Significance of the Stability of Fusarium Head Blight Resistance in the Variety Registration, Breeding, and Genetic Research of Winter Wheat Using Disease Index, Fusarium-Damaged Kernels, and Deoxynivalenol Contamination
Abstract
1. Introduction
2. Results
2.1. Experiment Conducted from 1990 to 1993 [15]
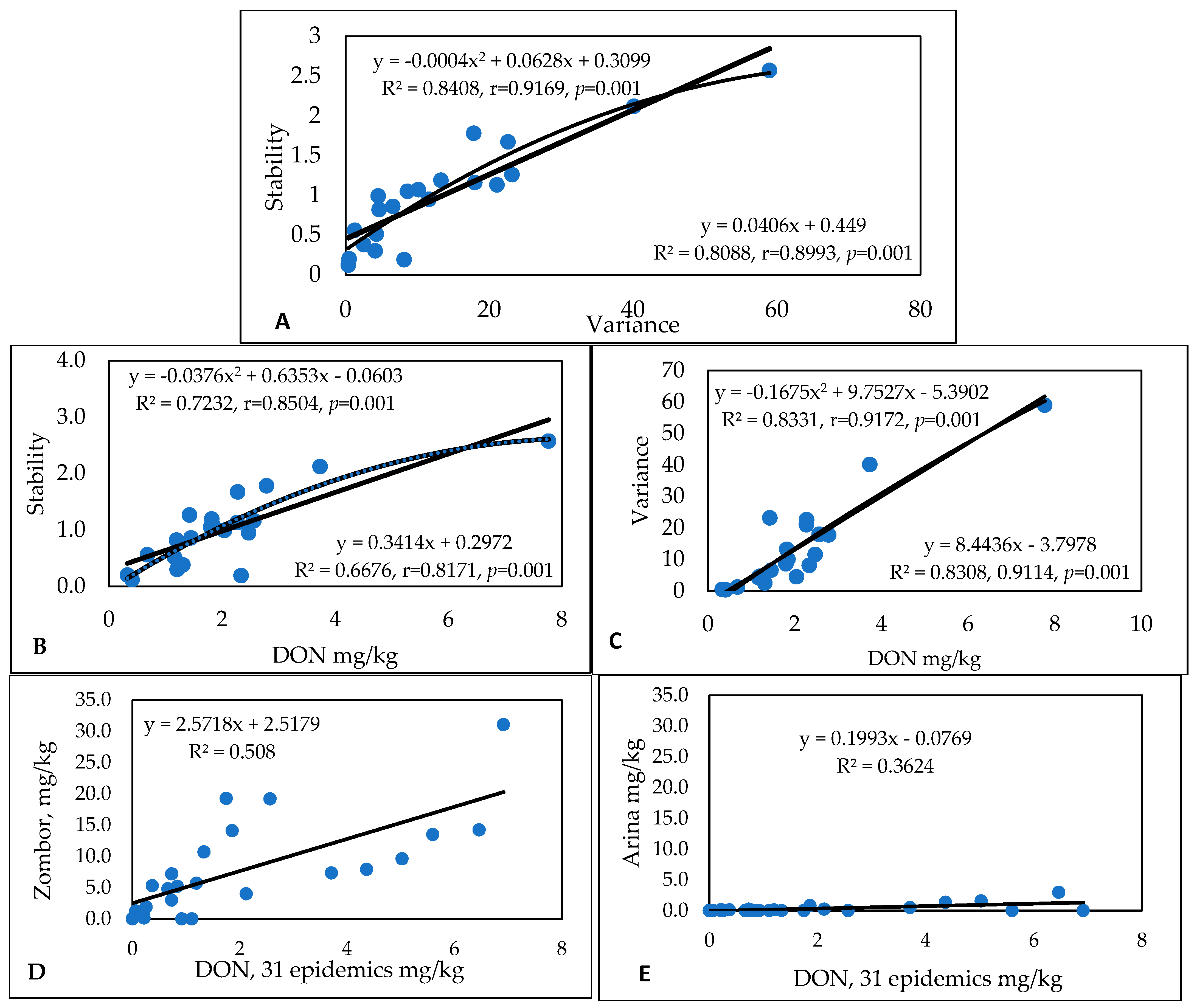
2.1.1. FHB Traits and Their Relationships
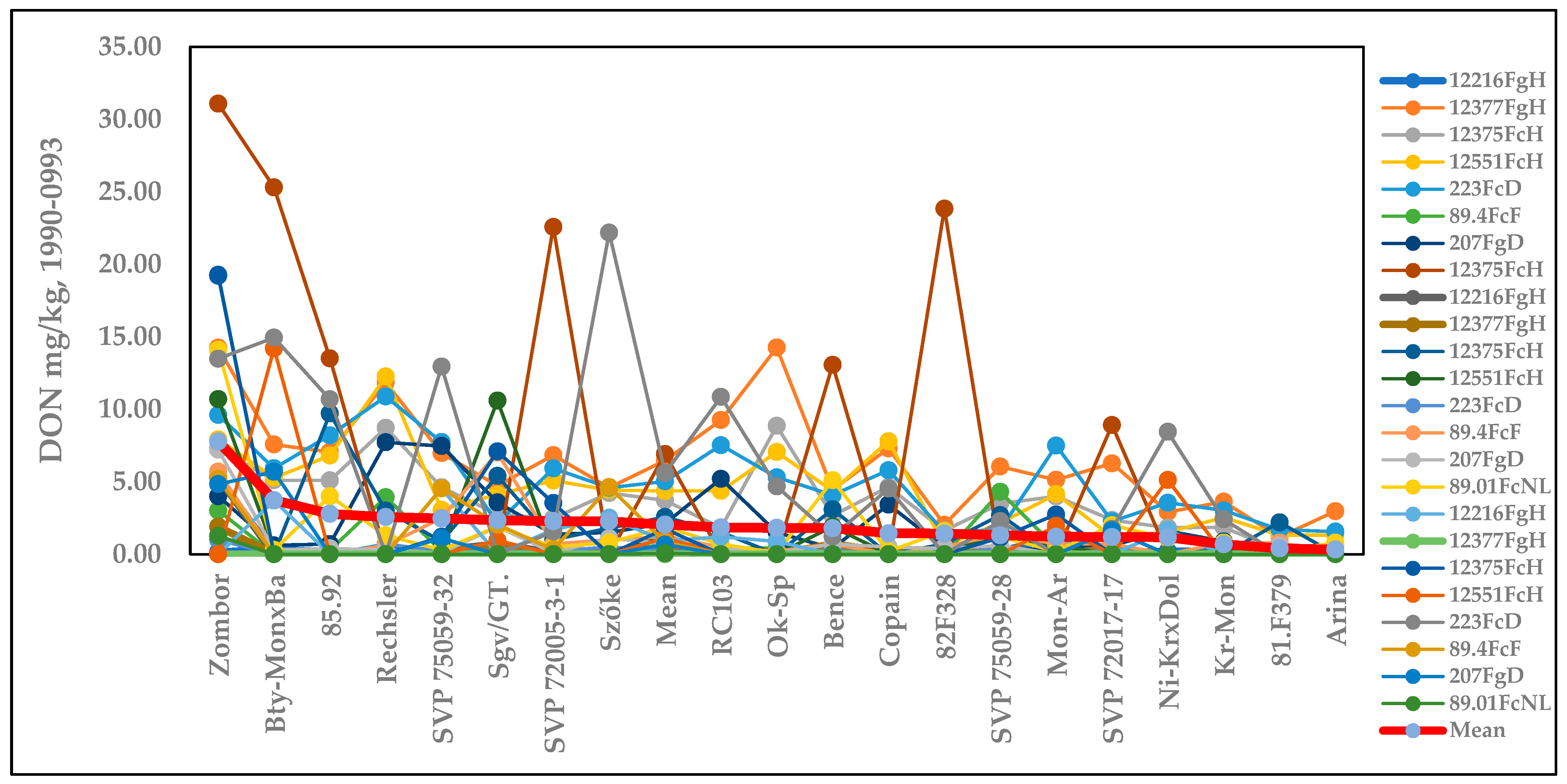
2.1.2. Influence of Isolates on the Variety Ranking of Genotypes in Experiment 1
2.2. Experiment Conducted from 1994 to 1996 (Mesterhazy et al., 1999) [15]
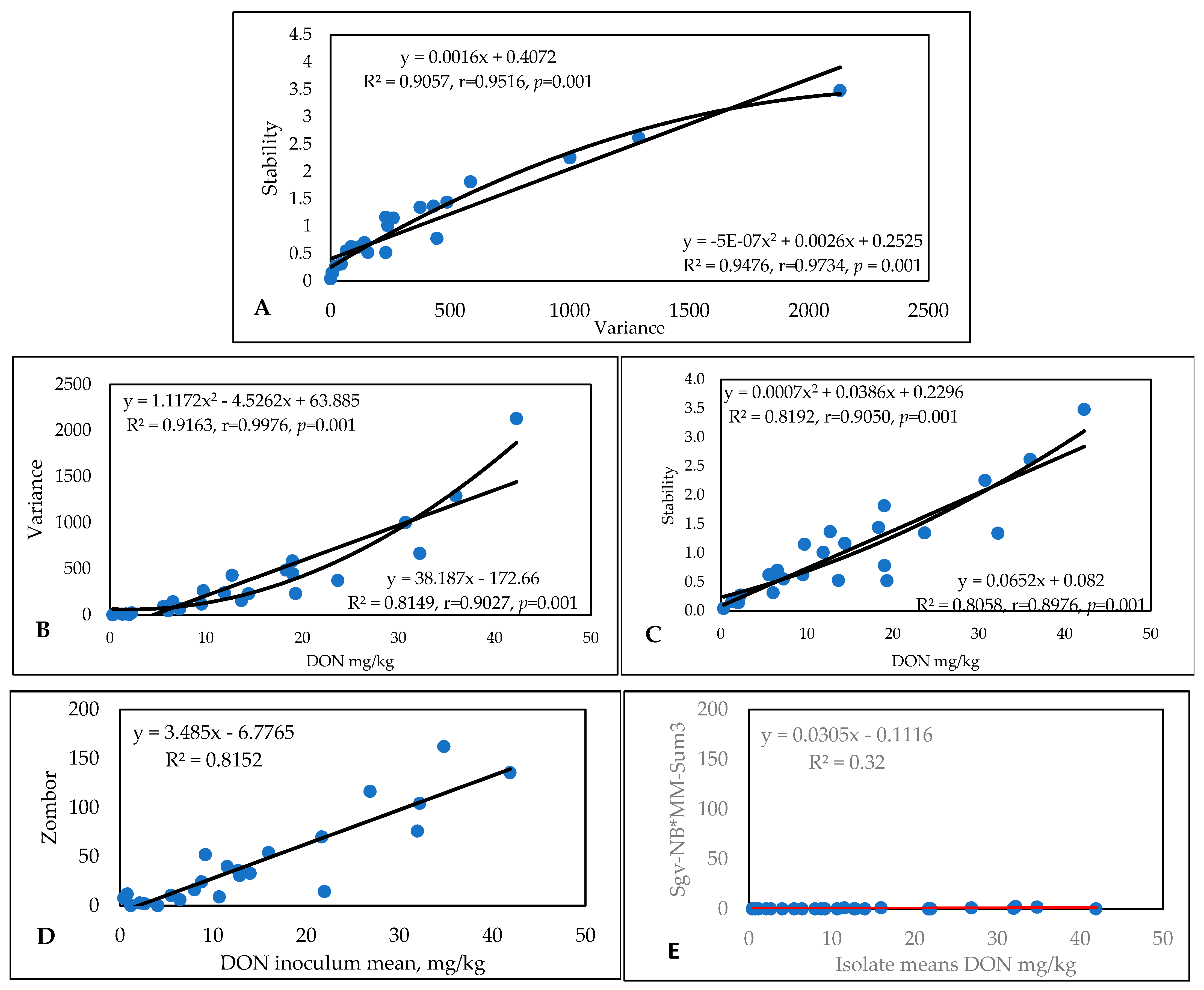
2.2.1. FHB Traits and Their Relations
2.2.2. Influence of Isolates on the Variety Ranking of Genotypes for Experiment 2
2.3. Experiment Conducted Between 2009 and 2012 [14]
2.3.1. FHB Traits and Their Relations
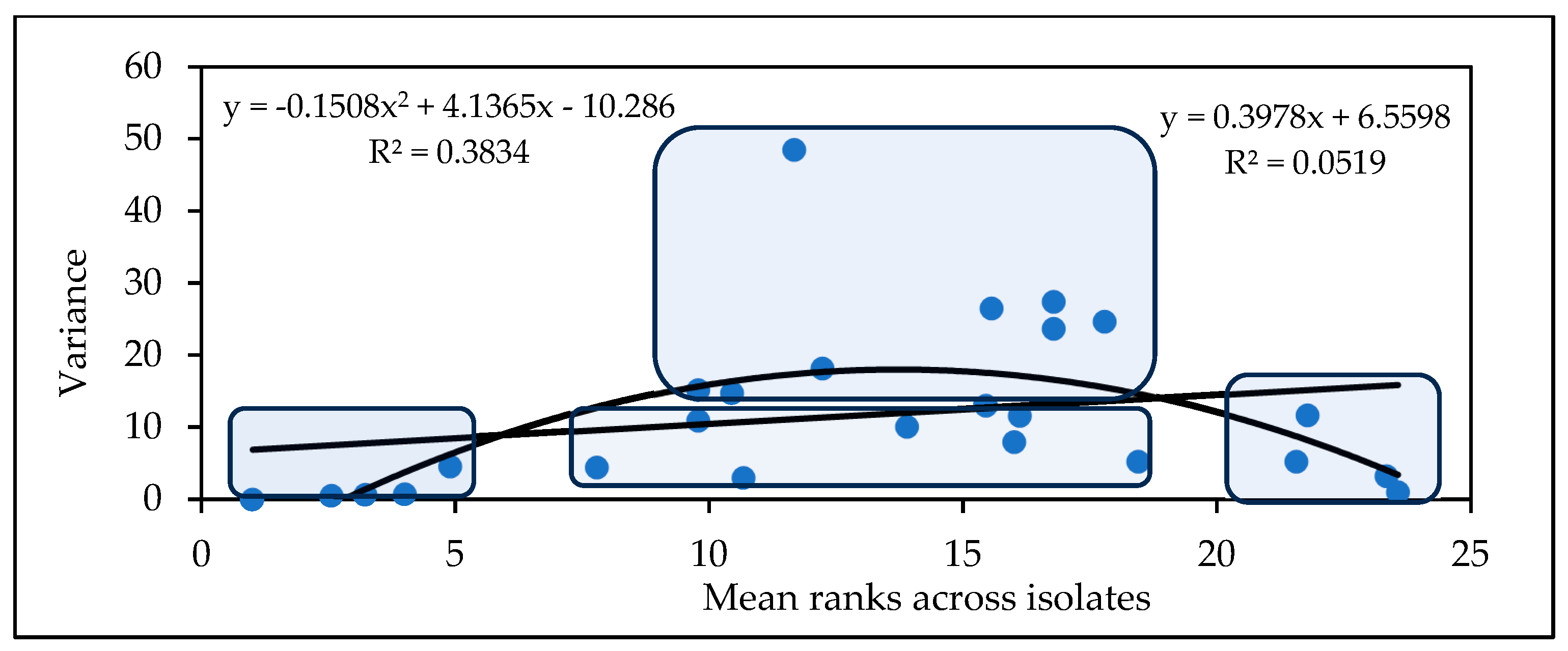
2.3.2. Influence of Isolates on the Variety Ranking of Genotypes in the 3rd Experiment
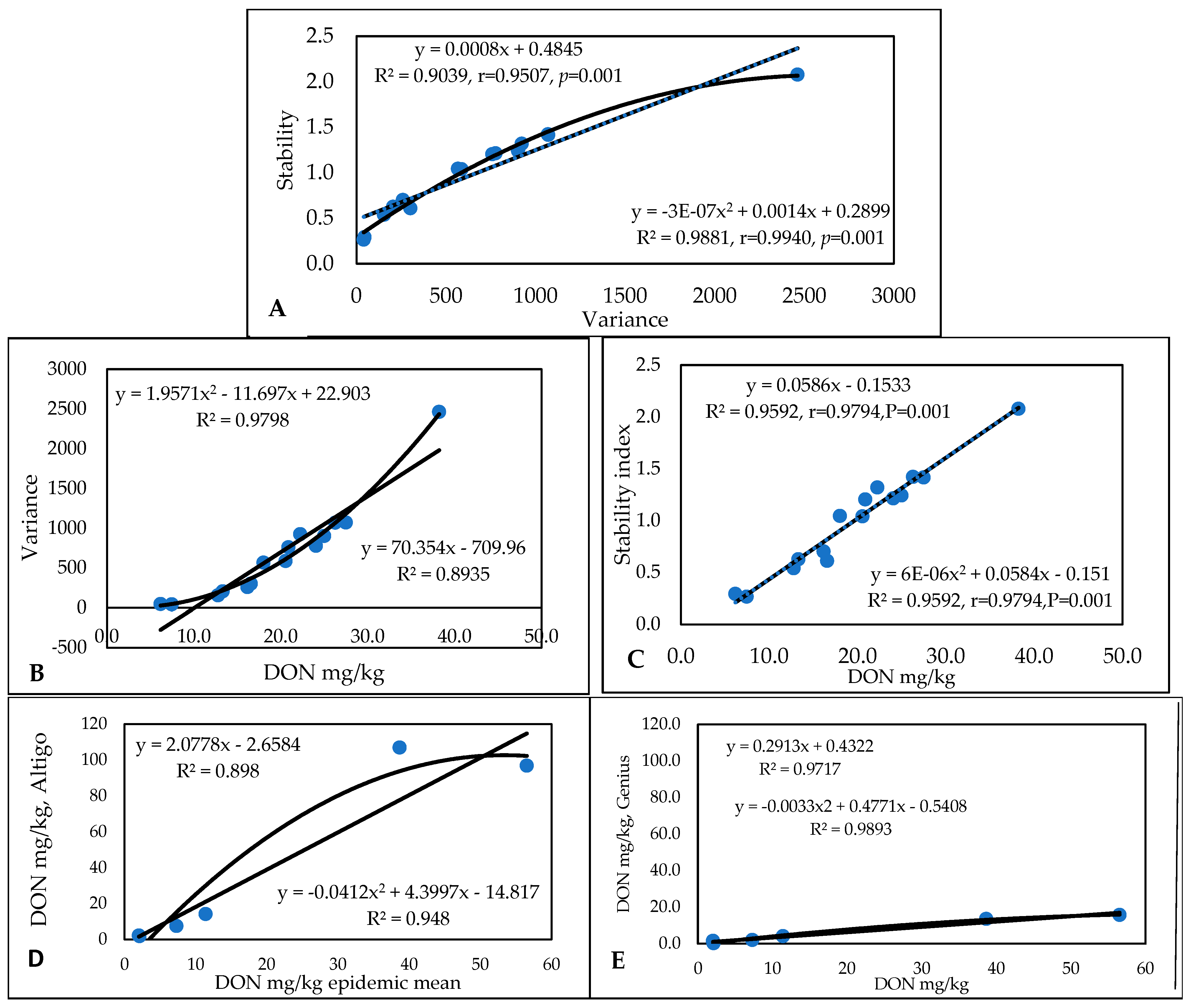
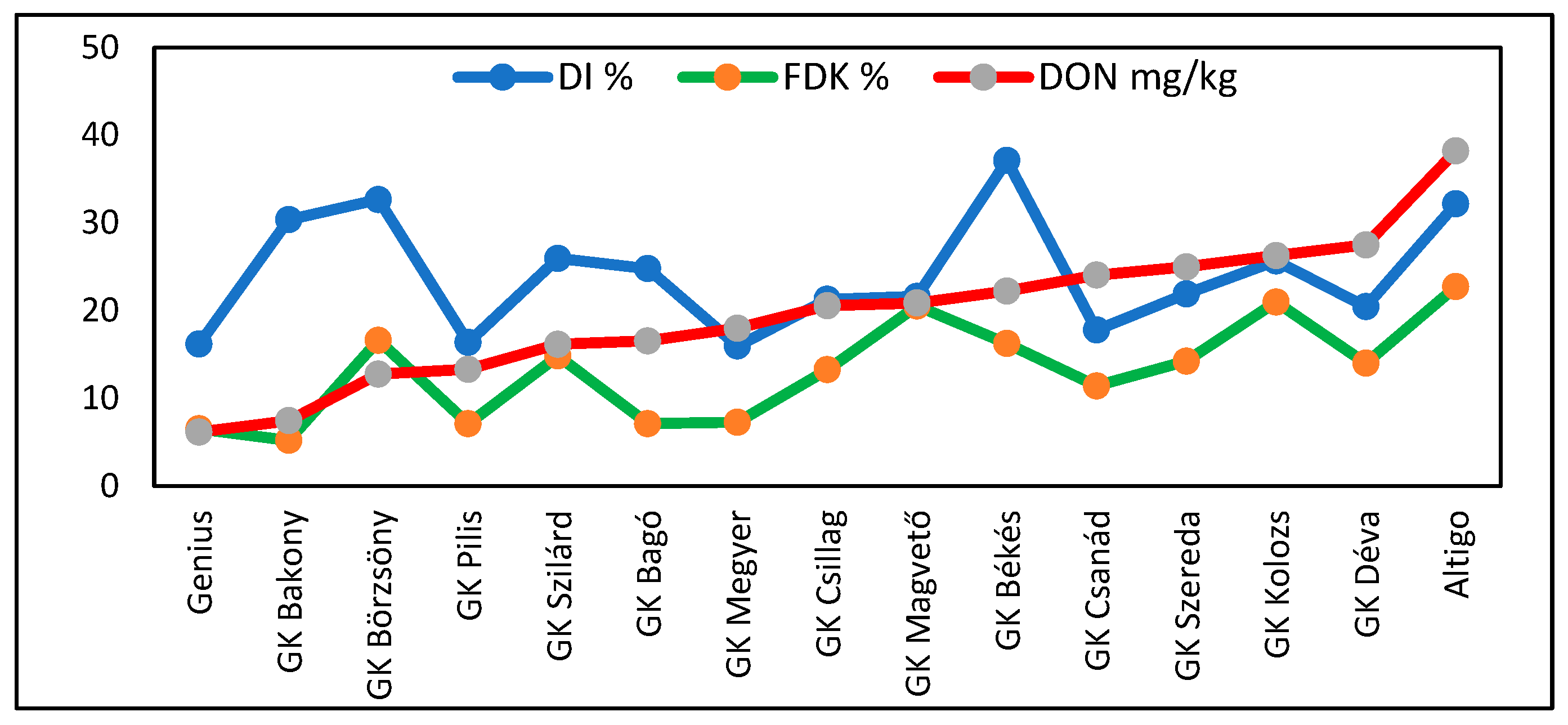
2.4. Experiment 4: 2019–2021
2.5. PC Analyses for Experiments 1–4
2.5.1. Experiment 1
2.5.2. Experiment 2
2.5.3. Experient 3
2.5.4. Experiment 4
3. Discussion
3.1. Stability
3.2. Isolates, Epidemics, and Environment
3.3. Correlations for Traits and Epidemics
3.4. Relationships Between Traits
3.5. Principal Component Analyses
3.6. The Multi-Toxin Problem
3.7. Breeding Aspects
4. Material and Methods
4.1. Plant Material and Experimental Design
4.2. Inoculations and Their Evaluation
4.2.1. Isolates
4.2.2. Inoculum Production
4.2.3. Inoculation
4.2.4. Disease Evaluation
4.2.5. Evaluation of Stability
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AUDPC | Area under disease progress curve |
| DH | Doubled haploid |
| DI | Disease index |
| DON | Deoxynivalenol |
| FDK | Fusarium-damaged kernels |
| FHB | Fusarium head blight |
| GK | Gabona Kutató (Cereal Res. Ltd.) |
| LSD | Limit of smallest difference |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| QTL | Quantitative trait locus |
| RD | Resistance to DON accumulation |
| SI | Stability index; b value in the linear function y = a + bx |
References
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; DelPonte, E.M.; Chala, A.; et al. Key Global Actions for mycotoxin management in wheat and other small grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Park, D.L.; Njapau, H.; Boutrif, E. Minimizing risks posed by mycotoxins utilizing the HACCP concept. FAO Food. Nutr. Agric. J. 1999, 23, 49–56. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Oláh, J.; Popp, J. Losses in the grain supply chain: Causes and solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef]
- Anonymous. DSM-Firmenich World Mycotoxin Survey, The Global Threat January—December 2024. 2024. Available online: https://magyarmezogazdasag.hu/2025/02/26/riaszto-szinteket-ert-el-a-mikotoxin-szennyezettseg-az-allati-takarmanyokban (accessed on 17 February 2025).
- Bai, G.; Su, Z.; Ca, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Nakagawa, H.O.; Gocho, H.; Nishio, K.; Watanabe, S. Nature and inheritance of ear scab resistance in wheat. I. Heritability estimates and heritable relationships of ear-scab and some agronomic characters in F2 of the cross Shinchu-naga x Norin 12. Bull. Tokai-Kinki Agric. Exp. Stn. 1966, 15, 43–54. [Google Scholar]
- Hanson, E.W.; Ausemus, A.R.; Stakman, S.C. Varietal resistance of spring wheats to fusarial head blight. Phytopathology 1950, 30, 61–90. [Google Scholar]
- Scott, I.T. Varietal Resistance and Susceptibility to Scab; Research Bulletin 111; University of Missouri, College of Agriculture, Agricultural Experiment Station: Columbia, MO, USA, 1927; 14p. [Google Scholar]
- Liu, Z.Z.; Wang, Z.Y. Improved scab resistance in China: Source of resistance and problems. In Proceedings of the Wheat for Nontraditional, Warm Areas: A Proceedings of the International Conference, Foz Do Iguacu, Brazil, 29 July–3 August 1990; Saunders, D.A., Ed.; CIMMYT: México-Veracruz, Mexico, 1991; pp. 178–188. [Google Scholar]
- Atanasoff, D. Fusarium blight (scab) of wheat and other cereals. J. Agric. Res. 1920, 20, 1–32. [Google Scholar]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Mesterházy, Á. Types and components of resistance against Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.M.; Komoróczy, R. Nature of resistance of wheat to Fusarium head blight and deoxynivalenol contamination and their consequences for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins, Chemistry, Genetics, and Biology; APS Press: St. Paul, MN, USA, 2006; 260p, ISBN 10:0-89054-335-6. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. In Epidemiology of Mycotoxin Producing Fungi; Xu, X., Bailey, J.A., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 109, pp. 645–667. [Google Scholar]
- Miedaner, T. Breeding wheat and rye for resistance to Fusarium diseases. Plant Breed. 1997, 116, 201–220. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Hernández-Córdoba, M.; Vinas, P. High-resolution mass spectrometry for the determination of mycotoxins in biological samples. A review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, W.; Zhao, X.; Cao, H.; Fan, K.; Meng, J.; Nie, D.; Wu, Y.; Han, Z. Universal screening of 200 mycotoxins and their variations in stored cereals in Shanghai, China by UHPLC-Q-TOF MS. Food Chem. 2022, 387, 132869. [Google Scholar] [CrossRef] [PubMed]
- Lemming, E.W.; Montano, M.A.; Schmidt, J.; Cramer, B.; Humpf, H.U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents—Possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef]
- Beres, B.L.; Brûlé-Babel, A.L.; Ye, Z.; Graf, R.J.; Turkington, T.K.; Harding, M.W.; Kutcher, H.R.; Hooker, D.C. Exploring Genotype × Environment × Management synergies to manage Fusarium head blight in wheat. Can. J. Plant Pathol. 2018, 40, 179–188. [Google Scholar] [CrossRef]
- Van Eeuwijk, F.A.; Mesterhazy, A.; Kling, C.I.; Ruckenbauer, P.; Saur, L.; Buerstmayr, H.; Lemmens, M.; Maurin, M.; Snijders, C.H.A. Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale, using a multiplicative model for interaction. Theor. Appl. Genet. 1995, 90, 221–228. [Google Scholar] [CrossRef]
- Mesterházy, A.; Lehoczki-Krsjak, S.; Varga, M.; S zabó-Hevér, Á.; Tóth, B.; Lemmens, M. Breeding for FHB resistance via Fusarium damaged kernels and deoxynivalenol accumulation as well as inoculation methods in winter wheat. Agric. Sci. 2015, 6, 970–1002. [Google Scholar] [CrossRef]
- Fuentes, R.G.; Mickelson, H.R.; Busch, R.H.; Dill-Macky, R.; Evans, C.K.; Wiersma, J.V.; Xie, W.; Dong, Y.; Anderson, J.A. Resource allocation and cultivar stability in breeding for Fusarium head blight resistance in spring wheat. Crop Sci. 2005, 45, 1965–1972. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russel, W.A. Stability parameters for comparing parameters. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Varga, M.; Toth, B. Methodical considerations and resistance evaluation against F. graminearum and F. culmorum Head Blight in wheat. The influence of mixture of isolates on aggressiveness and resistance expression. Microorganisms 2020, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Gyorgy, A.; Varga, M.; Mesterhazy, A. The Influence of the dilution rate on the aggressiveness of inocula and the expression of resistance against Fusarium head blight in wheat. Plants 2020, 9, 943. [Google Scholar] [CrossRef]
- Mesterhazy, A. Updating the breeding philosophy of wheat to Fusarium Head Blight (FHB): Resistance components, QTL identification and phenotyping—A review. Plants 2020, 9, 1702. [Google Scholar] [CrossRef]
- Szabó-Hevér, Á.; Lehoczki-Krsjak, S.; Varga, M.; Purnhauser, L.; Pauk, J.; Lantos, C.; Mesterházy, Á. Differential influence of QTL linked to Fusarium head blight, Fusarium-damaged kernel, deoxynivalenol contents and associated morphological traits in a Frontana-derived wheat population. Euphytica 2014, 200, 9–26. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Tóth, B.; Varga, M.; Bartók, T.; Szabó-Hevér, Á.; Farády, L.; Lehoczki-Krsjak, S. Role of Fungicides, of Nozzle Types, and the Resistance Level of Wheat Varieties in the Control of Fusarium Head Blight and Deoxynivalenol. Toxins 2011, 3, 1453–1483. [Google Scholar] [CrossRef]
- Lana, D.F.; Paul, P.A.; Minyo, R.; Thomison, P.; Madden, L.V. Stability of hybrid maize reaction to Gibberella ear rot and deoxynivalenol contamination of grain. Phytopathology 2020, 110, 1908–1922. [Google Scholar] [CrossRef]
- Gaire, R.; Sneller, C.; Brown-Guedira, G.; Van Sanford, D.; Mohammadi, M.; Kolb, F.L.; Olson, E.; Sorrels, M.; Rutkpski, J. Genetic trends in FHB resistance from 20 years of winter wheat breeding and cooperative testing in the northern USA. Plant Dis. 2022, 106, 364–372. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Varga, M.; György, A.; Lehoczki-Krsjak, S.; Tóth, B. The role of adapted and non-adapted resistance sources in breeding resistance of winter wheat to Fusarium head blight and deoxynivalenol contamination. World Mycotoxin J. 2018, 11, 539. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, W.Q.; van der Lee, T.; Waalwijk, C.; van Diepeninge, A.D.; Feng, J.; Zhang, H.; Liu, T.G. Evaluation of Fusarium Head Blight resistance in 410 Chinese wheat cultivars selected for their climate conditions and ecological niche using natural infection across three distinct experimental sites. Front. Plant Sci. 2022, 13, 916282. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Wellings, C.R.; Park, R.F. Wheat Rusts an Atlas of Resistance Genes; CSIRO: Pullenvale, Australia, 1995; ISBN 0 643 05428 6. [Google Scholar]
- Roelfs, A.P. Resistance to leaf and stem rusts in wheat. In Breeding Strategies for Resistance to the Rusts of Wheat; Simmonds, N.W., Rajaram, S., Eds.; CIMMYT: México-Veracruz, Mexico, 1988; pp. 10–22. [Google Scholar]
- Pirko, Y.V.; Karelov, A.V.; Kozub, N.O.; Ivashchuk, B.V.; Sozinov, I.A.; Topchii, T.V.; Morgun, V.V.; Blume, Y.B. identification of genes for resistance to yellow rust of Asian origin in winter wheat cultivars and lines. Cytol. Genet. 2021, 55, 227–235. [Google Scholar] [CrossRef]
- Lagudah, E.S. Molecular genetics of race non-specific rust resistance in wheat. Euphytica 2011, 179, 81–91. [Google Scholar] [CrossRef]
- Miedaner, T.; Sieber, A.-N.; Desaint, H.; Buerstmayr, H.; Friedrich, C.; Longin, H.; Würschum, T. The potential of genomic-assisted breeding to improve Fusarium head blight resistance in winter durum wheat. Plant Breed. 2017, 136, 610–619. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Du, Z.; Che, M.; Yibin Zhang, Y.; Quan, W.; Jiang, X.; Ma, Y.; Zhao, Y.; Zhang, Z. Identification of a major QTL on chromosome arm 2AL for reducing yellow rust severity from a Chinese wheat landrace with evidence for durable resistance. Theor. Appl. Genet. 2019, 132, 457–471. [Google Scholar] [CrossRef]
- Melichar, J.P.E.; Berry, S.; Newell, C.; MacCormack, R.; Boyd, L.A. QTL identification and micro phenotype characterization of the developmentally regulated yellow rust resistance in the UK wheat cultivar Guardian. Theor. Appl. Genet. 2008, 117, 391–399. [Google Scholar] [CrossRef]
- Bariana, H.S.; Bansal, U.K.; Schmidt, A.; Lehmensiek, A.; Kaur, J.; Miah, H.; Howes, N.; McIntyre, C.L. Molecular mapping of adult plant stripe rust resistance in wheat and identification of pyramided QTL genotypes. Euphytica 2010, 176, 251–260. [Google Scholar] [CrossRef]
- Qureshi, N.; Bariana, H.S.; Zhang, P.; McIntosh, R.; Bansal, U.K.; Wong, D.; Hayden, M.J.; Dubcovsky, J.; Shankar, M. Genetic relationship of stripe rust resistance genes Yr34 and Yr48 in wheat and identification of linked KASP markers. Plant Dis. 2018, 102, 413–420. [Google Scholar] [CrossRef]
- Rollar, S.; Serfling, A.; Geyer, M.; Hart, L.; Mohler, V.; Ordon, F. QTL mapping of adult plant and seedling resistance to leaf rust (Puccinia triticina Eriks.) in a multiparent advanced generation intercross (MAGIC) wheat population. Theor. Appl. Gen. 2021, 134, 37–51. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Buerstmayr, M.; Schweiger, W.; Steiner, B. Genomics-assisted breeding for Fusarium head blight resistance in wheat. In Translational Genomics for Crop Breeding; Varshney, R.K., Tuberosa, R., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 45–61. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- Draeger, R.; Gosman, N.; Steed, A.; Chandler, E.; Thomsett, M.; Srinivasachary; Schondelmaier, J.; Buerstmayr, H.; Lemmens, M.; Schmolke, M.; et al. Identification of QTLs for resistance to Fusarium head blight, DON accumulation and associated traits in the winter wheat variety Arina. Theor. Appl. Genet. 2005, 115, 617–625. [Google Scholar] [CrossRef]
- van Sanford, D.; Anderson, J.; Campbell, J.; Costa, K.; Cregan, J.; Griffey, P.; Hayes, C.; Ward, R. Discovery and development of molecular markers linked to Fusarium Head Blight resistance. Crop Sci. 2001, 41, 638–644. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Kászonyi, G.; Varga, M.; Tóth, B.; Varga, J. Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. Eur. J. Plant Pathol. 2005, 112, 267–281. [Google Scholar] [CrossRef]
- Mesterházy, A. What is Fusarium Head Blight (FHB) resistance and what are its food safety risks in wheat? Problems and solutions—A review. Toxins 2024, 16, 31. [Google Scholar] [CrossRef]
- Wollenweber, H.; Reinking, O. Die Fusarien: Ihre Beschreibung, Schadwirkung und Bekämpfung. In The Fusaria: Their Description, Damages and Control; Paul Parey Verlag: Singhofen, Germany, 1935; 355p. [Google Scholar]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Surrey, UK, 1971; 237p. [Google Scholar]
- Bai, G.-H.; Chen, L.-F.; Shaner, G. Breeding for resistance to Fusarium head blight of wheat in China. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 296–317. ISBN 0-89054-302-X. [Google Scholar]
- Miedaner, T.; Vasquez, A.; Castiblanco, V.; Castillo, H.E.; Foroud, N.; Würschum, T.; Leiser, W. Genome-wide association study for deoxynivalenol production and aggressiveness in wheat and rye head blight by resequencing 92 isolates of Fusarium culmorum. BMC Genom. 2021, 22, 630. [Google Scholar] [CrossRef]
- Miedaner, T.; Gang, G.; Geiger, H.H. Quantitative genetic basis of aggressiveness of 42 isolates of Fusarium culmorum for winter rye head blight. Plant Dis. 2006, 80, 500–504. [Google Scholar] [CrossRef]
- Sakr, N. Resistance to Fusarium head blight in some Syrian wheat and barley cultivars. Sarhad J. Agric. 2023, 39, 80–94. [Google Scholar] [CrossRef]
- Gale, L.R. Population biology of Fusarium species causing head blight of grain crops. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 120–143. ISBN 0-89054-302-X. [Google Scholar]
- Miedaner, T.; Lieberherr, B.; Gaikpa, D.S. Aggressiveness of Fusarium culmorum isolates for head blight symptoms is highly stable across four cereal crops. J. Phytopathol. 2021, 169, 387–392. [Google Scholar] [CrossRef]
- Miedaner, T.; Bolduan, C.; Melchinger, A.E. Aggressiveness and mycotoxin production of eight isolates each of Fusarium graminearum and Fusarium verticillioides for ear rot on susceptible and resistant early maize inbred lines. Eur. J. Plant Pathol. 2010, 127, 113–123. [Google Scholar] [CrossRef]
- Liu, S.; Hall, M.D.; Griffey, C.A.; McKendry, A.L. Meta-Analysis of QTL associated with Fusarium head blight resistance in wheat. Crop Sci. 2009, 49, 1955–1968. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.R.; Tolosa, J.; Graziani, G.; Gaspari, A. Target analysis and retrospective screening of mycotoxins and pharmacologically active substances in milk using an ultrahigh-performance liquid chromatography/high-resolution mass spectrometry approach. J. Dairy Sci. 2020, 103, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Berényi, A.; Szabó, B.; Meszlényi, T. Preharvest natural multitoxin contamination of winter wheat genotypes in Hungary with special attention to aflatoxins and HT-2 Toxin. Agriculture 2024, 14, 2024. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Wagner, C.; Nosenko, T.; Omony, J.; Steiner, B.; Nussbaumer, T.; Klaus, F.X.; Mayer, K.F.X.; Buerstmayr, H. Fusarium head blight resistance in European winter wheat: Insights from genome-wide transcriptome analysis. BMC Genom. 2021, 22, 470. [Google Scholar] [CrossRef]
- Bönnighausen, J.; Schauer, N.; Schäfer, W.; Bormann, J. Metabolic profiling of wheat rachis node infection by Fusarium graminearum—Decoding deoxynivalenol-dependent susceptibility. New Phytol. 2019, 21, 459–469. [Google Scholar] [CrossRef]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defense responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Gardiner, D.M. Transcriptomics of cereal—Fusarium graminearum interactions: What we have learned so far. Mol. Plant Pathol. 2018, 19, 764–778. [Google Scholar] [CrossRef]
- Ali, N.; Degen, G.H. Urinary biomarkers of exposure to the mycoestrogen zearalenone and its modified forms in German adults. Arch. Toxicol. 2018, 92, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- De Santis, B.; Debegnach, F.; Toscano, P.; Crisci, A.; Battilani, P.; Brera, C. Overall exposure of European adult population to mycotoxins by statistically modelled biomonitoring data. Toxins 2021, 13, 695. [Google Scholar] [CrossRef]
- Schmidt, J.; Cramer, B.; Turner, P.C.; Stoltzfus, R.J.; Humphrey, J.H.; Smith, L.E.; Humpf, H.-U. Determination of urinary mycotoxin biomarkers using a sensitive online solid phase extraction-UHPLC-MS/MS method. Toxins 2021, 13, 418. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human biomonitoring of mycotoxins in blood, plasma and serum in recent years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesterházy, Á.; Bartók, T.; Lamper, C. Influence of cultivar resistance, epidemic severity, and Fusarium species on the efficacy of fungicide control of Fusarium head blight in wheat and deoxynivalenol (DON) contamination of grain. Plant Dis. 2003, 87, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.-H.; Shaner, G. Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 1996, 80, 975–979. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. Fusarium Laboratory Manual; Blackwell Publishing Ltd.: Oxford, UK, 2006; 388p, ISBN 10: 0-8138-1919-9. [Google Scholar]
- Tóth, B.; Mesterházy, Á.; Nicholson, P.; Téren, J.; Varga, J. Mycotoxin production and molecular variability of European and American Fusarium culmorum isolates. Eur. J. Plant Pathol. 2004, 110, 587–599. [Google Scholar] [CrossRef]
- Tóth, B.; Mesterházy, Á.; Horváth, Z.; Bartók, T.; Varga, M.; Varga, J. Genetic variability of Central European Fusarium graminearum clade isolates. Eur. J. Plant Pathol. 2005, 113, 35–45. [Google Scholar] [CrossRef]
- György Andrea, T.B.; Varga, M.; Mesterhazy, A. Methodical considerations and resistance evaluation against Fusarium graminearum and F. culmorum head blight in wheat. Part 3. Susceptibility window and resistance expression. Microorganisms 2020, 8, 627. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Toldine Toth, E.; Szel, S.; Varga, M.; Toth, B. Resistance of maize hybrids to Fusarium graminearum, F. culmorum, and F. verticillioides ear rots with toothpick and silk channel inoculation, as well as their toxin production. Agronomy 2020, 10, 1283. [Google Scholar] [CrossRef]
- Sváb, J. Biometriai Módszerek a Kutatásban (Methods for Biometrics in Research), 3rd ed.; Mezogazdasagi Kiado (Agr. Publ. House): Budapest, Hungary, 1981; p. 557. [Google Scholar]
- Weber, E. Grundriss der Biologischen Statistik. In Fundaments of the Biological Statistics; VEB Fisher Verlag: Jena, Germany, 1967; p. 706. [Google Scholar]
- Anonymous. Statistica 13.0 Program was Used; Informer Technologies Inc.: Santa Clara, CA, USA; Tibco: Santa Clara, CA, USA, 2022; Available online: www.informer.com (accessed on 12 February 2022).
- Mesterhazy, Á.; Varga, M.; Tóth, B.; Kótai, C.; Bartók, T.; Véha, A.; Ács, K.; Vágvölgyi, C.; Lehoczki-Krsjak, S. Reduction of deoxynivalenol (DON) contamination by improved fungicide use in wheat. Part 2. Farm scale tests with different nozzle types and updating the integrated approach. Eur. J. Plant Pathol. 2018, 151, 1–20. [Google Scholar] [CrossRef]
- Willyerd, K.T.; Li, C.; Madden, L.V.; Bradley, C.A.; Bergstrom, G.C.; Sweets, L.E.; McMullen, M.; Ransom, J.K.; Grybauskas, A.; Osborne, L.; et al. Efficacy and stability of integrating fungicide and cultivar resistance to manage Fusarium head blight and deoxynivalenol in wheat. Plant Dis. 2012, 96, 957–967. [Google Scholar] [CrossRef]







| Line No. | Isolate/Genotype | Sgv/GT… | 85.92 | Bence | Zombor | Szõke | Ok-Sp | Bty-Mo*Kr | Mon-Ar | Ni-Kr*Dol | Kri-Mon | Arina | SVP 75059-28 | SVP72059-32 | SVP 72017 | SVP 72005 | Copain | Rechsler | RC103 | 82F328 | 81F349 | Mean | Variance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12216 | 1 | 3 | 2 | 6 | 6 | 5 | 4 | 1 | 1 | 1 | 1 | 3 | 1 | 6 | 2 | 1 | 4 | 1 | 2 | 4 | 2.8 | 3.5 |
| 2 | 39.01 | 6 | 1 | 1 | 5 | 3 | 7 | 1 | 13 | 3 | 2 | 6 | 7 | 4 | 8 | 10 | 3 | 7 | 2 | 3 | 3 | 4.8 | 10.2 |
| 3 | 223 | 12 | 2 | 8 | 8 | 4 | 2 | 3 | 15 | 4 | 4 | 4 | 1 | 6 | 1 | 3 | 13 | 12 | 4 | 7 | 1 | 5.7 | 18.1 |
| 4 | 89.4 | 16 | 5 | 7 | 9 | 5 | 3 | 2 | 11 | 5 | 6 | 5 | 8 | 8 | 5 | 7 | 8 | 3 | 3 | 5 | 2 | 6.2 | 10.8 |
| 5 | 207 | 15 | 4 | 3 | 2 | 2 | 1 | 5 | 18 | 2 | 3 | 3 | 2 | 2 | 4 | 6 | 7 | 11 | 5 | 1 | 11 | 5.4 | 21.7 |
| 6 | 39.01 | 9 | 7 | 4 | 7 | 12 | 6 | 7 | 5 | 6 | 10 | 7 | 5 | 3 | 7 | 1 | 4 | 1 | 8 | 12 | 14 | 6.8 | 11.4 |
| 7 | 12377 | 2 | 6 | 6 | 4 | 1 | 8 | 10 | 3 | 14 | 8 | 14 | 6 | 18 | 9 | 4 | 11 | 9 | 6 | 9 | 5 | 7.7 | 17.6 |
| 8 | 12216 | 5 | 12 | 17 | 12 | 9 | 4 | 14 | 9 | 11 | 9 | 9 | 15 | 7 | 3 | 18 | 5 | 13 | 11 | 11 | 7 | 10.1 | 16.7 |
| 9 | 207 | 14 | 8 | 5 | 3 | 15 | 11 | 9 | 8 | 10 | 12 | 15 | 10 | 9 | 11 | 5 | 6 | 5 | 7 | 10 | 6 | 9.0 | 11.2 |
| 10 | 12551 | 3 | 13 | 21 | 17 | 16 | 13 | 6 | 6 | 13 | 5 | 2 | 9 | 5 | 2 | 12 | 18 | 14 | 10 | 4 | 8 | 9.9 | 30.8 |
| 11 | 12551 | 7 | 16 | 12 | 11 | 21 | 9 | 13 | 4 | 8 | 11 | 22 | 14 | 10 | 10 | 8 | 14 | 2 | 9 | 18 | 12 | 11.6 | 24.4 |
| 12 | 12375 | 13 | 19 | 13 | 1 | 11 | 14 | 17 | 12 | 9 | 13 | 13 | 4 | 15 | 12 | 11 | 2 | 16 | 14 | 16 | 13 | 11.9 | 21.2 |
| 13 | 12216 | 11 | 9 | 10 | 16 | 8 | 10 | 8 | 16 | 15 | 22 | 10 | 16 | 11 | 16 | 16 | 12 | 23 | 15 | 20 | 15 | 14.0 | 18.6 |
| 14 | 12377 | 4 | 11 | 9 | 15 | 10 | 15 | 15 | 14 | 16 | 16 | 12 | 17 | 17 | 13 | 14 | 9 | 20 | 17 | 19 | 16 | 14.0 | 14.1 |
| 15 | 89.4 | 17 | 20 | 11 | 18 | 18 | 19 | 12 | 7 | 7 | 17 | 20 | 11 | 16 | 15 | 9 | 10 | 6 | 12 | 14 | 9 | 13.4 | 20.1 |
| 16 | 223 | 19 | 14 | 15 | 13 | 13 | 12 | 11 | 19 | 21 | 18 | 17 | 13 | 13 | 14 | 13 | 15 | 17 | 20 | 17 | 10 | 15.2 | 9.3 |
| 17 | 12216 | 8 | 18 | 19 | 10 | 19 | 24 | 22 | 2 | 17 | 15 | 29 | 21 | 12 | 18 | 17 | 20 | 8 | 13 | 13 | 19 | 16.2 | 37.1 |
| 18 | 12377 | 10 | 21 | 24 | 24 | 22 | 18 | 23 | 10 | 20 | 7 | 8 | 24 | 19 | 22 | 15 | 23 | 10 | 16 | 6 | 26 | 17.4 | 41.6 |
| 19 | 12551 | 28 | 15 | 14 | 14 | 7 | 17 | 16 | 26 | 18 | 27 | 16 | 18 | 14 | 17 | 20 | 16 | 28 | 21 | 21 | 18 | 18.6 | 27.7 |
| 20 | 89.4 | 20 | 17 | 16 | 19 | 17 | 16 | 18 | 23 | 22 | 28 | 19 | 12 | 21 | 21 | 24 | 17 | 27 | 22 | 22 | 25 | 20.3 | 15.2 |
| 21 | 223 | 21 | 24 | 20 | 22 | 24 | 31 | 25 | 17 | 12 | 21 | 31 | 22 | 23 | 29 | 26 | 24 | 15 | 29 | 15 | 22 | 22.7 | 26.1 |
| 22 | 12375 | 18 | 31 | 25 | 25 | 26 | 30 | 24 | 20 | 19 | 14 | 11 | 26 | 31 | 23 | 27 | 31 | 22 | 26 | 8 | 31 | 23.4 | 42.8 |
| 23 | 207 | 30 | 22 | 18 | 21 | 20 | 22 | 21 | 29 | 23 | 29 | 23 | 19 | 25 | 19 | 29 | 19 | 29 | 28 | 25 | 27 | 23.9 | 15.9 |
| 24 | 12375 | 22 | 26 | 27 | 28 | 28 | 21 | 27 | 21 | 25 | 20 | 18 | 28 | 20 | 20 | 19 | 29 | 18 | 19 | 28 | 20 | 23.2 | 15.4 |
| 25 | 207 | 23 | 10 | 28 | 27 | 27 | 20 | 31 | 27 | 29 | 26 | 25 | 27 | 28 | 25 | 21 | 25 | 19 | 18 | 24 | 17 | 23.9 | 24.1 |
| 26 | 12375 | 31 | 23 | 22 | 20 | 14 | 27 | 19 | 31 | 24 | 31 | 24 | 23 | 22 | 26 | 30 | 21 | 31 | 31 | 23 | 24 | 24.9 | 22.1 |
| 27 | 12551 | 24 | 29 | 26 | 26 | 25 | 26 | 28 | 24 | 27 | 19 | 26 | 25 | 24 | 31 | 25 | 26 | 25 | 27 | 27 | 21 | 25.6 | 6.4 |
| 28 | 39.01 | 27 | 25 | 23 | 23 | 23 | 25 | 20 | 30 | 26 | 30 | 21 | 20 | 27 | 27 | 31 | 22 | 30 | 30 | 26 | 30 | 25.8 | 12.7 |
| 29 | 12377 | 29 | 27 | 30 | 31 | 29 | 28 | 26 | 22 | 28 | 25 | 30 | 30 | 26 | 30 | 28 | 30 | 26 | 24 | 31 | 23 | 27.7 | 6.9 |
| 30 | 223 | 26 | 30 | 29 | 29 | 30 | 29 | 30 | 25 | 30 | 23 | 28 | 29 | 30 | 28 | 23 | 28 | 21 | 25 | 30 | 28 | 27.6 | 7.5 |
| 31 | 89.4 | 25 | 28 | 31 | 30 | 31 | 23 | 29 | 28 | 31 | 24 | 27 | 31 | 29 | 24 | 22 | 27 | 24 | 23 | 29 | 29 | 27.3 | 9.6 |
| Mean | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 18.4 | |
| DI Mean | 28.1 | 26.2 | 23.4 | 23.0 | 23.0 | 20.5 | 20.1 | 18.8 | 18.0 | 17.9 | 16.6 | 16.0 | 14.8 | 12.8 | 12.8 | 12.0 | 11.5 | 10.7 | 8.5 | 7.3 | 16.0 | 17.1 | |
| Genotypes | Disease Index | FDK | DON | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | Stability | ||
| Arina | 8.51 | 77.35 | 0.612 | 5.9 | 66.2 | 0.320 | 0.33 | 0.497 | 0.200 | 17.77 |
| SVP 75059-28 | 10.67 | 97.91 | 0.625 | 10.9 | 224.8 | 0.660 | 1.31 | 2.509 | 0.380 | 38.87 |
| SVP 72017 | 7.26 | 42.21 | 0.395 | 13.0 | 359.4 | 0.810 | 1.19 | 4.673 | 0.820 | 47.75 |
| 81F349 | 11.46 | 219.82 | 0.679 | 10.9 | 252.9 | 0.660 | 0.41 | 0.382 | 0.120 | 55.27 |
| 82F328 | 11.97 | 97.56 | 0.664 | 16.0 | 409.6 | 0.910 | 1.43 | 23.152 | 1.260 | 62.50 |
| Kri-Mon | 16.03 | 208.90 | 1.016 | 12.8 | 340.5 | 0.730 | 0.68 | 1.257 | 0.560 | 64.72 |
| Szõke | 16.65 | 192.54 | 0.834 | 14.8 | 379.6 | 0.710 | 2.27 | 21.061 | 1.130 | 69.96 |
| Copain | 14.75 | 104.22 | 0.728 | 18.7 | 595.1 | 0.820 | 1.45 | 6.564 | 0.860 | 82.57 |
| RC103 | 12.83 | 111.26 | 0.722 | 18.3 | 628.7 | 1.050 | 1.84 | 10.107 | 1.070 | 87.32 |
| 85.92 | 12.78 | 131.34 | 0.733 | 19.0 | 612.7 | 1.170 | 2.79 | 17.842 | 1.780 | 88.91 |
| Bty-Mo*Kr | 22.99 | 429.32 | 1.380 | 17.1 | 476.8 | 0.990 | 3.73 | 40.113 | 2.120 | 110.51 |
| Sgv/GT... | 17.91 | 336.44 | 0.932 | 21.4 | 613.3 | 0.590 | 2.34 | 8.143 | 0.190 | 111.25 |
| SVP72059-32 | 20.46 | 238.02 | 1.016 | 25.9 | 735.7 | 1.330 | 2.47 | 11.604 | 0.950 | 115.28 |
| Bence | 18.82 | 329.63 | 0.947 | 24.3 | 701.0 | 1.170 | 1.80 | 8.605 | 1.050 | 120.81 |
| SVP 72005 | 26.20 | 422.11 | 1.388 | 34.8 | 611.4 | 0.928 | 2.28 | 22.611 | 1.670 | 124.81 |
| Ni-Kr*Dol | 23.03 | 614.99 | 1.750 | 17.2 | 478.4 | 0.970 | 1.16 | 4.271 | 0.510 | 126.93 |
| Mon-Ar | 23.37 | 629.52 | 1.715 | 17.6 | 478.6 | 1.010 | 1.21 | 4.110 | 0.300 | 128.60 |
| Rechsler | 18.00 | 337.01 | 1.329 | 28.7 | 1048.5 | 1.600 | 2.56 | 17.980 | 1.160 | 161.88 |
| Ok-Sp | 20.07 | 440.55 | 1.425 | 21.9 | 1022.4 | 1.390 | 1.82 | 13.268 | 1.190 | 169.34 |
| Zombor | 28.14 | 325.17 | 1.126 | 39.4 | 1192.1 | 1.490 | 7.77 | 58.999 | 2.570 | 184.08 |
| Mean | 17.09 | 269.29 | 1.001 | 19.4 | 561.4 | 0.965 | 2.04 | 13.887 | 0.994 | 98.45 |
| LSD 5% | 3.09 | 2.38 | 0.54 | |||||||
| FHB Trait | Trait | Disease Index | FDK | DON | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | ||
| DI | Variance | 0.815 *** | |||||||
| Stability | 0.837 *** | 0.959 *** | |||||||
| FDK | Mean | 0.757 *** | 0.393 | 0.436 | |||||
| Variance | 0.608 ** | 0.355 | 0.420 | 0.858 *** | |||||
| Stability | 0.540 | 0.363 | 0.456 * | 0.740 *** | 0.895 *** | ||||
| DON | Mean | 0.602 ** | 0.178 | 0.209 | 0.746 *** | 0.695 *** | 0.582 ** | ||
| Variance | 0.572 ** | 0.164 | 0.220 | 0.655 ** | 0.570 ** | 0.512 * | 0.920 *** | ||
| Stability | 0.487 * | 0.074 | 0.171 | 0.658 ** | 0.596 ** | 0.615 ** | 0.817 *** | 0.908 *** | |
| Disease Index | FDK | DON | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotypes | Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | Stability |
| Sgv-NB/MM-Sum3 | 2.61 | 4.3 | 0.10 | 0.92 | 2.33 | 0.04 | 0.32 | 0.4 | 0.04 |
| Arina | 5.28 | 46.6 | 0.36 | 3.88 | 24.02 | 0.17 | 1.28 | 7.8 | 0.16 |
| Sgv-NB/MM-Sum3 .252 | 3.45 | 10.1 | 0.13 | 2.03 | 25.75 | 0.07 | 1.70 | 9.2 | 0.15 |
| Ringo Sztár | 10.61 | 132.6 | 0.68 | 7.08 | 112.48 | 0.46 | 2.06 | 6.0 | 0.14 |
| RSt-MM/NB | 7.95 | 37.8 | 0.23 | 5.02 | 110.42 | 0.12 | 2.26 | 21.2 | 0.27 |
| DH 84.42 | 20.58 | 501.0 | 1.39 | 17.26 | 424.72 | 0.99 | 5.59 | 89.1 | 0.62 |
| 81.60-NB/Kő | 20.83 | 234.2 | 0.76 | 11.97 | 201.99 | 0.77 | 6.07 | 45.1 | 0.31 |
| P4371.88 | 19.09 | 241.0 | 0.92 | 17.19 | 425.69 | 1.00 | 6.15 | 86.3 | 0.63 |
| SK8090 | 14.82 | 101.0 | 0.57 | 15.54 | 434.45 | 1.21 | 6.55 | 141.7 | 0.70 |
| Siouxland | 13.10 | 119.3 | 0.48 | 25.00 | 714.71 | 1.58 | 7.28 | 65.7 | 0.55 |
| P2118.89 | 16.92 | 95.7 | 0.61 | 30.42 | 335.40 | 0.77 | 9.54 | 116.9 | 0.62 |
| Sum3^2-81.60 | 15.07 | 238.3 | 0.93 | 11.59 | 310.00 | 0.74 | 9.72 | 262.0 | 1.15 |
| Rechsler | 23.33 | 343.0 | 1.18 | 28.09 | 527.06 | 1.48 | 11.90 | 239.0 | 1.01 |
| Sgv-GT... | 28.31 | 402.0 | 1.31 | 15.73 | 393.76 | 1.22 | 12.71 | 430.3 | 1.37 |
| Kincső | 24.10 | 314.9 | 1.10 | 18.43 | 536.37 | 1.29 | 13.67 | 155.9 | 0.52 |
| Kende | 27.90 | 585.1 | 1.63 | 22.32 | 259.66 | 0.90 | 14.41 | 230.4 | 1.17 |
| Bence | 20.91 | 364.9 | 1.25 | 16.06 | 444.99 | 1.30 | 18.34 | 486.7 | 1.44 |
| Szőke | 26.15 | 535.8 | 1.55 | 18.49 | 355.44 | 0.80 | 18.99 | 585.2 | 1.81 |
| 78.1.04 | 31.72 | 479.3 | 0.70 | 40.10 | 877.13 | 1.16 | 19.03 | 444.4 | 0.78 |
| Góbé | 31.51 | 335.3 | 0.98 | 32.09 | 801.40 | 1.47 | 19.31 | 230.9 | 0.52 |
| Jbj-5o | 30.78 | 463.6 | 1.25 | 42.34 | 487.36 | 1.28 | 23.69 | 374.3 | 1.35 |
| Csaba | 38.92 | 838.5 | 1.90 | 26.45 | 445.01 | 1.39 | 30.72 | 1001.1 | 2.25 |
| Zugoly | 38.37 | 577.2 | 1.48 | 50.43 | 774.84 | 1.37 | 32.23 | 666.9 | 1.34 |
| Őrség | 34.52 | 580.0 | 1.64 | 43.17 | 808.92 | 1.74 | 35.97 | 1288.4 | 2.61 |
| Zombor | 35.74 | 820.6 | 1.86 | 35.43 | 648.78 | 1.53 | 42.25 | 2130.2 | 3.48 |
| Mean | 21.70 | 336.1 | 1.00 | 21.48 | 419.31 | 0.99 | 14.07 | 364.60 | 1.00 |
| LSD 5% | 0.98 | 2,70 | 8.58 | ||||||
| Correlations/Traits | Triplets | Disease Index | FDK | DON | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | ||
| DI | Variance | 0.91 *** | |||||||
| Stability | 0.86 *** | 0.944 *** | |||||||
| FDK | Mean | 0.83 *** | 0.66 *** | 0.59 | |||||
| Variance | 0.75 *** | 0.569 * | 0.50 | 0.86 *** | |||||
| Stability | 0.79 *** | 0.65 *** | 0.686 *** | 0.777 *** | 0.889 *** | ||||
| DON | Mean | 0.88 *** | 0.86 *** | 0.80 *** | 0.808 *** | 0.698 *** | 0.717 *** | ||
| Variance | 0.68 *** | 0.78 *** | 0.71 *** | 0.56 ** | 0.49 * | 0.55 ** | 0.90 *** | ||
| Stability | 0.73 *** | 0.84 *** | 0.83 *** | 0.576 ** | 0.49 * | 0.61 ** | 0.89 *** | 0.95 *** | |
| Genotype | DI % | FDK % | DON mg/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | Stability | |
| Rst/NBb | 8.71 * | 76.9 | 0.75 | 0.91 | 4.77 | 0.09 | 1.87 | 13 | 0.23 |
| Zu//Ré/NB/5/DH … | 5.30 | 51.4 | 0.85 | 7.87 | 312.54 | 0.91 | 2.44 | 24 | 0.39 |
| Zu//Ré/NBb | 8.84 | 99.5 | 1.30 | 7.23 | 298.03 | 0.88 | 3.80 | 40 | 0.41 |
| Zu//Ré/NBa | 10.00 | 122.3 | 0.94 | 5.71 | 150.99 | 0.59 | 4.07 | 35 | 0.32 |
| SzD 5319 | 4.53 | 28.0 | 0.98 | 7.61 | 155.39 | 0.73 | 4.45 | 54 | 0.50 |
| MIDAS | 3.78 | 31.4 | 1.02 | 7.41 | 151.49 | 0.77 | 5.26 | 112 | 0.70 |
| GALLUS (SzD 8583) | 5.31 | 58.3 | 0.72 | 5.30 | 131.51 | 0.64 | 5.58 | 145 | 0.84 |
| SzD 4511 | 4.77 | 33.0 | 0.86 | 11.82 | 308.51 | 1.04 | 5.74 | 64 | 0.50 |
| BALATON | 10.66 | 121.2 | 1.00 | 14.45 | 428.05 | 1.32 | 6.00 | 175 | 0.89 |
| SzD 5066 | 7.19 | 68.9 | 1.40 | 9.90 | 269.69 | 1.06 | 6.02 | 207 | 0.97 |
| SzD 0016 S | 3.72 | 37.2 | 0.91 | 8.25 | 175.82 | 0.70 | 6.10 | 95 | 0.61 |
| Ttj/RC103/3/Sgv/NB//MM/Sum3 | 8.78 | 91.9 | 0.45 | 9.11 | 262.45 | 1.00 | 6.26 | 126 | 0.76 |
| SzD 0016 F | 6.80 | 59.0 | 0.54 | 7.77 | 192.21 | 0.89 | 6.45 | 141 | 0.83 |
| SzD 5501 | 6.38 | 74.8 | 0.71 | 9.70 | 204.00 | 0.83 | 6.51 | 79 | 0.55 |
| GK HOLLÓ | 9.31 | 99.9 | 0.93 | 9.44 | 184.08 | 0.80 | 6.81 | 76 | 0.57 |
| SzD 6401 | 4.75 | 58.2 | 1.11 | 10.79 | 376.29 | 1.19 | 7.33 | 201 | 1.01 |
| GK FÉNY | 15.10 | 235.7 | 1.45 | 9.66 | 366.43 | 1.16 | 7.35 | 194 | 0.96 |
| GK CSILLAG | 15.64 | 170.7 | 1.09 | 11.71 | 402.86 | 1.06 | 7.42 | 213 | 0.96 |
| Attila/3/Sgv/NB//MM/Sum2 | 12.22 | 165.9 | 1.21 | 10.65 | 317.77 | 1.04 | 7.69 | 178 | 0.92 |
| GK BÉKÉS | 13.80 | 187.0 | 1.35 | 9.90 | 127.36 | 0.67 | 7.97 | 99 | 0.67 |
| GK PETUR | 8.25 | 96.7 | 1.27 | 11.00 | 286.36 | 0.82 | 8.03 | 185 | 0.91 |
| GK HATTYU | 8.79 | 99.4 | 0.90 | 14.73 | 467.86 | 1.35 | 8.03 | 273 | 1.11 |
| RAINER | 8.94 | 92.4 | 1.12 | 13.78 | 444.51 | 1.26 | 8.32 | 282 | 1.14 |
| Be/SK 48.21// FHB 142 | 10.17 | 113.5 | 1.06 | 12.45 | 427.35 | 1.21 | 9.04 | 216 | 0.98 |
| SzD 6626 | 11.06 | 182.7 | 1.32 | 11.61 | 254.52 | 1.01 | 9.37 | 307 | 1.19 |
| Rst/Nba | 7.16 | 150.6 | 0.50 | 8.51 | 178.35 | 0.58 | 9.48 | 254 | 1.08 |
| SzD 5377 | 7.93 | 96.8 | 1.64 | 14.92 | 460.58 | 1.32 | 10.05 | 250 | 1.08 |
| GK HUNYAD | 8.09 | 120.5 | 1.07 | 13.48 | 438.10 | 1.34 | 10.87 | 461 | 1.27 |
| SzD 18364 (B-18364) | 6.88 | 84.7 | 0.52 | 9.92 | 193.30 | 0.77 | 11.06 | 292 | 1.17 |
| GK ÉLET | 19.82 | 264.3 | 1.53 | 18.03 | 427.79 | 0.98 | 11.11 | 275 | 0.93 |
| SzD 5205 | 11.25 | 203.5 | 0.81 | 11.51 | 310.45 | 1.13 | 11.90 | 588 | 1.70 |
| GK SZALA | 7.64 | 108.1 | 0.53 | 14.95 | 439.84 | 1.33 | 12.17 | 379 | 1.37 |
| SzD 5417 | 15.89 | 353.3 | 0.82 | 18.29 | 519.56 | 1.37 | 12.27 | 547 | 1.63 |
| SzD 6701 | 9.50 | 175.1 | 1.22 | 11.53 | 309.41 | 1.06 | 12.31 | 447 | 1.49 |
| SzD 5275 | 8.12 | 80.9 | 0.50 | 19.45 | 557.92 | 1.01 | 12.54 | 228 | 0.88 |
| GK KALÁSZ | 16.86 | 193.0 | 1.57 | 15.37 | 426.83 | 1.13 | 12.96 | 267 | 0.99 |
| GK PIACOS | 16.39 | 196.4 | 0.99 | 16.53 | 442.22 | 0.98 | 13.19 | 405 | 1.26 |
| SzD 6515 | 10.66 | 211.1 | 1.19 | 22.04 | 621.11 | 1.47 | 13.33 | 751 | 1.87 |
| SzD 7257 | 10.10 | 154.0 | 0.98 | 16.41 | 480.71 | 1.34 | 14.86 | 937 | 2.16 |
| GK KAPOS | 12.50 | 270.5 | 0.89 | 17.64 | 434.28 | 1.17 | 17.52 | 962 | 2.18 |
| Mean | 9.54 | 127.97 | 1.00 | 11.68 | 323.53 | 1.00 | 8.59 | 264.46 | 1.00 |
| LSD 5% | 3.72 | 1.41 | 2.14 | ||||||
| Correlations | Disease Index (DI) | FDK | DON | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | Stability | |
| DI | Variance | 0.861 *** | ||||||||
| Stability | 0.432 ** | 0.297 | ||||||||
| FDK | Mean | 0.478 ** | 0.557 ** | 0.194 | ||||||
| Variance | 0.414 * | 0.458 ** | 0.226 | 0.911 *** | ||||||
| Stability | 0.264 | 0.368 * | 0.258 | 0.786 *** | 0.886 *** | |||||
| DON | Mean | 0.493 ** | 0.637 *** | 0.074 | 0.820 *** | 0.673 *** | 0.562 *** | |||
| Variance | 0.340 * | 0.609 *** | 0.028 | 0.693 *** | 0.607 *** | 0.588 *** | 0.848 *** | |||
| Stability | 0.330 * | 0.606 *** | 0.037 | 0.701 *** | 0.627 *** | 0.659 *** | 0.854 *** | 0.970 *** | ||
| Genotype | DI % | FDK % | DON mg/kg | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | Stability | |
| Genius | 16.19 | 215.97 | 0.80 | 6.53 | 72.97 | 0.53 | 6.16 | 44.71 | 0.29 |
| GK Bakony | 30.38 | 529.55 | 1.36 | 5.23 | 147.96 | 0.67 | 7.46 | 40.32 | 0.26 |
| GK Börzsöny | 32.67 | 845.62 | 1.69 | 16.63 | 407.81 | 1.04 | 12.77 | 153.60 | 0.54 |
| GK Pilis | 16.38 | 105.90 | 0.43 | 7.10 | 162.51 | 0.76 | 13.31 | 204.57 | 0.63 |
| GK Szilárd | 25.96 | 324.39 | 1.08 | 14.83 | 187.45 | 0.84 | 16.16 | 258.51 | 0.70 |
| GK Bagó | 24.79 | 220.95 | 0.89 | 7.13 | 59.85 | 0.38 | 16.55 | 300.82 | 0.61 |
| GK Megyer | 15.98 | 165.97 | 0.62 | 7.28 | 125.16 | 0.69 | 18.01 | 566.25 | 1.04 |
| GK Csillag | 21.29 | 215.95 | 0.73 | 13.27 | 198.06 | 0.81 | 20.56 | 586.32 | 1.04 |
| GK Magvető | 21.63 | 223.90 | 0.87 | 20.54 | 690.43 | 1.75 | 20.87 | 758.07 | 1.20 |
| GK Békés | 37.13 | 898.64 | 1.78 | 16.25 | 634.89 | 1.01 | 22.24 | 922.53 | 1.32 |
| GK Csanád | 17.79 | 133.39 | 0.71 | 11.43 | 205.10 | 0.89 | 24.05 | 776.31 | 1.21 |
| GK Szereda | 21.92 | 451.80 | 0.47 | 14.25 | 382.20 | 1.13 | 24.99 | 901.94 | 1.24 |
| GK Kolozs | 25.67 | 331.45 | 1.12 | 20.98 | 595.66 | 1.58 | 26.28 | 1068.50 | 1.42 |
| GK Déva | 20.46 | 285.74 | 0.99 | 14.00 | 347.65 | 1.22 | 27.49 | 1070.21 | 1.41 |
| Altigo | 32.17 | 499.97 | 1.48 | 22.75 | 672.80 | 1.72 | 38.22 | 2461.90 | 2.08 |
| Mean | 24.03 | 363.28 | 1.00 | 13.21 | 326.03 | 1.00 | 19.68 | 674.30 | 1.00 |
| LSD 5% | 7.28 | 6.69 | 7.45 | ||||||
| Trait | Sub-Trait | DI % | FDK % | DON mg/kg | |||||
|---|---|---|---|---|---|---|---|---|---|
| Correlations | Mean | Variance | Stability | Mean | Variance | Stability | Mean | Variance | |
| DI | Variance | 0.894 *** | |||||||
| Stability | 0.919 *** | 0.856 *** | |||||||
| FDK | Mean | 0.461 | 0.360 | 0.430 | |||||
| Variance | 0.539 * | 0.489 | 0.503 | 0.906 *** | |||||
| Stability | 0.278 | 0.196 | 0.266 | 0.91 *** | 0.912 *** | ||||
| DON | Mean | 0.191 | 0.052 | 0.106 | 0.726 ** | 0.652 ** | 0.719 ** | ||
| Variance | 0.277 | 0.134 | 0.229 | 0.707 *** | 0.690 ** | 0.736 ** | 0.945 *** | ||
| Stability | 0.181 | 0.075 | 0.128 | 0.744 *** | 0.719 ** | 0.763 *** | 0.979 *** | 0.951 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesterhazy, Á.; Tóth, B.; Berényi, A.; Ács, K.; Meszlényi, T. Significance of the Stability of Fusarium Head Blight Resistance in the Variety Registration, Breeding, and Genetic Research of Winter Wheat Using Disease Index, Fusarium-Damaged Kernels, and Deoxynivalenol Contamination. Toxins 2025, 17, 288. https://doi.org/10.3390/toxins17060288
Mesterhazy Á, Tóth B, Berényi A, Ács K, Meszlényi T. Significance of the Stability of Fusarium Head Blight Resistance in the Variety Registration, Breeding, and Genetic Research of Winter Wheat Using Disease Index, Fusarium-Damaged Kernels, and Deoxynivalenol Contamination. Toxins. 2025; 17(6):288. https://doi.org/10.3390/toxins17060288
Chicago/Turabian StyleMesterhazy, Ákos, Beata Tóth, Attila Berényi, Katalin Ács, and Tamas Meszlényi. 2025. "Significance of the Stability of Fusarium Head Blight Resistance in the Variety Registration, Breeding, and Genetic Research of Winter Wheat Using Disease Index, Fusarium-Damaged Kernels, and Deoxynivalenol Contamination" Toxins 17, no. 6: 288. https://doi.org/10.3390/toxins17060288
APA StyleMesterhazy, Á., Tóth, B., Berényi, A., Ács, K., & Meszlényi, T. (2025). Significance of the Stability of Fusarium Head Blight Resistance in the Variety Registration, Breeding, and Genetic Research of Winter Wheat Using Disease Index, Fusarium-Damaged Kernels, and Deoxynivalenol Contamination. Toxins, 17(6), 288. https://doi.org/10.3390/toxins17060288






