Isolation and Mechanistic Investigation of the Efficient Zearalenone-Removing Strain Bacillus licheniformis YJ25
Abstract
1. Introduction
2. Results and Discussion
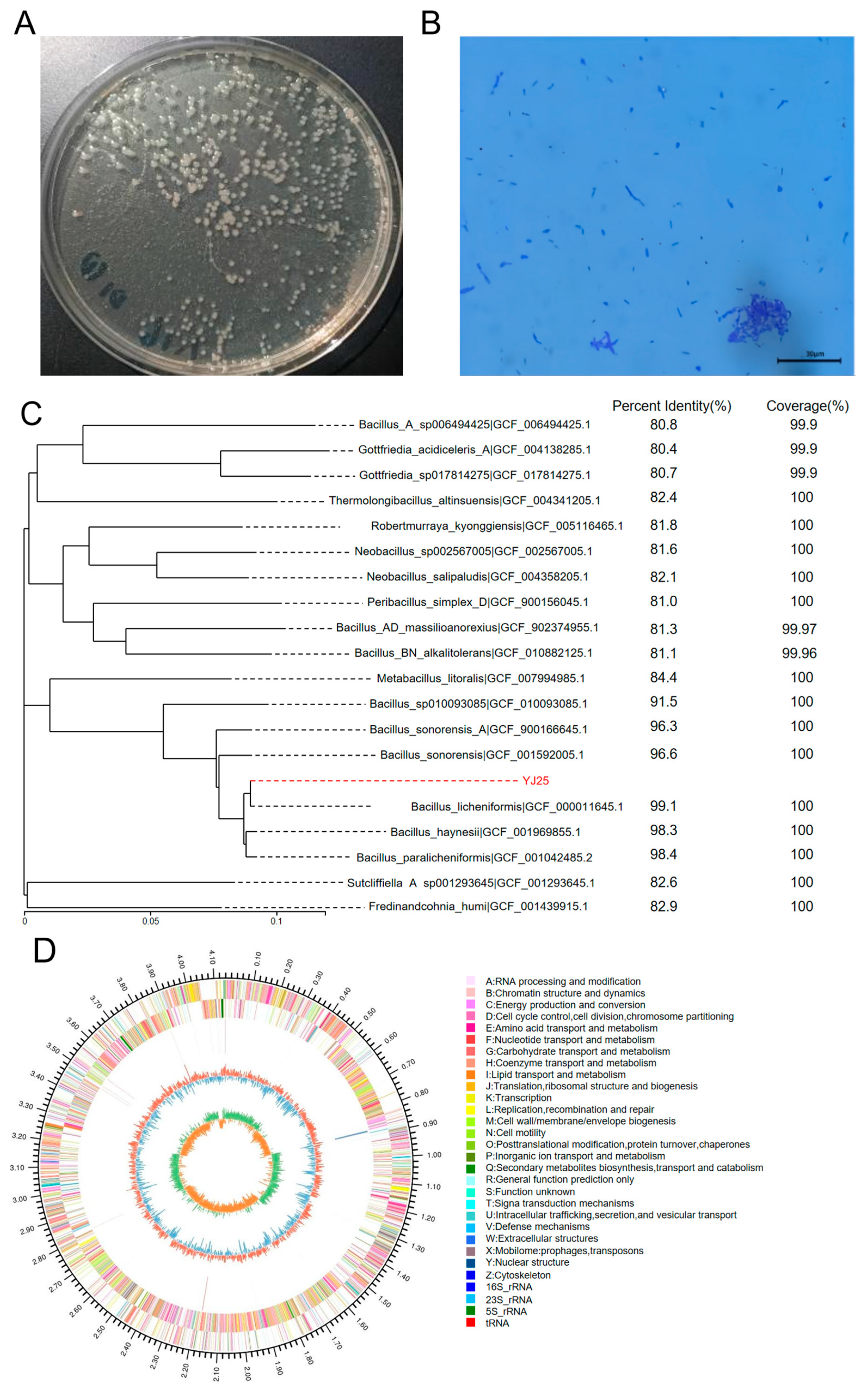
2.1. Isolation and Characterization of ZEN-Removing Bacteria
2.1.1. Isolation of ZEN-Removal Strains
2.1.2. Identification of YJ25
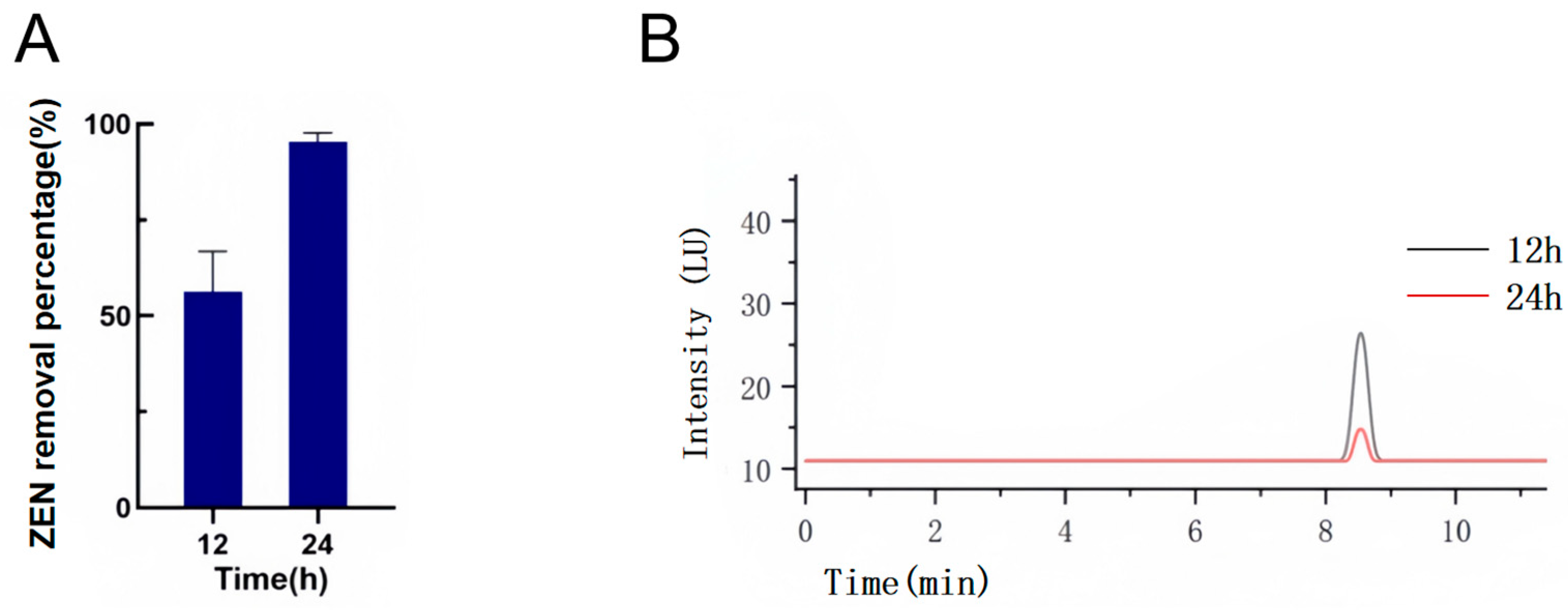
2.2. Effect of the Time Gradient on the Removal of ZEN by YJ25
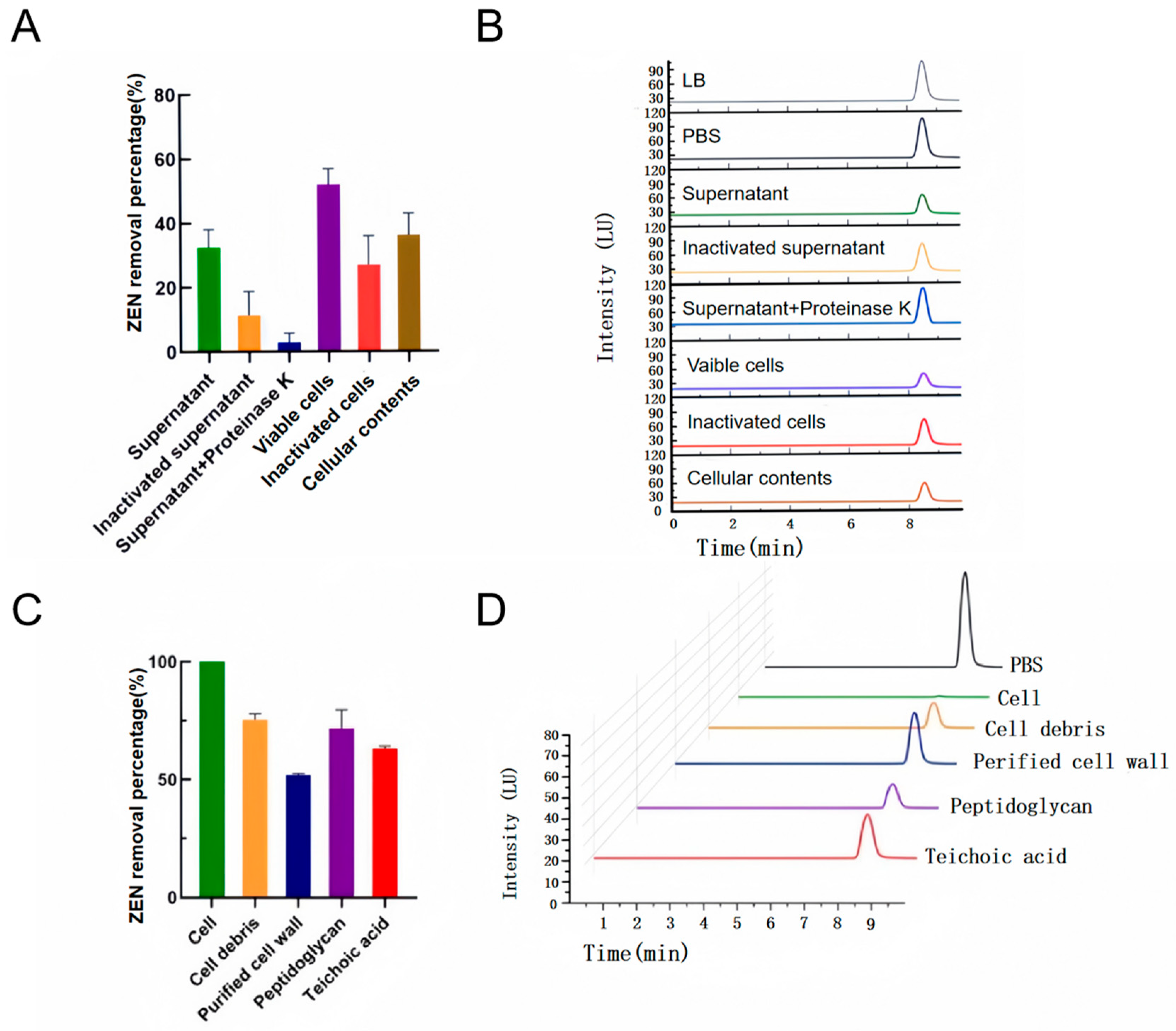
2.3. Localization and Analysis of the Removal Active Site of YJ25
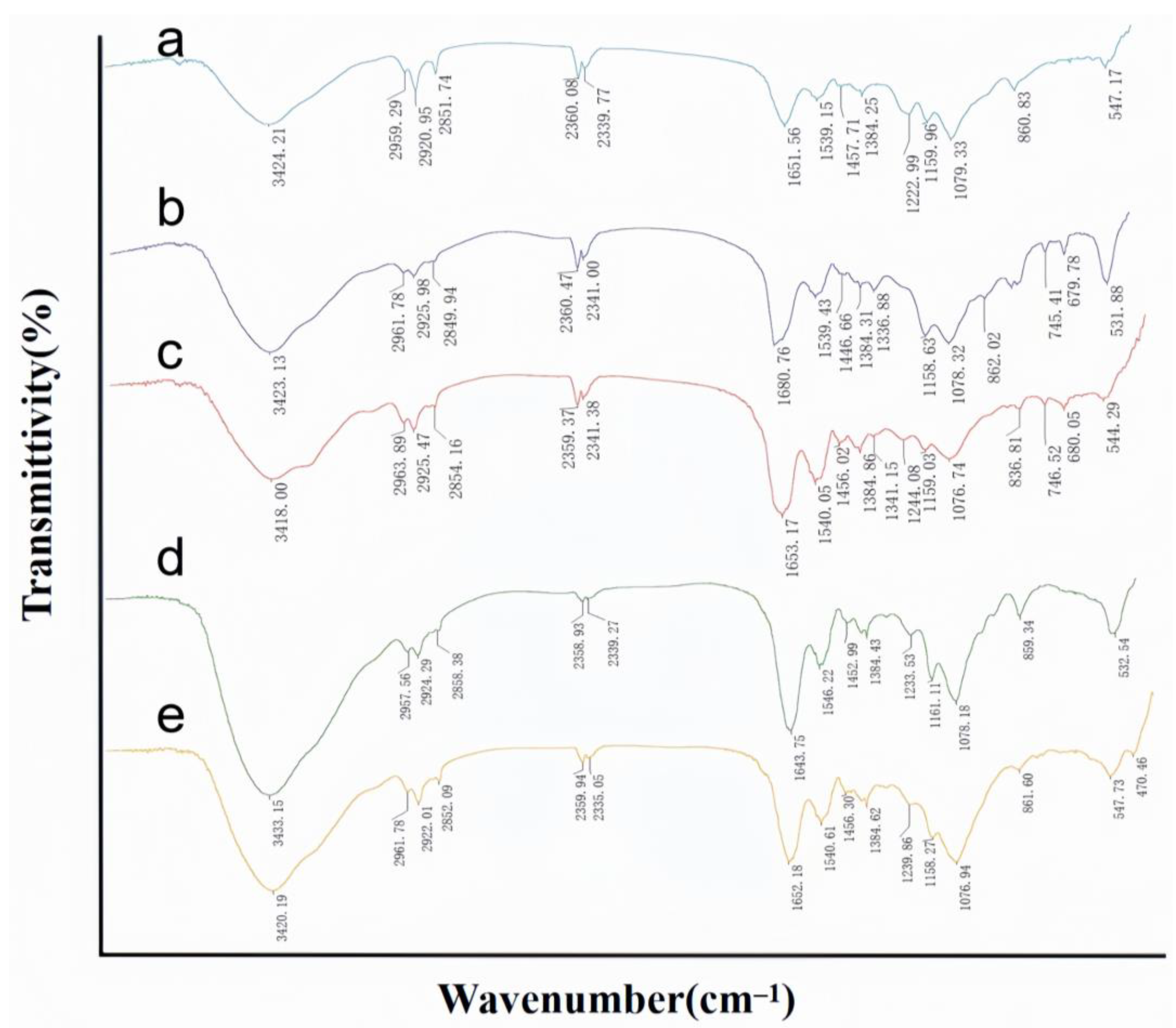
2.4. Investigation of the Cell Wall Adsorption Mechanism
FTIR Analysis Results
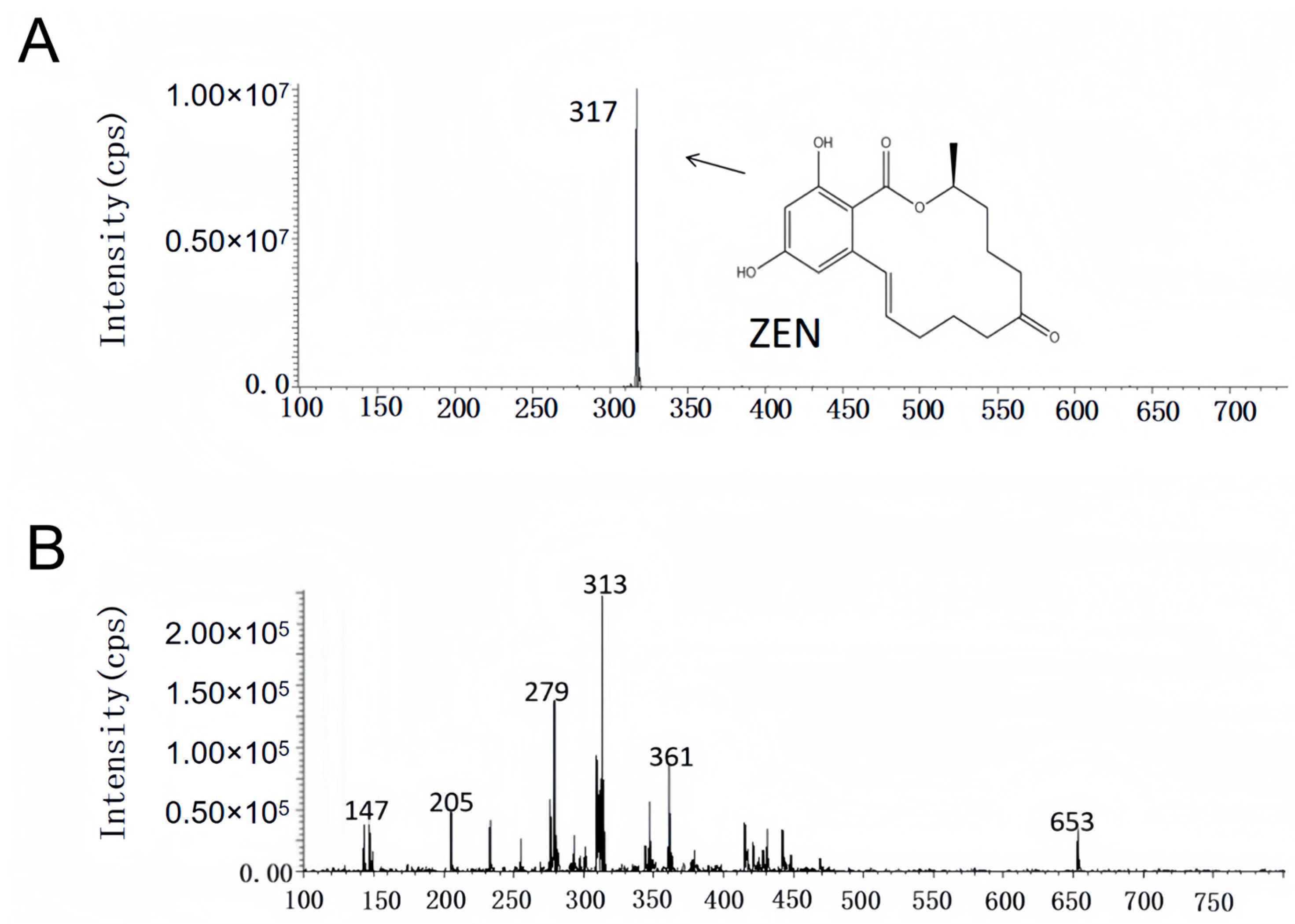
2.5. Results of Degradation Product Analysis
3. Conclusions
4. Materials and Methods
4.1. Sample and Primary Reagents
4.1.1. Moldy Corn Sample
4.1.2. Primary Reagents and Media
4.2. Main Instruments
4.3. Methods for the Determination of ZEN
4.4. Isolation and Characterization of Efficient Removal Strains of ZEN
4.4.1. Isolation of ZEN-Removal Strains
4.4.2. Identification of YJ25
- (1)
- Morphological identification
- (2)
- 16S rDNA identification
- (3)
- Whole genome sequencing analysis
4.5. Effect of Time Gradient on ZEN Removal by YJ25
4.6. Localization and Analysis of the Removal Active Site of YJ25
4.7. Investigation of the Cell Wall Adsorption Mechanism
- (1)
- H2O (100 °C,15 min), cell debris;
- (2)
- 2% (w/V) SDS (100 °C, 15 min), purified cell wall fraction;
- (3)
- 0.1 M HCl (100 °C, 15 min) to expose the teichoic acid portion;
- (4)
- 10% (w/V) TCA (100 °C, 15 min) to expose the peptidoglycan fraction.
4.8. Analysis of the Degradation Products of YJ25
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZEN | Zearalenone |
| LC–MS | Liquid Chromatography–Mass Spectrometry |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| AF | Aflatoxins |
| FUM | Fumonisin |
| OTA | Ochratoxin A |
| DON | Deoxynivalenol |
| EU | European Union |
| Hbl | Hemolysin BL |
| Nhe | Non-hemolytic enterotoxin |
| HPLC | High Performance Liquid Chromatography |
| TCA | Trichloroacetic Acid |
| SDS | Sodium Dodecyl Sulfate |
| PBS | Phosphate-Buffered Saline |
References
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.K.K.; Lee, J.C.-Y.; Turner, P.C.; El-Nezami, H. Low dose of zearalenone elevated colon cancer cell growth through G protein-coupled estrogenic receptor. Sci. Rep. 2021, 11, 7403. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twaruzek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-T.; Gao, Y.-J.; Zhang, W.-J.; Bi, T.-C.; Wang, X.; Ma, C.-X.; Rong, R. Development a multi-immunoaffinity column LC-MS-MS method for comprehensive investigation of mycotoxins contamination and co-occurrence in traditional Chinese medicinal materials. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2021, 1178, 122730. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Zhu, Z.; Ji, F.; Yin, X.; Hong, Q.; Shi, J. Isolation and characterization of Bacillus amyloliquefaciens ZDS-1: Exploring the degradation of Zearalenone by Bacillus spp. Food Control 2016, 68, 244–250. [Google Scholar] [CrossRef]
- Borzekowski, A.; Drewitz, T.; Keller, J.; Pfeifer, D.; Kunte, H.-J.; Koch, M.; Rohn, S.; Maul, R. Biosynthesis and Characterization of Zearalenone-14-Sulfate, Zearalenone-14-Glucoside and Zearalenone-16-Glucoside Using Common Fungal Strains. Toxins 2018, 10, 104. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- GB 13078-2017; Hygienical standard for feeds. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of China: Beijing, China, 2017.
- Ning, C.-m.; An, J.-x.; Zhao, Y.; Yang, Y. Toxic effects of zearalenone on animal reproductive performance and its mechanism. Chin. J. Anim. Nutr. 2023, 35, 2166–2174. [Google Scholar]
- Hojnik, N.; Modic, M.; Tavcar-Kalcher, G.; Babic, J.; Walsh, J.L.; Cvelbar, U. Mycotoxin Decontamination Efficacy of Atmospheric Pressure Air Plasma. Toxins 2019, 11, 219. [Google Scholar] [CrossRef]
- Yang, K.; Li, K.; Pan, L.; Luo, X.; Xing, J.; Wang, J.; Wang, L.; Wang, R.; Zhai, Y.; Chen, Z. Effect of Ozone and Electron Beam Irradiation on Degradation of Zearalenone and Ochratoxin A. Toxins 2020, 12, 138. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Ji, J.; Wu, H.; Pi, F.; Zhang, Y.; Sun, X. Chemical and toxicological alterations of zearalenone under ozone treatment. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2019, 36, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Niu, L.; Yang, W.; Chen, Y.; Huang, Y.; Li, C. Degradation of Zearalenone by Dielectric Barrier Discharge Cold Plasma and Its Effect on Maize Quality. Foods 2023, 12, 1129. [Google Scholar] [CrossRef]
- Fruhauf, S.; Puhringer, D.; Thamhesl, M.; Fajtl, P.; Kunz-Vekiru, E.; Hobartner-Gussl, A.; Schatzmayr, G.; Adam, G.; Damborsky, J.; Djinovic-Carugo, K.; et al. Bacterial Lactonases ZenA with Noncanonical Structural Features Hydrolyze the Mycotoxin Zearalenone. ACS Catal. 2024, 14, 3392–3410. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Tinyiro, S.E.; Wokadala, C.; Xu, D.; Yao, W. Adsorption and degradation of zearalenone by bacillus strains. Folia Microbiol. 2011, 56, 321–327. [Google Scholar] [CrossRef]
- El-Nezami, H.; Polychronaki, N.; Salminen, S.; Mykkanen, H. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative α-zearalenol. Appl. Environ. Microbiol. 2002, 68, 3545–3549. [Google Scholar] [CrossRef]
- Niderkorn, V.; Morgavi, D.P.; Aboab, B.; Lemaire, M.; Boudra, H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria. J. Appl. Microbiol. 2009, 106, 977–985. [Google Scholar] [CrossRef]
- He, Z.; Qin, F.; Guo, B.; Yu, W.; Wang, Y. Screening, identification, and mechanism elucidation of zearalenone-reducing lactic acid bacteria from Chinese sourdough. Microbiol. China 2024, 51, 2521–2533. [Google Scholar]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini-Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef]
- Swamy, H.V.L.N.; Smith, T.K.; MacDonald, E.J.; Boermans, H.J.; Squires, E.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2002, 80, 3257–3267. [Google Scholar] [CrossRef]
- Wang, L.; Yue, T.; Yuan, Y.; Wang, Z.; Ye, M.; Cal, R. A new insight into the adsorption mechanism of patulin by the heat-inactive lactic acid bacteria cells. Food Control 2015, 50, 104–110. [Google Scholar] [CrossRef]
- Yi, P.-J.; Pai, C.-K.; Liu, J.-R. Isolation and characterization of a Bacillus licheniformis strain capable of degrading zearalenone. World J. Microbiol. Biotechnol. 2011, 27, 1035–1043. [Google Scholar] [CrossRef]
- Fu, G.; Ma, J.; Wang, L.; Yang, X.; Liu, J.; Zhao, X. Effect of Degradation of Zearalenone-Contaminated Feed by Bacillus licheniformis CK1 on Postweaning Female Piglets. Toxins 2016, 8, 300. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Yi, P.-J.; Lee, T.-Y.; Liu, J.-R. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef] [PubMed]
- Sorokulova, I.B.; Pinchuk, I.V.; Denayrolles, M.; Osipova, I.G.; Huang, J.M.; Cutting, S.M.; Urdaci, M.C. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008, 53, 954–963. [Google Scholar] [CrossRef]
- Waldeck, J.; Daum, G.; Bisping, B.; Meinhardt, F. Isolation and molecular characterization of chitinase-deficient Bacillus licheniformis strains capable of deproteinization of shrimp shell waste to obtain highly viscous chitin. Appl. Environ. Microbiol. 2006, 72, 7879–7885. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, P.; Zhang, H.; Liu, M.; Ding, Q.; Cai, J. In Vitro Degradation of Zearalenone by Culture Supernatant of Bacillus subtilis. Food Bioprocess Technol. 2024, 17, 2206–2215. [Google Scholar] [CrossRef]
- El-Nezami, H.; Polychronaki, N.; Lee, Y.K.; Haskard, C.; Juvonen, R.; Salminen, S.; Mykkanen, H. Chemical moieties and interactions involved in the binding of zearalenone to the surface of Lactobacillus rhamnosus strains GG. J. Agric. Food Chem. 2004, 52, 4577–4581. [Google Scholar] [CrossRef]
- Hancock, I.C. Bacterial cell surface carbohydrates: Structure and assembly. Biochem. Soc. Trans. 1997, 25, 183–187. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb. Cell Factories 2020, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Ake, A.H.J.; Rochdi, N.; Jemo, M.; Hafidi, M.; Ouhdouch, Y.; El Fels, L. Cr(VI) removal performance from wastewater by microflora isolated from tannery effluents in a semi-arid environment: A SEM, EDX, FTIR and zeta potential study. Front. Microbiol. 2024, 15, 1423741. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.H.; Smith, A.C.; Kacurakova, M.; Saunders, P.K.; Wellner, N.; Waldron, K.W. The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol. 2000, 124, 397–405. [Google Scholar] [CrossRef]
- Guo, C.; Tianli, Y.; Yahong, Y.; Zhouli, W.; Ling, W.; Rui, C. Study on Mechanism of Inactivated Cider Yeast Adsorbing Patulin by Fourier Transform Infrared Spectroscopy. Spectrosc. Spectr. Anal. 2013, 33, 672–676. [Google Scholar]
- De Troyer, L.; De Zutter, N.; De Saeger, S.; Dumoulin, F.; Croubels, S.; De Baere, S.; De Gelder, L.; Audenaert, K. Actinobacteria as Promising Biocontrol Agents for In Vitro and In Planta Degradation and Detoxification of Zearalenone. Toxins 2024, 16, 253. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Wang, J.; Lyu, Z.; Liu, J.; Zheng, Y. Isolation and Identification of Zearalenone-Degrading Bacterium. J. Chin. Cereals Oils 2020, 35, 104–108+116. [Google Scholar]
- Adacsi, C.; Kovacs, S.; Pocsi, I.; Pusztahelyi, T. Elimination of Deoxynivalenol, Aflatoxin B1, and Zearalenone by Gram-Positive Microbes (Firmicutes). Toxins 2022, 14, 591. [Google Scholar] [CrossRef]

| Feature | Value |
|---|---|
| Genome size (bp) | 4,140,472 |
| G+C content (%) | 46.19 |
| Protein-coding genes (CDSs) | 4216 |
| rRNA genes | 10 |
| tRNA genes | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wu, F.; Zhao, P.; Gao, Y.; Li, M.; Luo, M.; Zhou, Q.; Zhou, S.; Li, X.; Hong, Y.; et al. Isolation and Mechanistic Investigation of the Efficient Zearalenone-Removing Strain Bacillus licheniformis YJ25. Toxins 2025, 17, 263. https://doi.org/10.3390/toxins17060263
Wu Y, Wu F, Zhao P, Gao Y, Li M, Luo M, Zhou Q, Zhou S, Li X, Hong Y, et al. Isolation and Mechanistic Investigation of the Efficient Zearalenone-Removing Strain Bacillus licheniformis YJ25. Toxins. 2025; 17(6):263. https://doi.org/10.3390/toxins17060263
Chicago/Turabian StyleWu, Yuting, Feina Wu, Pan Zhao, Yan Gao, Mengyao Li, Mengjiao Luo, Qian Zhou, Siyuan Zhou, Xinhui Li, Yaling Hong, and et al. 2025. "Isolation and Mechanistic Investigation of the Efficient Zearalenone-Removing Strain Bacillus licheniformis YJ25" Toxins 17, no. 6: 263. https://doi.org/10.3390/toxins17060263
APA StyleWu, Y., Wu, F., Zhao, P., Gao, Y., Li, M., Luo, M., Zhou, Q., Zhou, S., Li, X., Hong, Y., Wu, Y., Zhou, Z., Liu, Y., Xia, Y., Zou, L., & Yin, J. (2025). Isolation and Mechanistic Investigation of the Efficient Zearalenone-Removing Strain Bacillus licheniformis YJ25. Toxins, 17(6), 263. https://doi.org/10.3390/toxins17060263







