Botulinum Toxin for Bruxism: An Overview
Abstract
1. Introduction
2. Results
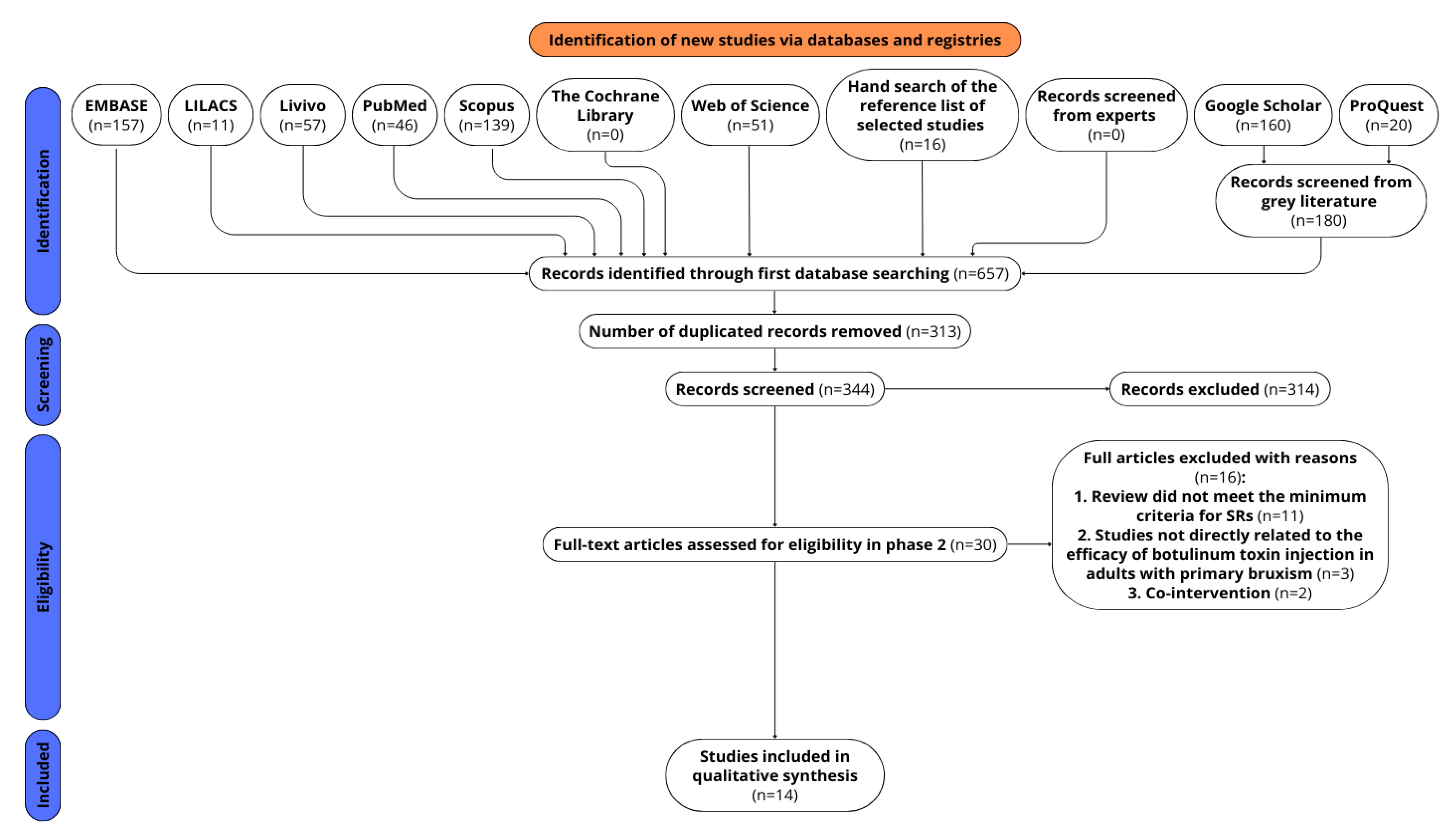
2.1. Study Selection
2.2. Characteristics of Systematic Reviews
2.3. Bibliometric Analysis
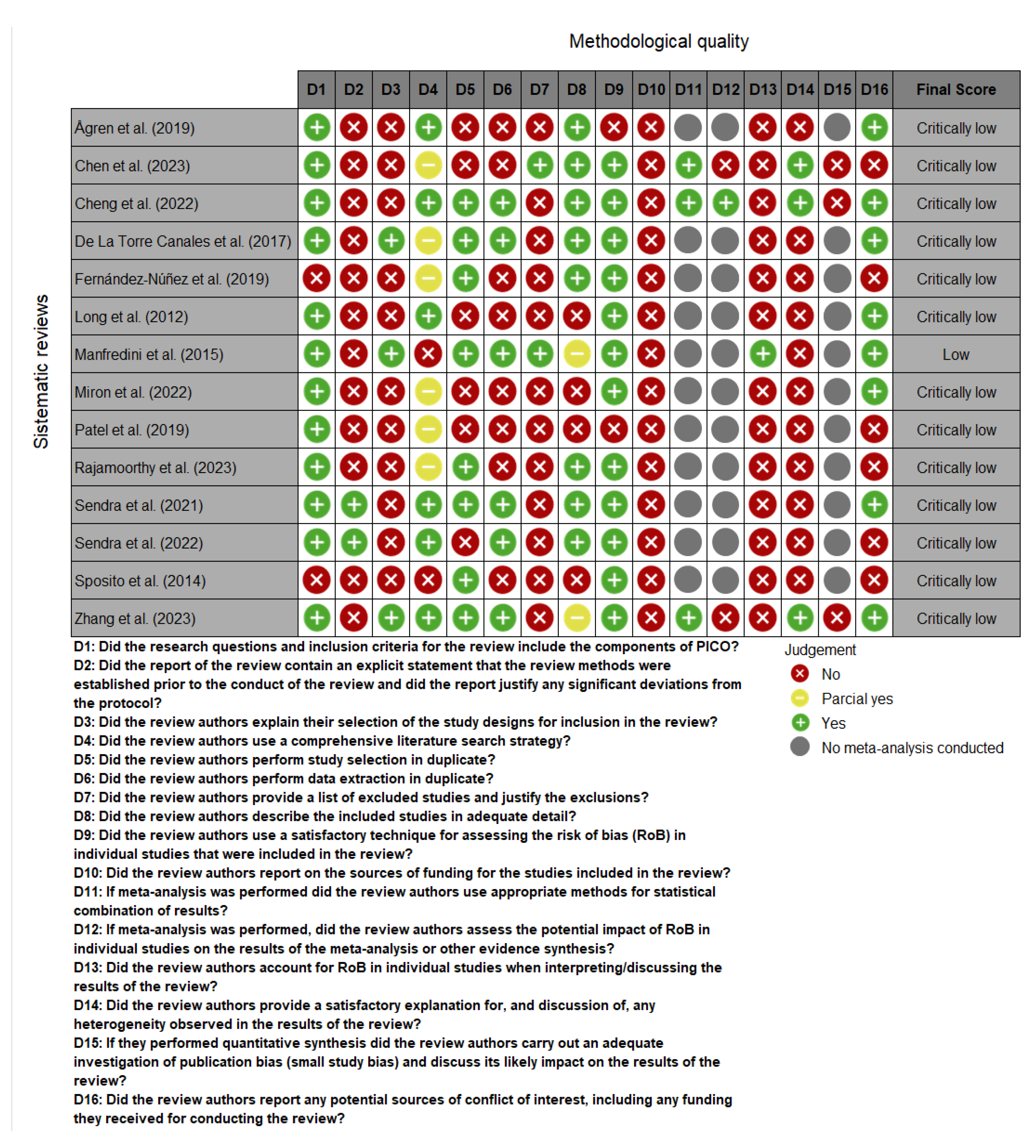
2.4. Methodological Quality
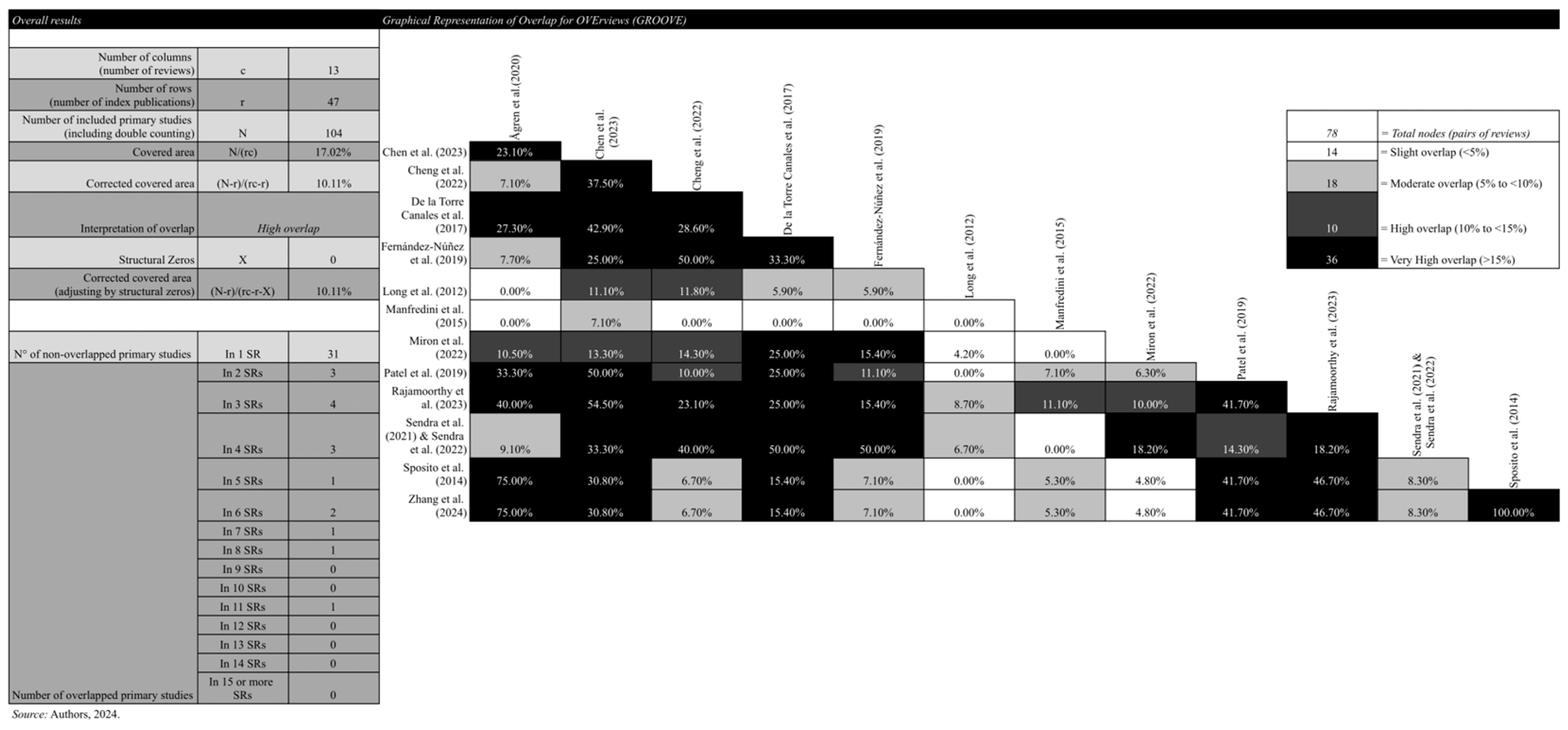
2.5. Primary Study Overlap
2.6. Synthesis of Results of Systematic Reviews
2.7. Certainty Assessment
3. Discussion
4. Conclusions
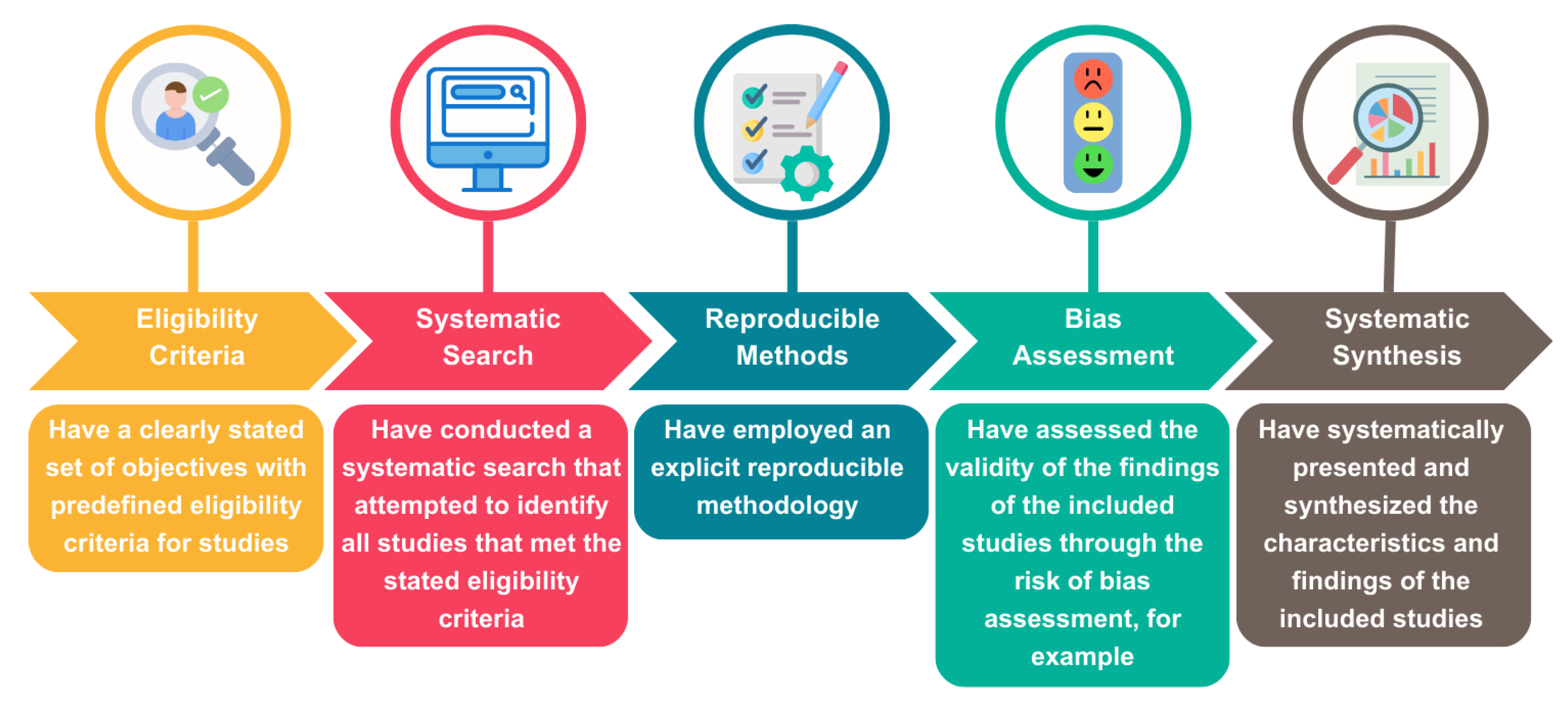
5. Materials and Methods
- Research Question
- Eligibility criteria
- Participants
- Intervention
- Comparison
- Primary outcomes included the following:
- Secondary outcomes included the following:
- SRs that included participants outside the adult age range;
- SRs that included cases of bruxism caused by or associated with neurological disorders;
- SR that included participants using BoNT-A therapy for conditions other than bruxism, such as the management of temporomandibular disorders or the use for aesthetic purposes;
- Exclusion of publication types other than SRs, such as primary studies, editorials, letters to the editor, case reports, conference papers and proceedings, book chapters, preprints, and patents.
- Information sources
- Search strategy
- Selection process
- Data collection process
- Data items
- Methodological quality
- Synthesis methods
- Certainty assessment
6. Strengths and Limitations of This Study
7. Protocol Alterations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Awake Bruxism |
| AMSTAR-2 | A MeaSurement Tool to Assess systematic Reviews |
| BoNT-A | Botulinum Toxin Type A |
| BTX-A | Botulinum Toxin Type A |
| CCA | Corrected Covered Area |
| COBE | Brazilian Centre for Evidence-Based Research |
| DC/TMD | Diagnostic Criteria for Temporomandibular Disorders |
| EMG | Electromyography |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| GROOVE | Graphical Representation of Overlap for OVErviews |
| MA | Meta-Analysis |
| OSF | Open Science Framework |
| PICOS | Participants, Intervention, Control, Outcomes, and Study design |
| PRIOR | Preferred Reporting Items for Overviews of Reviews |
| PRISMA-P | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols |
| PSG | Polysomnography |
| RCT | Randomized Controlled Trial |
| ROBIS | Risk of Bias in Systematic Reviews |
| RMMA | Rhythmic Masticatory Muscle Activity |
| SB | Sleep Bruxism |
| SR | Systematic Review |
| STAB | Standardized Tool for the Assessment of Bruxism |
| TMD | Temporomandibular Disorder |
| VAS | Visual Analog Scale |
References
- Lobbezoo, F.; Ahlberg, J.; Glaros, A.; Kato, T.; Koyano, K.; Lavigne, G.; de Leeuw, R.; Manfredini, D.; Svensson, P.; Winocur, E. Bruxism Defined and Graded: An International Consensus. J. Oral Rehabil. 2013, 40, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.; Wetselaar, P.; Glaros, A.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R. International Consensus on the Assessment of Bruxism: Report of a Work in Progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.; Kato, T.; Kolta, A.; Sessle, B. Neurobiological Mechanisms Involved in Sleep Bruxism. Crit. Rev. Oral Biol. Med. 2003, 14, 30–46. [Google Scholar] [CrossRef]
- de Oliveira, J.M.D.; Coelho, M.S.; Pereira, R.d.P.L.; Pauletto, P.; de Sá, J.D.; Brancher, J.A.; Feltrin-Souza, J.; Guerra, E.N.S.; Massignan, C.; Canto, G.D.L. Genetic Polymorphisms and Bruxism: A Scoping Review. Sleep Med. 2024, 124, 554–575. [Google Scholar] [CrossRef]
- Beddis, H.; Pemberton, M.; Davies, S. Sleep Bruxism: An Overview for Clinicians. Br. Dent. J. 2018, 225, 497–501. [Google Scholar] [CrossRef]
- Bertazzo-Silveira, E.; Kruger, C.M.; De Toledo, I.P.; Porporatti, A.L.; Dick, B.; Flores-Mir, C.; Canto, G.D.L. Association between Sleep Bruxism and Alcohol, Caffeine, Tobacco, and Drug Abuse: A Systematic Review. J. Am. Dent. Assoc. 2016, 147, 859–866. [Google Scholar] [CrossRef]
- Carra, M.C.; Huynh, N.; Fleury, B.; Lavigne, G. Overview on Sleep Bruxism for Sleep Medicine Clinicians. Sleep Med. Clin. 2015, 10, 375–384. [Google Scholar] [CrossRef]
- Polmann, H.; Domingos, F.L.; Melo, G.; Stuginski-Barbosa, J.; Guerra, E.N.d.S.; Porporatti, A.L.; Dick, B.D.; Flores-Mir, C.; De Luca Canto, G. Association between Sleep Bruxism and Anxiety Symptoms in Adults: A Systematic Review. J. Oral Rehabil. 2019, 46, 482–491. [Google Scholar] [CrossRef]
- Polmann, H.; Réus, J.C.; Massignan, C.; Serra-Negra, J.M.; Dick, B.D.; Flores-Mir, C.; Lavigne, G.J.; De Luca Canto, G. Association between Sleep Bruxism and Stress Symptoms in Adults: A Systematic Review and Meta-analysis. J. Oral Rehabil. 2021, 48, 621–631. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Verhoeff, M.C.; Ahlberg, J.; Manfredini, D.; Aarab, G.; Koutris, M.; Svensson, P.; Thymi, M.; Visscher, C.M.; Lavigne, G.J. A Century of Bruxism Research in Top-Ranking Medical Journals. Cephalalgia Rep. 2024, 7, 25158163241235574. [Google Scholar] [CrossRef]
- Thomas, D.C.; Manfredini, D.; Patel, J.; George, A.; Chanamolu, B.; Pitchumani, P.K.; Sangalli, L. Sleep Bruxism: The Past, the Present, and the Future Evolution of a Concept. J. Am. Dent. Assoc. 2024, 155, 329–343. [Google Scholar] [CrossRef]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of Bruxism in Adults: A Systematic Review of the Literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Maluly, M.; Andersen, M.; Dal-Fabbro, C.; Garbuio, S.; Bittencourt, L.; De Siqueira, J.; Tufik, S. Polysomnographic Study of the Prevalence of Sleep Bruxism in a Population Sample. J. Dent. Res. 2013, 92, S97–S103. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism Physiology and Pathology: An Overview for Clinicians. J. Oral Rehabil. 2008, 35, 476–494. [Google Scholar] [CrossRef]
- Oliveira, J.M.D.; Pauletto, P.; Massignan, C.; D’Souza, N.; de Godoi Gonçalves, D.A.; Flores-Mir, C.; Canto, G.D.L. Prevalence of Awake Bruxism: A Systematic Review. J. Dent. 2023, 138, 104715. [Google Scholar] [CrossRef]
- Matusz, K.; Maciejewska-Szaniec, Z.; Gredes, T.; Pobudek-Radzikowska, M.; Glapiński, M.; Górna, N.; Przystańska, A. Common Therapeutic Approaches in Sleep and Awake Bruxism—An Overview. Neurol. I Neurochir. Pol. 2022, 56, 455–463. [Google Scholar] [CrossRef]
- Melo, G.; Duarte, J.; Pauletto, P.; Porporatti, A.L.; Stuginski-Barbosa, J.; Winocur, E.; Flores-Mir, C.; De Luca Canto, G. Bruxism: An Umbrella Review of Systematic Reviews. J. Oral Rehabil. 2019, 46, 666–690. [Google Scholar] [CrossRef]
- Lima, L.S.R.; Guedes, J.L.d.S.; Tuñas, I.T.C. Toxina Botulínica Em Odontologia: Uma Revisão de Literatura. Rev. Bras. De Odontol. 2020, 77, 1–8. [Google Scholar] [CrossRef]
- Mainieri, V.C.; Saueressig, A.C.; Fagondes, S.C.; Teixeira, E.R.; Rehm, D.D.S.; Grossi, M.L. Analysis of the Effects of a Mandibular Advancement Device on Sleep Bruxism Using Polysomnography, the BiteStrip, the Sleep Assessment Questionnaire, and Occlusal Force. Int. J. Prosthodont. 2014, 27, 309. [Google Scholar] [CrossRef]
- de Souza Melo, G.; Batistella, E.Â.; Bertazzo-Silveira, E.; Gonçalves, T.M.S.V.; de Souza, B.D.M.; Porporatti, A.L.; Flores-Mir, C.; Canto, G.D.L. Association of Sleep Bruxism with Ceramic Restoration Failure: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2018, 119, 354–362. [Google Scholar] [CrossRef]
- Réus, J.C.; Polmann, H.; Mendes Souza, B.D.; Flores-Mir, C.; Trevisol Bittencourt, P.C.; Winocur, E.; Okeson, J.; De Luca Canto, G. Association between Primary Headache and Bruxism: An Updated Systematic Review. J. Oral Facial Pain Headache 2021, 35, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bussadori, S.K.; Motta, L.J.; Horliana, A.C.R.T.; Santos, E.M.; Martimbianco, A.L.C. The Current Trend in Management of Bruxism and Chronic Pain: An Overview of Systematic Reviews. J. Pain Res. 2020, 13, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Ahlberg, J.; Winocur, E.; Lobbezoo, F. Management of Sleep Bruxism in Adults: A Qualitative Systematic Literature Review. J. Oral Rehabil. 2015, 42, 862–874. [Google Scholar] [CrossRef]
- Van Zandijcke, M.; Marchau, M. Treatment of bruxism with botulinum toxin injections. J. Neurol. Neurosurg. Psychiatry 1990, 53, 530. [Google Scholar] [CrossRef]
- Onan, D.; Farham, F.; Martelletti, P. Clinical conditions targeted by OnabotulinumtoxinA in different ways in medicine. Toxins 2024, 16, 309. [Google Scholar] [CrossRef]
- Soares-Silva, L.; de Amorim, C.S.; Magno, M.B.; Tavares-Silva, C.; Maia, L.C. Effects of Different Interventions on Bruxism: An Overview of Systematic Reviews. Sleep Breath. 2024, 28, 1465–1476. [Google Scholar] [CrossRef]
- Ågren, M.; Sahin, C.; Pettersson, M. The Effect of Botulinum Toxin Injections on Bruxism: A Systematic Review. J. Oral Rehabil. 2020, 47, 395–402. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, C.-H.; Bae, T.H.; Huang, C.-Y.; Chen, C.; Kang, Y.-N.; Chiu, W.-K. Effectiveness of Botulinum Toxin Injection on Bruxism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Aesthetic Plast. Surg. 2023, 47, 775–790. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, L.; Ma, L.; Pang, F.; Qu, X.; Zhang, A. Efficacy of Botulinum-A for Nocturnal Bruxism Pain and the Occurrence of Bruxism Events: A Meta-Analysis and Systematic Review. Br. J. Oral Maxillofac. Surg. 2022, 60, 174–182. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Câmara-Souza, M.B.; Do Amaral, C.F.; Garcia, R.C.M.R.; Manfredini, D. Is There Enough Evidence to Use Botulinum Toxin Injections for Bruxism Management? A Systematic Literature Review. Clin. Oral Investig. 2017, 21, 727–734. [Google Scholar] [CrossRef]
- Fernández-Núñez, T.; Amghar-Maach, S.; Gay-Escoda, C. Efficacy of Botulinum Toxin in the Treatment of Bruxism: Systematic Review. Med. Oral Patol. Oral Y Cir. Bucal 2019, 24, e416. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liao, Z.; Wang, Y.; Liao, L.; Lai, W. Efficacy of Botulinum Toxins on Bruxism: An Evidence-based Review. Int. Dent. J. 2012, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Miron, M.I.; Ciora, E.; Vedinas, T.; Mocuta, D.-E. Therapeutic Approaches to Nocturnal Bruxism—A Systematic Review. Arch. Balk. Med. Union 2022, 57, 372–383. [Google Scholar] [CrossRef]
- Patel, J.; Cardoso, J.A.; Mehta, S. A Systematic Review of Botulinum Toxin in the Management of Patients with Temporomandibular Disorders and Bruxism. Br. Dent. J. 2019, 226, 667–672. [Google Scholar] [CrossRef]
- Rajamoorthy, S.N. Botulinum Toxin-A Injections into Facial Muscles for the Treatment of Temporomandibular Disorders and Bruxism: A Systematic Review. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 428–452. [Google Scholar]
- Sendra, L.A.; Montez, C.; Vianna, K.C.; Barboza, E.P. Clinical Outcomes of Botulinum Toxin Type A Injections in the Management of Primary Bruxism in Adults: A Systematic Review. J. Prosthet. Dent. 2021, 126, 33–40. [Google Scholar] [CrossRef]
- Sendra, L.A.; Antunes, L.A.A.; Barboza, E.P. Use of Botulinum Neurotoxin Type A in the Management of Primary Bruxism in Adults: An Updated Systematic Review. J. Prosthet. Dent. 2024, 132, 93–99. [Google Scholar] [CrossRef]
- Sposito, M.M.d.M.; Teixeira, S.A.F. Botulinum Toxin A for Bruxism: A Systematic Review. Acta Fisiátrica 2014, 21, 201–204. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J.; Zhou, X.; Sun, L.; Li, T. Can Botulinum Toxin Injection Alleviate the Pain of Bruxism? A Bayesian Network Analysis and a Single-Arm Analysis. J. Dent. Sci. 2024, 19, 885–893. [Google Scholar] [CrossRef]
- Pollock, M.; Fernandes, R.; Becker, L.; Pieper, D.; Hartling, L. Chapter V: Overviews of Reviews [Last Updated August 2023]. In Cochrane Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 16 April 2025).
- Bracchiglione, J.; Meza, N.; Pérez-Carrasco, I.; Vergara-Merino, L.; Madrid, E.; Urrútia, G.; Cosp, X.B. A Methodological Review Finds Mismatch between Overall and Pairwise Overlap Analysis in a Sample of Overviews. J. Clin. Epidemiol. 2023, 159, 31–39. [Google Scholar] [CrossRef]
- Pollock, M.; Fernandes, R.M.; Becker, L.A.; Featherstone, R.; Hartling, L. What Guidance Is Available for Researchers Conducting Overviews of Reviews of Healthcare Interventions? A Scoping Review and Qualitative Metasummary. Syst. Rev. 2016, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Glenton, C.; Dahm, P.; Garner, P.; Akl, E.A.; Alper, B.; Brignardello-Petersen, R.; Carrasco-Labra, A.; De Beer, H.; Hultcrantz, M. GRADE Guidelines 26: Informative Statements to Communicate the Findings of Systematic Reviews of Interventions. J. Clin. Epidemiol. 2020, 119, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. Br. Med. J. 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. An International Registry of Systematic-Review Protocols. Lancet 2011, 377, 108–109. [Google Scholar] [CrossRef]
- Chalmers, I.; Glasziou, P. Avoidable Waste in the Production and Reporting of Research Evidence. Lancet 2009, 374, 86–89. [Google Scholar] [CrossRef]
- Pauletto, P.; Polmann, H.; Réus, J.C.; de Oliveira, J.M.D.; Chaves, D.; Lehmkuhl, K.; Massignan, C.; Stefani, C.M.; Martins, C.C.; Flores-Mir, C. Critical Appraisal of Systematic Reviews of Intervention in Dentistry Published between 2019–2020 Using the AMSTAR 2 Tool. Evid.-Based Dent. 2022, 1–8. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. Br. Med. J. 2008, 336, 924–926. [Google Scholar] [CrossRef]
- de Oliveira, J.M.D.; Oliveira, L.B.; Pauletto, P.; Stefani, C.M.; Massignan, C.; Martins, C.C.; Porfírio, G.J.M.; Peres, M.A.; Flores-Mir, C.; Canto, G.D.L. Reporting of the Certainty of Evidence Through GRADE in Systematic Reviews in Dentistry Indexed during Late 2019–Late 2020. [Preprint]. 2023. Available online: https://www.researchsquare.com/article/rs-3408361/v1 (accessed on 16 April 2025).
- Lobbezoo, F.; Naeije, M. Bruxism Is Mainly Regulated Centrally, Not Peripherally. J. Oral Rehabil. 2001, 28, 1085–1091. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Câmara-Souza, M.B.; Poluha, R.L.; de Figueredo, O.M.C.; Nobre, B.B.d.S.; Ernberg, M.; Conti, P.C.R.; Rizzatti-Barbosa, C.M. Long-Term Effects of a Single Application of Botulinum Toxin Type A in Temporomandibular Myofascial Pain Patients: A Controlled Clinical Trial. Toxins 2022, 14, 741. [Google Scholar] [CrossRef]
- Sitnikova, V.; Kämppi, A.; Teronen, O.; Kemppainen, P. Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial. Toxins 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.; Nanchaipruek, Y.; Phumariyapong, P.; Apinuntham, C.; Rakchart, S.; Pettersson, M.; Wanitphakdeedecha, R. Duration of Bite Force Reduction Following a Single Injection of Botulinum Toxin in the Masseter Muscle Bilaterally: A One-year Non-randomized Trial. J. Oral Rehabil. 2023, 50, 343–350. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Canales, G.; Poluha, R.L.; Pinzón, N.A.; Da Silva, B.R.; Almeida, A.M.; Ernberg, M.; Manso, A.C.; Bonjardim, L.R.; Rizzatti-Barbosa, C.M. Efficacy of Botulinum Toxin Type-A I in the Improvement of Mandibular Motion and Muscle Sensibility in Myofascial Pain TMD Subjects: A Randomized Controlled Trial. Toxins 2022, 14, 441. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Wetselaar, P.; Svensson, P.; Lobbezoo, F. The Bruxism Construct: From Cut-off Points to a Continuum Spectrum. J. Oral Rehabil. 2019, 46, 991–997. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Aarab, G.; Bender, S.; Bracci, A.; Cistulli, P.A.; Conti, P.C.; De Leeuw, R.; Durham, J.; Emodi-Perlman, A. Standardised Tool for the Assessment of Bruxism. J. Oral Rehabil. 2024, 51, 29–58. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Lavigne, G.J.; Svensson, P.; Lobbezoo, F. Five Years after the 2018 Consensus Definitions of Sleep and Awake Bruxism: An Explanatory Note. J. Oral Rehabil. 2024, 51, 623–624. [Google Scholar] [CrossRef]
- Popescu, M.N.; Beiu, C.; Iliescu, C.A.; Racoviță, A.; Berteanu, M.; Iliescu, M.G.; Stănescu, A.M.A.; Radaschin, D.S.; Popa, L.G. Ultrasound-Guided Botulinum Toxin-A Injections into the Masseter Muscle for Both Medical and Aesthetic Purposes. Toxins 2024, 16, 413. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef]
- Coelho, M.; Pauletto, P.; Polmann, H.; de Oliveira, J.M.D.; de Luca Macial, L.; Stefani, C.M.; De Luca Canto, G. Botulinum Toxin for the Management of Bruxism: An Overview of Reviews. Open Sci. Framew. 2023. [Google Scholar] [CrossRef]
- Coelho, M.; de Oliveira, J.; Polmann, H.; Pauletto, P.; de Luca Maciel, L.; Stefani, C.; De Luca Canto, G. Botulinum Toxin for the Management of Bruxism: An Overview of Review Protocol. Br. Med. J. Open 2024, 14, e082861. [Google Scholar] [CrossRef]
- Gates, M.; Gates, A.; Pieper, D.; Fernandes, R.M.; Tricco, A.C.; Moher, D.; Brennan, S.E.; Li, T.; Pollock, M.; Lunny, C. Reporting Guideline for Overviews of Reviews of Healthcare Interventions: Development of the PRIOR Statement. Br. Med. J. 2022, 378, e070849. [Google Scholar] [CrossRef]
- Becker, L.A.; Oxman, A.D. 22 Overviews of Reviews. Cochrane Handb. Syst. Rev. Interv. 2008, 607, 607–631. [Google Scholar]
- Casett, E.; Réus, J.; Stuginski-Barbosa, J.; Porporatti, A.; Carra, M.; Peres, M.; de Luca Canto, G.; Manfredini, D. Validity of Different Tools to Assess Sleep Bruxism: A Meta-analysis. J. Oral Rehabil. 2017, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.L.; Park, S.H. EndNote X7 for Medical Writing. J. Korean Fract. Soc. 2014, 27, 237–244. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.S.; Oliveira, J.M.D.d.; Polmann, H.; Pauletto, P.; Stefani, C.M.; Maciel, L.C.D.L.; Canto, G.D.L. Botulinum Toxin for Bruxism: An Overview. Toxins 2025, 17, 249. https://doi.org/10.3390/toxins17050249
Coelho MS, Oliveira JMDd, Polmann H, Pauletto P, Stefani CM, Maciel LCDL, Canto GDL. Botulinum Toxin for Bruxism: An Overview. Toxins. 2025; 17(5):249. https://doi.org/10.3390/toxins17050249
Chicago/Turabian StyleCoelho, Manuella Salm, Júlia Meller Dias de Oliveira, Helena Polmann, Patrícia Pauletto, Cristine Miron Stefani, Lara Catarine De Luca Maciel, and Graziela De Luca Canto. 2025. "Botulinum Toxin for Bruxism: An Overview" Toxins 17, no. 5: 249. https://doi.org/10.3390/toxins17050249
APA StyleCoelho, M. S., Oliveira, J. M. D. d., Polmann, H., Pauletto, P., Stefani, C. M., Maciel, L. C. D. L., & Canto, G. D. L. (2025). Botulinum Toxin for Bruxism: An Overview. Toxins, 17(5), 249. https://doi.org/10.3390/toxins17050249




