30, E195–E208. [Google Scholar] [CrossRef]
- Hasan, I.; Gerdol, M.; Fujii, Y.; Rajia, S.; Koide, Y.; Yamamoto, D.; Kawsar, S.M.A.; Ozeki, Y. CDNA and Gene Structure of MytiLec-1, A Bacteriostatic R-Type Lectin from the Mediterranean Mussel (Mytilus galloprovincialis). Mar. Drugs 2016, 14, 92. [Google Scholar] [CrossRef]
- Pinaud, S.; Tetreau, G.; Poteaux, P.; Galinier, R.; Chaparro, C.; Lassalle, D.; Portet, A.; Simphor, E.; Gourbal, B.; Duval, D. New Insights Into Biomphalysin Gene Family Diversification in the Vector Snail Biomphalaria glabrata. Front. Immunol. 2021, 12, 635131. [Google Scholar] [CrossRef]
- Sher, D.; Zlotkin, E. A Hydra with Many Heads: Protein and Polypeptide Toxins from Hydra and Their Biological Roles. Toxicon 2009, 54, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Sher, D.; Fishman, Y.; Melamed-Book, N.; Zhang, M.; Zlotkin, E. Osmotically Driven Prey Disintegration in the Gastrovascular Cavity of the Green Hydra by a Pore-Forming Protein. FASEB J. 2008, 22, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Maček, P.; Belmonte, G.; Pederzolli, C.; Menestrina, G. Mechanism of Action of Equinatoxin II, a Cytolysin from the Sea Anemone Actinia Equina L. Belonging to the Family of Actinoporins. Toxicology 1994, 87, 205–227. [Google Scholar] [CrossRef]
- Cabezas, S.; Ho, S.; Ros, U.; Lanio, M.E.; Alvarez, C.; van der Goot, F.G. Damage of Eukaryotic Cells by the Pore-Forming Toxin Sticholysin II: Consequences of the Potassium Efflux. Biochim. Biophys. Acta BBA-Biomembr. 2017, 1859, 982–992. [Google Scholar] [CrossRef]
- Parker, M.W.; Feil, S.C. Pore-Forming Protein Toxins: From Structure to Function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef]
- Belmonte, G.; Pederzolli, C.; Maček, P.; Menestrina, G. Pore Formation by the Sea Anemone Cytolysin Equinatoxin II in Red Blood Cells and Model Lipid Membranes. J. Membr. Biol. 1993, 131, 11–22. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of Separate Predation- and Defence-Evoked Venoms in Carnivorous Cone Snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.-H.; Israel, M.R.; Inserra, M.C.; Smith, J.J.; Lewis, R.J.; Alewood, P.F.; Vetter, I.; Dutertre, S. δ-Conotoxin SuVIA Suggests an Evolutionary Link between Ancestral Predator Defence and the Origin of Fish-Hunting Behaviour in Carnivorous Cone Snails. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150817. [Google Scholar] [CrossRef]
- Mertens, P.; Blond, S.; David, R.; Rigoard, P. Anatomy, Physiology and Neurobiology of the Nociception: A Focus on Low Back Pain (Part A). Neurochirurgie 2015, 61, S22–S34. [Google Scholar] [CrossRef]
- Šuput, D.; Frangež, R.; Bunc, M. Cardiovascular Effects of Equinatoxin III from the Sea Anemone Actinia equina (L.). Toxicon 2001, 39, 1421–1427. [Google Scholar] [CrossRef]
- Frangež, R.; Šuput, D.; Molgó, J. Effects of Equinatoxin II on Isolated Guinea Pig Taenia Caeci Muscle Contractility and Intracellular Ca2+. Toxicon 2008, 51, 1416–1423. [Google Scholar] [CrossRef]
- Key, B.; Brown, D. Designing Brains for Pain: Human to Mollusc. Front. Physiol. 2018, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.J. Human Injuries and Fatalities Due to Venomous Marine Snails of the Family Conidae. Int. J. Clin. Pharmacol. Ther. 2016, 54, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Savory, P.; Rojko, N.; Kisovec, M.; Wood, N.; Hambley, R.; Pugh, J.; Wallace, E.J.; McNeill, L.; Bruce, M.; et al. Crystal Structure of an Invertebrate Cytolysin Pore Reveals Unique Properties and Mechanism of Assembly. Nat. Commun. 2016, 7, 11598. [Google Scholar] [CrossRef]
- Ratibou, Z.; Inguimbert, N.; Dutertre, S. Predatory and Defensive Strategies in Cone Snails. Toxins 2024, 16, 94. [Google Scholar] [CrossRef] [PubMed]
| Diet | Clade | Nr. of Species | Nr. of Sequences | References |
|---|---|---|---|---|
| Vermivorous | Virroconus | 3 | 25 | [68,71,78] |
| Lautoconus | 5 | 10 | [79,80] | |
| Strategoconus | 2 | 8 | [78,81] | |
| Rhizoconus | 1 | 8 | [71] | |
| Kalloconus | 2 | 6 | [79,82] 1 | |
| Lividoconus | 2 | 5 | [71,83] | |
| Rhombiconus | 1 | 2 | [71] | |
| Splinoconus | 2 | 2 | [84] | |
| Turriconus | 1 | 1 | [85] | |
| Dendroconus | 1 | 1 | [86] | |
| Piscivorous | Pionoconus | 3 | 12 | [67,87,88,89] |
| Gastridium | 1 | 9 | [90] | |
| Chelyconus | 1 | 6 | [91] | |
| Molluscivorous | / | 0 | 0 | / |
| Biological Role | Description | References |
|---|---|---|
| Disruption of cells and epithelia | Pore formation causes cell swelling and lysis due to colloid-osmotic shock. This leads to disintegration of epithelia, allowing for smaller conotoxins to pass through epithelial barriers (e.g., blood-brain barrier). | [92,93,94,95,96,97,98,99] |
| Entry of small conotoxins into cells through pores | Pores formed by conoporins allow for the translocation of smaller conotoxins into the target cell. | [100,101,102,103,104,105,106] |
| Part of the immune system | Conoporins are employed in the destruction of pathogens by forming pores on their cell membranes, causing lysis. | [27,62,73,75,76,80,107,108,109,110,111,112,113,114,115,116,117] |
| Digestion | Conoporins facilitate the breakdown of digested tissue by lysing digested cells via pore formation. | [40,118,119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruparčič, M.; Šolinc, G.; Caserman, S.; Galindo, J.C.G.; Tenorio, M.J.; Anderluh, G. The Biological Role of Conoporins, Actinoporin-like Pore-Forming Toxins from Cone Snails. Toxins 2025, 17, 291. https://doi.org/10.3390/toxins17060291
Ruparčič M, Šolinc G, Caserman S, Galindo JCG, Tenorio MJ, Anderluh G. The Biological Role of Conoporins, Actinoporin-like Pore-Forming Toxins from Cone Snails. Toxins. 2025; 17(6):291. https://doi.org/10.3390/toxins17060291
Chicago/Turabian StyleRuparčič, Matija, Gašper Šolinc, Simon Caserman, Juan Carlos Garcia Galindo, Manuel Jimenez Tenorio, and Gregor Anderluh. 2025. "The Biological Role of Conoporins, Actinoporin-like Pore-Forming Toxins from Cone Snails" Toxins 17, no. 6: 291. https://doi.org/10.3390/toxins17060291
APA StyleRuparčič, M., Šolinc, G., Caserman, S., Galindo, J. C. G., Tenorio, M. J., & Anderluh, G. (2025). The Biological Role of Conoporins, Actinoporin-like Pore-Forming Toxins from Cone Snails. Toxins, 17(6), 291. https://doi.org/10.3390/toxins17060291






