The Ghost of Predator Past: Interaction of Past Predator Exposure and Resource Availability on Toxin Retention and Cell Growth in a Dinoflagellate
Abstract
1. Introduction
2. Results
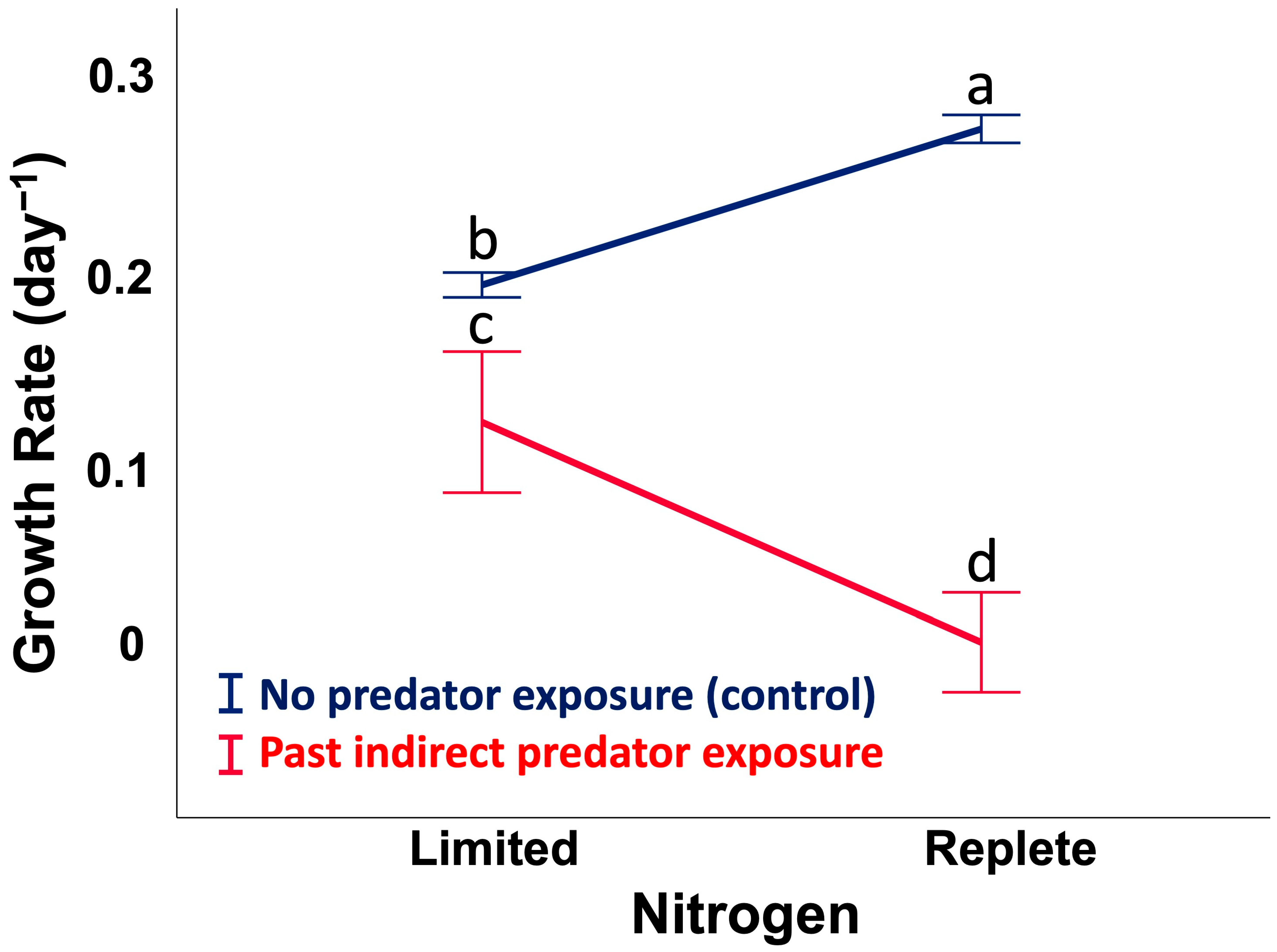
2.1. Assay 1: Cell Growth Rate (CGR), Past Predator Exposure vs. Nitrogen Availability
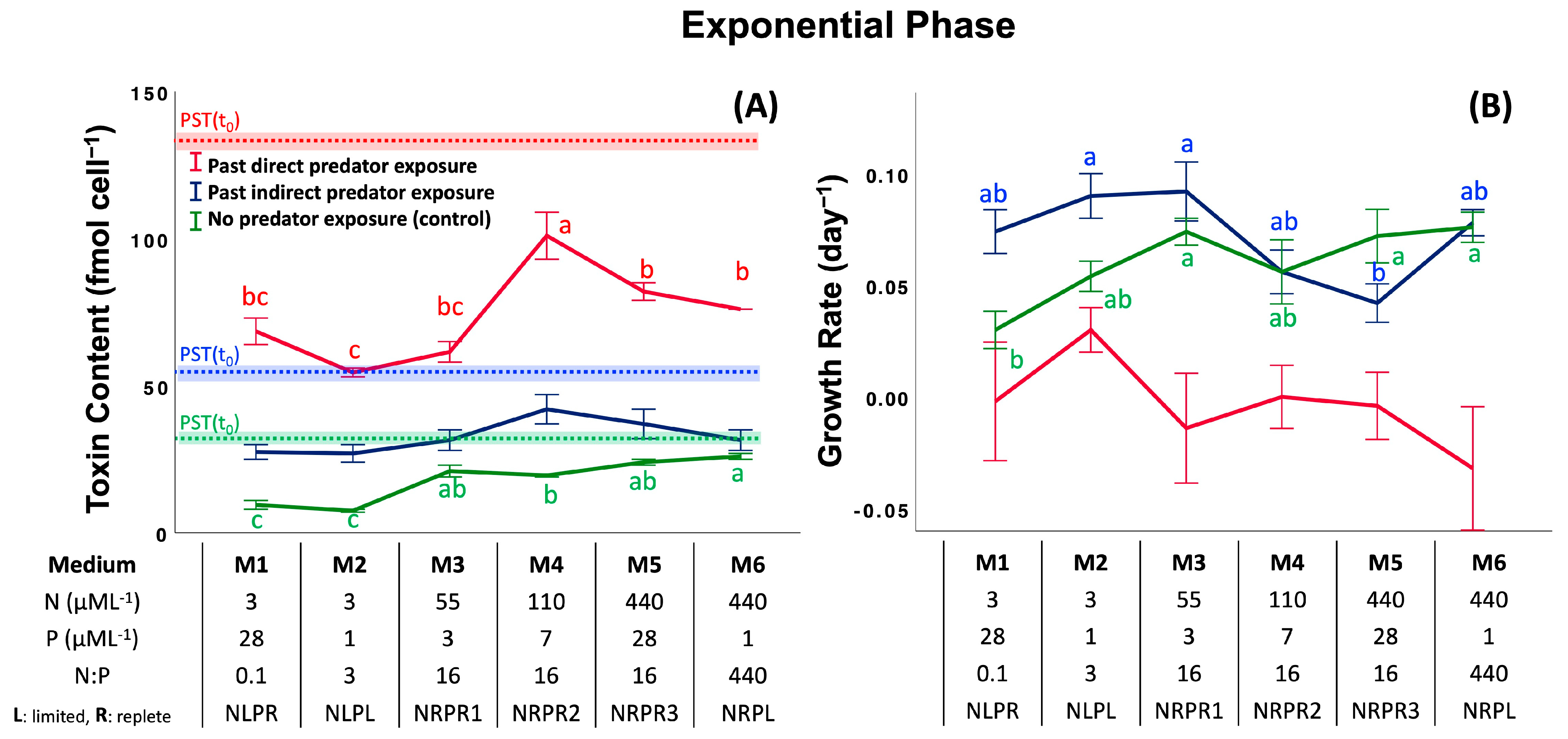
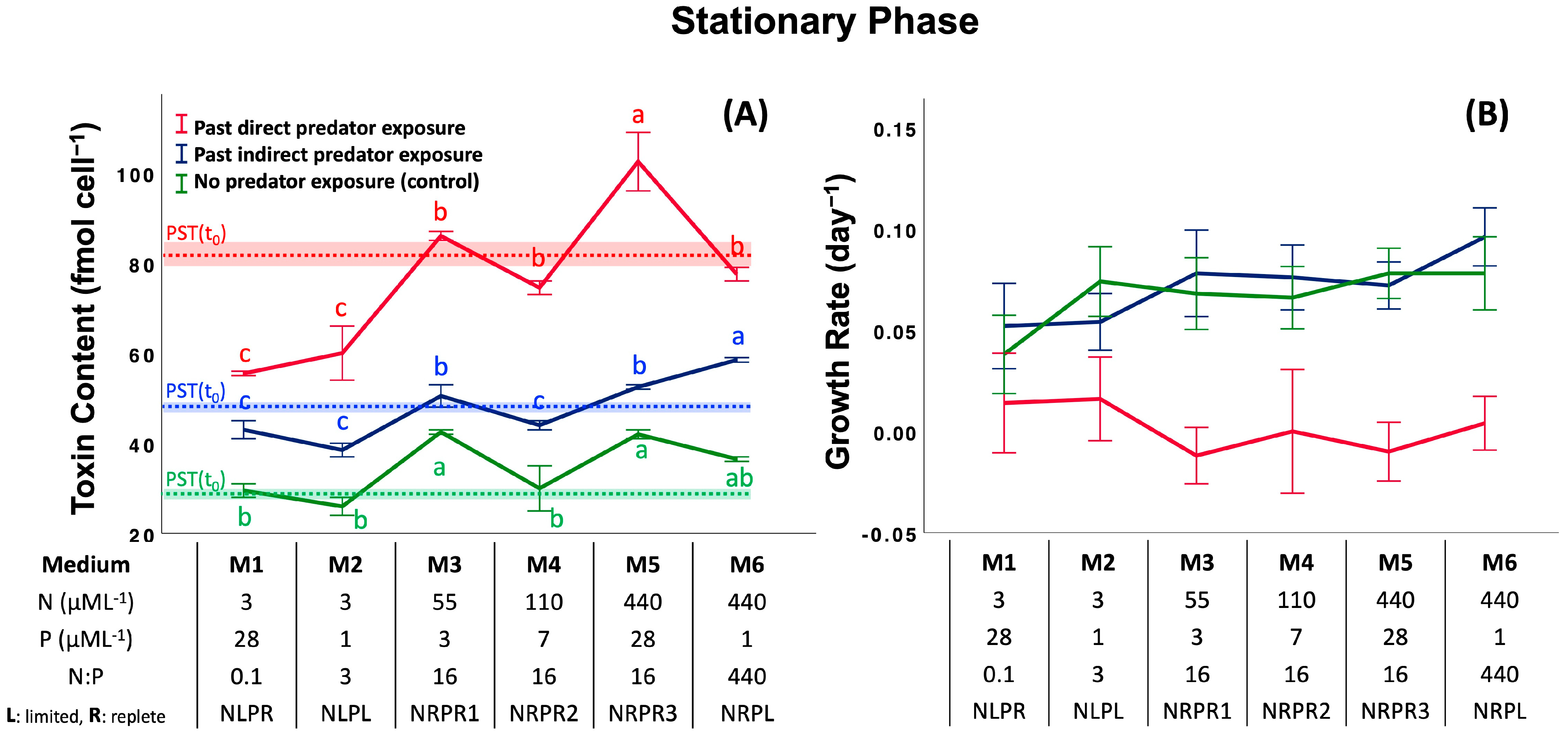
2.2. Assay 2: Effect of Past Predator Exposure and Nutrient Regime on CTC and CGR
2.3. Relationships Between Toxin Retention Rate and Cell Growth Rate
3. Discussion
4. Conclusions
5. Materials and Methods
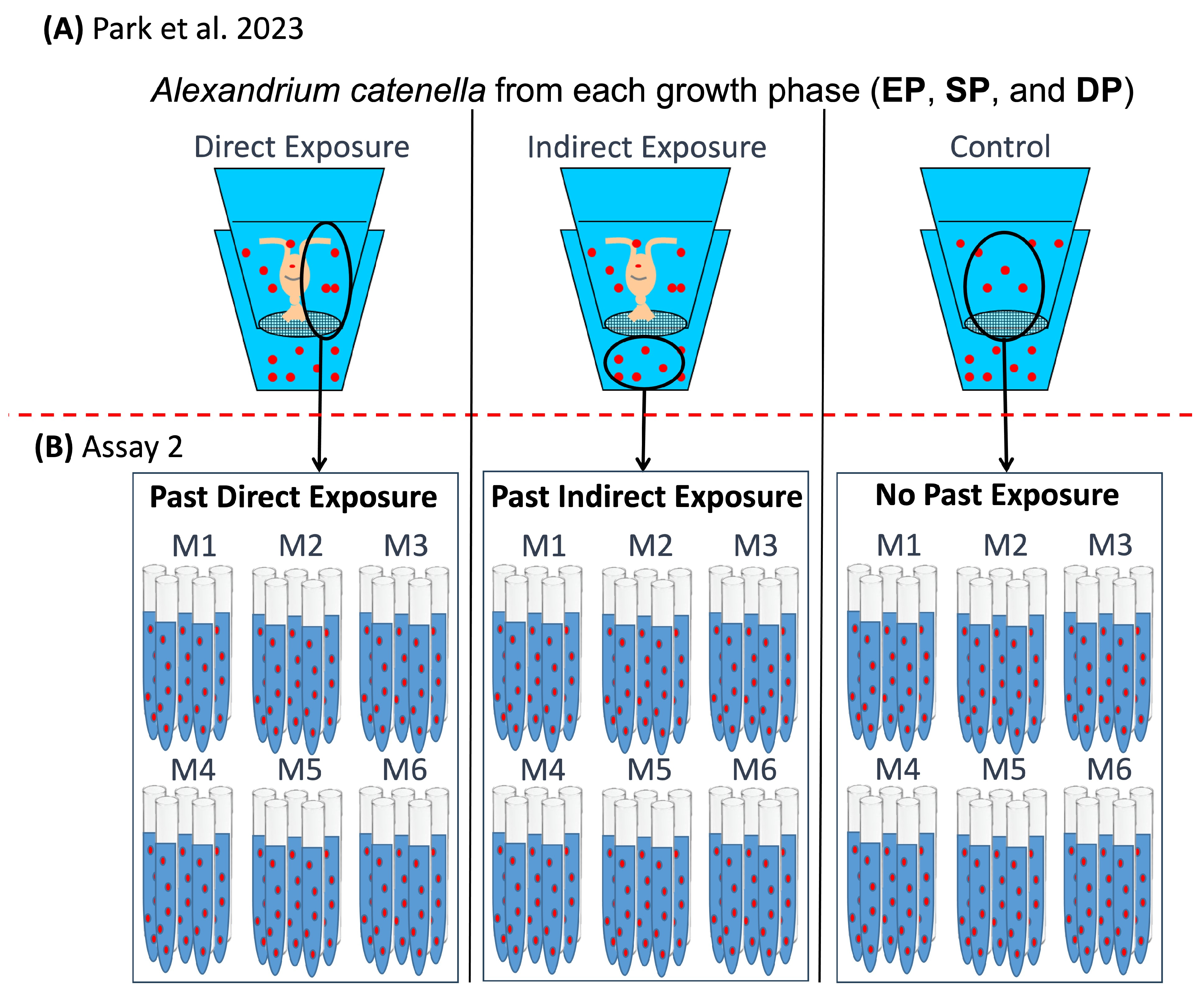
5.1. Experimental Design
5.2. Culturing of Prey and the Zooplankton Predator
5.3. Assay 1: Cell Growth Rate (CGR), Past Predator Exposure vs. Nitrogen Availability
5.4. Assay 2
5.4.1. CGR, Effect of Past Predator Exposure and Nutrient Regime
5.4.2. CTC, Effect of Past Predator Exposure and Nutrient Regime
5.5. Relationships Between Toxin Retention Rate and Growth Rate
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommer, H.; Meyer, K.F. Paralytic Shell-Fish Poisoning. Arch. Pathol. 1937, 24, 560–598. [Google Scholar]
- Schantz, E.J.; Ghazarossian, V.E.; Schnoes, H.K.; Strong, F.M.; Springer, J.P.; Pezzanite, J.O.; Clardy, J. Structure of Saxitoxin. J. Am. Chem. Soc. 1975, 97, 1238–1239. [Google Scholar] [CrossRef]
- Selander, E.; Berglund, E.C.; Engström, P.; Berggren, F.; Eklund, J.; Harðardóttir, S.; Lundholm, N.; Grebner, W.; Andersson, M.X. Copepods Drive Large-Scale Trait-Mediated Effects in Marine Plankton. Sci. Adv. 2019, 5, eaat5096. [Google Scholar] [CrossRef] [PubMed]
- Selander, E.; Cervin, G.; Pavia, H. Effects of Nitrate and Phosphate on Grazer-induced Toxin Production in Alexandrium minutum. Limnol. Oceanogr. 2008, 53, 523–530. [Google Scholar] [CrossRef]
- Selander, E.; Kubanek, J.; Hamberg, M.; Andersson, M.X.; Cervin, G.; Pavia, H. Predator Lipids Induce Paralytic Shellfish Toxins in Bloom-Forming Algae. Proc. Natl. Acad. Sci. USA 2015, 112, 6395–6400. [Google Scholar] [CrossRef]
- Bergkvist, J.; Selander, E.; Pavia, H. Induction of Toxin Production in Dinoflagellates: The Grazer Makes a Difference. Oecologia 2008, 156, 147–154. [Google Scholar] [CrossRef]
- Van Donk, E.; Ianora, A.; Vos, M. Induced Defences in Marine and Freshwater Phytoplankton: A Review. Hydrobiologia 2011, 668, 3–19. [Google Scholar] [CrossRef]
- Ryderheim, F.; Selander, E.; Kiørboe, T. Predator-Induced Defence in a Dinoflagellate Generates Benefits without Direct Costs. ISME J. 2021, 15, 2107–2116. [Google Scholar] [CrossRef]
- Brown, E.R.; Moore, S.G.; Gaul, D.A.; Kubanek, J. Predator Cues Target Signaling Pathways in Toxic Algal Metabolome. Limnol. Oceanogr. 2022, 67, 1227–1237. [Google Scholar] [CrossRef]
- Selander, E.; Fagerberg, T.; Wohlrab, S.; Pavia, H. Fight and Flight in Dinoflagellates? Kinetics of Simultaneous Grazer-induced Responses in Alexandrium tamarense. Limnol. Oceanogr. 2012, 57, 58–64. [Google Scholar] [CrossRef]
- Senft-Batoh, C.D.; Dam, H.G.; Shumway, S.E.; Wikfors, G.H.; Schlichting, C.D. Influence of Predator-Prey Evolutionary History, Chemical Alarm-Cues, and Feeding Selection on Induction of Toxin Production in a Marine Dinoflagellate: Marine Dinoflagellate Toxin Induction. Limnol. Oceanogr. 2015, 60, 318–328. [Google Scholar] [CrossRef]
- Senft-Batoh, C.D.; Dam, H.G.; Shumway, S.E.; Wikfors, G.H. A Multi-Phylum Study of Grazer-Induced Paralytic Shellfish Toxin Production in the Dinoflagellate Alexandrium fundyense: A New Perspective on Control of Algal Toxicity. Harmful Algae 2015, 44, 20–31. [Google Scholar] [CrossRef]
- Griffin, J.E.; Park, G.; Dam, H.G. Relative Importance of Nitrogen Sources, Algal Alarm Cues and Grazer Exposure to Toxin Production of the Marine Dinoflagellate Alexandrium catenella. Harmful Algae 2019, 84, 181–187. [Google Scholar] [CrossRef]
- Park, G.; Dam, H.G. Cell-Growth Gene Expression Reveals a Direct Fitness Cost of Grazer-Induced Toxin Production in Red Tide Dinoflagellate Prey. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202480. [Google Scholar] [CrossRef]
- Park, G.; Norton, L.; Avery, D.; Dam, H.G. Grazers Modify the Dinoflagellate Relationship between Toxin Production and Cell Growth. Harmful Algae 2023, 126, 102439. [Google Scholar] [CrossRef]
- Park, G.; Dam, H.G. Multiple Defense Is an Effective Antipredator Strategy in Dinoflagellates. ISME Commun. 2025, 5, ycaf029. [Google Scholar] [CrossRef]
- Peacor, S.D.; Peckarsky, B.L.; Trussell, G.C.; Vonesh, J.R. Costs of Predator-Induced Phenotypic Plasticity: A Graphical Model for Predicting the Contribution of Nonconsumptive and Consumptive Effects of Predators on Prey. Oecologia 2013, 171, 1–10. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Peacor, S.D.; Hawlena, D.; Thaker, M. Non-consumptive Predator Effects on Prey Population Size: A Dearth of Evidence. J. Anim. Ecol. 2020, 89, 1302–1316. [Google Scholar] [CrossRef]
- Wood, Z.T.; Fryxell, D.C.; Moffett, E.R.; Kinnison, M.T.; Simon, K.S.; Palkovacs, E.P. Prey Adaptation along a Competition-Defense Tradeoff Cryptically Shifts Trophic Cascades from Density-to Trait-Mediated. Oecologia 2020, 192, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Selander, E.; Thor, P.; Toth, G.; Pavia, H. Copepods Induce Paralytic Shellfish Toxin Production in Marine Dinoflagellates. Proc. R. Soc. B Biol. Sci. 2006, 273, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Trapp, A.; Heuschele, J.; Selander, E. Eavesdropping on Plankton—Can Zooplankton Monitoring Improve Forecasting of Biotoxins from Harmful Algae Blooms? Limnol. Oceanogr. 2021, 66, 3455–3471. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory, 1st ed.; University of Chicago Press: Chicago, IL, USA, 1997; ISBN 978-0-226-42495-8. [Google Scholar]
- Huntzinger, M.; Karban, R.; Young, T.P.; Palmer, T.M. Relaxation of Induced Indirect Defenses of Acacias Following Exclusion of Mammalian Herbivores. Ecology 2004, 85, 609–614. [Google Scholar] [CrossRef]
- Verschoor, A.M.; Bekmezci, O.K.; van Donk, E.; Vijverberg, J. The Ghost of Herbivory Past: Slow Defence Relaxation in the Chlorophyte Scenedesmus obliquus. J. Limnol. 2009, 68, 327–335. [Google Scholar] [CrossRef]
- Townsend, C.R.; Calow, P. Physiological Ecology: An Evolutionary Approach to Resource Use; Blackwell Scientific Publications: Oxford, UK, 1983; ISBN 978-0878938285. [Google Scholar]
- Sagers, C.L.; Coley, P.D. Benefits and Costs of Defense in a Neotropical Shrub. Ecology 1995, 76, 1835–1843. [Google Scholar] [CrossRef]
- Brönmark, C.; Lakowitz, T.; Nilsson, P.A.; Ahlgren, J.; Lennartsdotter, C.; Hollander, J. Costs of Inducible Defence along a Resource Gradient. PLoS ONE 2012, 7, e30467. [Google Scholar] [CrossRef] [PubMed]
- Van Donk, E.; Lürling, M.; Lampert, W. Consumer-Induced Changes in Phytoplankton: Inducibility, Costs, Benefits, and the Impact on Grazers. In The Ecology and Evolution of Inducible Defenses; Tollrian, R., Harvell, C.D., Eds.; Princeton University Press: Princeton, NJ, USA, 1999; pp. 89–103. ISBN 978-0-691-22819-8. [Google Scholar]
- Janzen, D.H. Evolutionary Physiology of Personal Defence. In Physiological Ecology: An Evolutionary Approach to Resource Use; Townsend, C.R., Calow, P., Eds.; Blackwell Science Inc.: Sunderland, MA, USA, 1981; pp. 145–164. ISBN 978-0-87893-828-5. [Google Scholar]
- Boyer, G.L.; Sullivan, J.J.; Andersen, R.J.; Harrison, P.J.; Taylor, F.J.R. Effects of Nutrient Limitation on Toxin Production and Composition in the Marine Dinoflagellate Protogonyaulax tamarensis. Mar. Biol. 1987, 96, 123–128. [Google Scholar] [CrossRef]
- Granéli, E.; Flynn, K. Chemical and Physical Factors Influencing Toxin Content. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2006; Volume 189, pp. 229–241. ISBN 978-3-540-32209-2. [Google Scholar]
- Anderson, D.M.; Kulis, D.M.; Sullivan, J.J.; Hall, S.; Lee, C. Dynamics and Physiology of Saxitoxin Production by the Dinoflagellates Alexandrium spp. Mar. Biol. 1990, 104, 511–524. [Google Scholar] [CrossRef]
- Guisande, C.; Frangópulos, M.; Maneiro, I.; Vergara, A.R.; Riveiro, I. Ecological Advantages of Toxin Production by the Dinoflagellate Alexandrium minutum under Phosphorus Limitation. Mar. Ecol. Prog. Ser. 2002, 225, 169–176. [Google Scholar] [CrossRef]
- Béchemin, C.; Grzebyk, D.; Hachame, F.; Hummert, C.; Maestrini, S.Y. Effect of Different Nitrogen/Phosphorus Nutrient Ratios on the Toxin Content in Alexandrium minutum. Aquat. Microb. Ecol. 1999, 20, 157–165. [Google Scholar] [CrossRef]
- Lim, P.-T.; Leaw, C.-P.; Kobiyama, A.; Ogata, T. Growth and Toxin Production of Tropical Alexandrium minutum Halim (Dinophyceae) under Various Nitrogen to Phosphorus Ratios. J. Appl. Phycol. 2010, 22, 203–210. [Google Scholar] [CrossRef]
- Agrawal, A.A. Benefits and Costs of Induced Plant Defense for Lepidium virginicum (Brassicaceae). Ecology 2000, 81, 1804–1813. [Google Scholar] [CrossRef]
- Relyea, R.A. Costs of Phenotypic Plasticity. Am. Nat. 2002, 159, 272–282. [Google Scholar] [CrossRef]
- Morris, W.F.; Traw, M.B.; Bergelson, J. On Testing for a Tradeoff between Constitutive and Induced Resistance. Oikos 2006, 112, 102–110. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A.; Rasmann, S.; Petry, W.K.; Carrillo-Gavilán, A.; Zas, R.; Sampedro, L. Trade-offs between Constitutive and Induced Defences Drive Geographical and Climatic Clines in Pine Chemical Defences. Ecol. Lett. 2014, 17, 537–546. [Google Scholar] [CrossRef]
- Boczar, B.A.; Beitler, M.K.; Liston, J.; Sullivan, J.J.; Cattolico, R.A. Paralytic Shellfish Toxins in Protogonyaulax tamarensis and Protogonyaulax catenella in Axenic Culture. Plant Physiol. 1988, 88, 1285–1290. [Google Scholar] [CrossRef]
- Parkhill, J.-P.; Cembella, A.D. Effects of Salinity, Light and Inorganic Nitrogen on Growth and Toxigenicity of the Marine Dinoflagellate Alexandrium tamarense from Northeastern Canada. J. Plankton Res. 1999, 21, 939–955. [Google Scholar] [CrossRef]
- Lim, P.; Leaw, C.; Usup, G.; Kobiyama, A.; Koike, K.; Ogata, T. Effects of Light and Temperature on Growth, Nitrate Uptake, and Toxin Production of Two Tropical Dinoflagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae)1. J. Phycol. 2006, 42, 786–799. [Google Scholar] [CrossRef]
- Dodson, S.I.; Crowl, T.A.; Peckarsky, B.L.; Kats, L.B.; Covich, A.P.; Culp, J.M. Non-Visual Communication in Freshwater Benthos: An Overview. J. N. Am. Benthol. Soc. 1994, 13, 268–282. [Google Scholar] [CrossRef]
- Hay, M.E. Marine Chemical Ecology: Chemical Signals and Cues Structure Marine Populations, Communities, and Ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- John, E.H.; Flynn, K.J. Growth Dynamics and Toxicity of Alexandrium fundyense (Dinophyceae): The Effect of Changing N [Ratio] P Supply Ratios on Internal Toxin and Nutrient Levels. Eur. J. Phycol. 2000, 35, 11–23. [Google Scholar] [CrossRef]
- Leong, S.C.Y.; Murata, A.; Nagashima, Y.; Taguchi, S. Variability in Toxicity of the Dinoflagellate Alexandrium tamarense in Response to Different Nitrogen Sources and Concentrations. Toxicon 2004, 43, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ignatiades, L.; Gotsis-Skretas, O.; Metaxatos, A. Field and Culture Studies on the Ecophysiology of the Toxic Dinoflagellate Alexandrium minutum (Halim) Present in Greek Coastal Waters. Harmful Algae 2007, 6, 153–165. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, H.J.; Kang, H.C.; Ok, J.H.; You, J.H.; Park, S.A. Growth Rates and Nitrate Uptake of Co-Occurring Red-Tide Dinoflagellates Alexandrium affine and A. fraterculus as a Function of Nitrate Concentration under Light-Dark and Continuous Light Conditions. Algae 2019, 34, 237–251. [Google Scholar] [CrossRef]
- Taroncher-Oldenburg, G.; Kulis, D.M.; Anderson, D.M. Coupling of Saxitoxin Biosynthesis to the G1 Phase of the Cell Cycle in the dinoflagellate Alexandrin fundyense: Temperature and Nutrient Effects. Nat. Toxins 1999, 7, 207–219. [Google Scholar] [CrossRef]
- Frangópulos, M.; Guisande, C.; DeBlas, E.; Maneiro, I. Toxin Production and Competitive Abilities under Phosphorus Limitation of Alexandrium Species. Harmful Algae 2004, 3, 131–139. [Google Scholar] [CrossRef]
- Cody, M.L. A General Theory of Clutch Size. Evolution 1966, 20, 174–184. [Google Scholar] [CrossRef]
- Pančić, M.; Kiørboe, T. Phytoplankton Defence Mechanisms: Traits and Trade-offs. Biol. Rev. 2018, 93, 1269–1303. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Rudgers, J.A.; Lau, J.A.; Irwin, R.E. Direct and Ecological Costs of Resistance to Herbivory. Trends Ecol. Evol. 2002, 17, 278–285. [Google Scholar] [CrossRef]
- Peckarsky, B.L.; Penton, M.A. Why Do Ephemerella Nymphs Scorpion Posture: A “Ghost of Predation Past”? Oikos 1988, 53, 185–193. [Google Scholar] [CrossRef]
- Neill, W.E. Induced Vertical Migration in Copepods as a Defence against Invertebrate Predation. Nature 1990, 345, 524–526. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Bitton, A.; DaVeiga, J. How Does the Presence of Predators Influence the Persistence of Antipredator Behavior? J. Theor. Biol. 2006, 239, 460–468. [Google Scholar] [CrossRef]
- Young, T.P.; Okello, B.D. Relaxation of an Induced Defense after Exclusion of Herbivores: Spines on Acacia drepanolobium. Oecologia 1998, 115, 508–513. [Google Scholar] [CrossRef]
- Stephens, T.G.; Ragan, M.A.; Bhattacharya, D.; Chan, C.X. Core Genes in Diverse Dinoflagellate Lineages Include a Wealth of Conserved Dark Genes with Unknown Functions. Sci. Rep. 2018, 8, 17175. [Google Scholar] [CrossRef]
- Pozdnyakov, I.; Matantseva, O.; Skarlato, S. Consensus Channelome of Dinoflagellates Revealed by Transcriptomic Analysis Sheds Light on Their Physiology. Algae 2021, 36, 315–326. [Google Scholar] [CrossRef]
- Li, J.; Halitschke, R.; Li, D.; Paetz, C.; Su, H.; Heiling, S.; Xu, S.; Baldwin, I.T. Controlled Hydroxylations of Diterpenoids Allow for Plant Chemical Defense without Autotoxicity. Science 2021, 371, 255–260. [Google Scholar] [CrossRef]
- Zanette, L.Y.; Clinchy, M. Ecology of Fear. Curr. Biol. 2019, 29, R309–R313. [Google Scholar] [CrossRef]
- Peplinski, J.; Malone, M.A.; Fowler, K.J.; Potratz, E.J.; Pergams, A.G.; Charmoy, K.L.; Rasheed, K.; Avdieiev, S.S.; Whelan, C.J.; Brown, J.S. Ecology of Fear: Spines, Armor and Noxious Chemicals Deter Predators in Cancer and in Nature. Front. Ecol. Evol. 2021, 9, 682504. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–80. ISBN 978-1-4615-8716-3. [Google Scholar]
- Colin, S.P.; Dam, H.G. Latitudinal Differentiation in the Effects of the Toxic Dinoflagellate Alexandrium spp. on the Feeding and Reproduction of Populations of the Copepod Acartia hudsonica. Harmful Algae 2002, 1, 113–125. [Google Scholar] [CrossRef]
- Flores, H.S.; Wikfors, G.H.; Dam, H.G. Reactive Oxygen Species Are Linked to the Toxicity of the Dinoflagellate Alexandrium spp. to Protists. Aquat. Microb. Ecol. 2012, 66, 199–209. [Google Scholar] [CrossRef]
- Colin, S.P.; Dam, H.G. Testing for Resistance of Pelagic Marine Copepods to a Toxic Dinoflagellate. Evol. Ecol. 2005, 18, 355–377. [Google Scholar] [CrossRef]
- Colin, S.P.; Dam, H.G. Comparison of the Functional and Numerical Responses of Resistant versus Non-Resistant Populations of the Copepod Acartia hudsonica Fed the Toxic Dinoflagellate Alexandrium tamarense. Harmful Algae 2007, 6, 875–882. [Google Scholar] [CrossRef]
- Senft-Batoh, C.D. Grazer-Induced Toxin Production in the Dinoflagellate Alexandrium fundyense: Cues, Mechanisms, and Cost; University of Connecticut: Storrs, CT, USA, 2012. [Google Scholar]
- Maclean, C.; Cembella, A.D.; Quilliam, M.A. Effects of Light, Salinity and Inorganic Nitrogen on Cell Growth and Spirolide Production in the Marine Dinoflagellate Alexandrium ostenfeldii (Paulsen) Balech et Tangen. Bot. Mar. 2003, 46, 466–476. [Google Scholar] [CrossRef]
- Oshima, Y. Post-column Derivatization HPLC Methods for Paralytic Shellfish Poisons. In Manual on Harmful Marine Microalgae; No. 33; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1995. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.; Batoh, C.; Dam, H.G. The Ghost of Predator Past: Interaction of Past Predator Exposure and Resource Availability on Toxin Retention and Cell Growth in a Dinoflagellate. Toxins 2025, 17, 290. https://doi.org/10.3390/toxins17060290
Park G, Batoh C, Dam HG. The Ghost of Predator Past: Interaction of Past Predator Exposure and Resource Availability on Toxin Retention and Cell Growth in a Dinoflagellate. Toxins. 2025; 17(6):290. https://doi.org/10.3390/toxins17060290
Chicago/Turabian StylePark, Gihong, Christina Batoh, and Hans G. Dam. 2025. "The Ghost of Predator Past: Interaction of Past Predator Exposure and Resource Availability on Toxin Retention and Cell Growth in a Dinoflagellate" Toxins 17, no. 6: 290. https://doi.org/10.3390/toxins17060290
APA StylePark, G., Batoh, C., & Dam, H. G. (2025). The Ghost of Predator Past: Interaction of Past Predator Exposure and Resource Availability on Toxin Retention and Cell Growth in a Dinoflagellate. Toxins, 17(6), 290. https://doi.org/10.3390/toxins17060290





