Cannabidiol Mitigates Deoxynivalenol-Induced Intestinal Toxicity by Regulating Inflammation, Oxidative Stress, and Barrier Integrity
Abstract
1. Introduction
2. Results
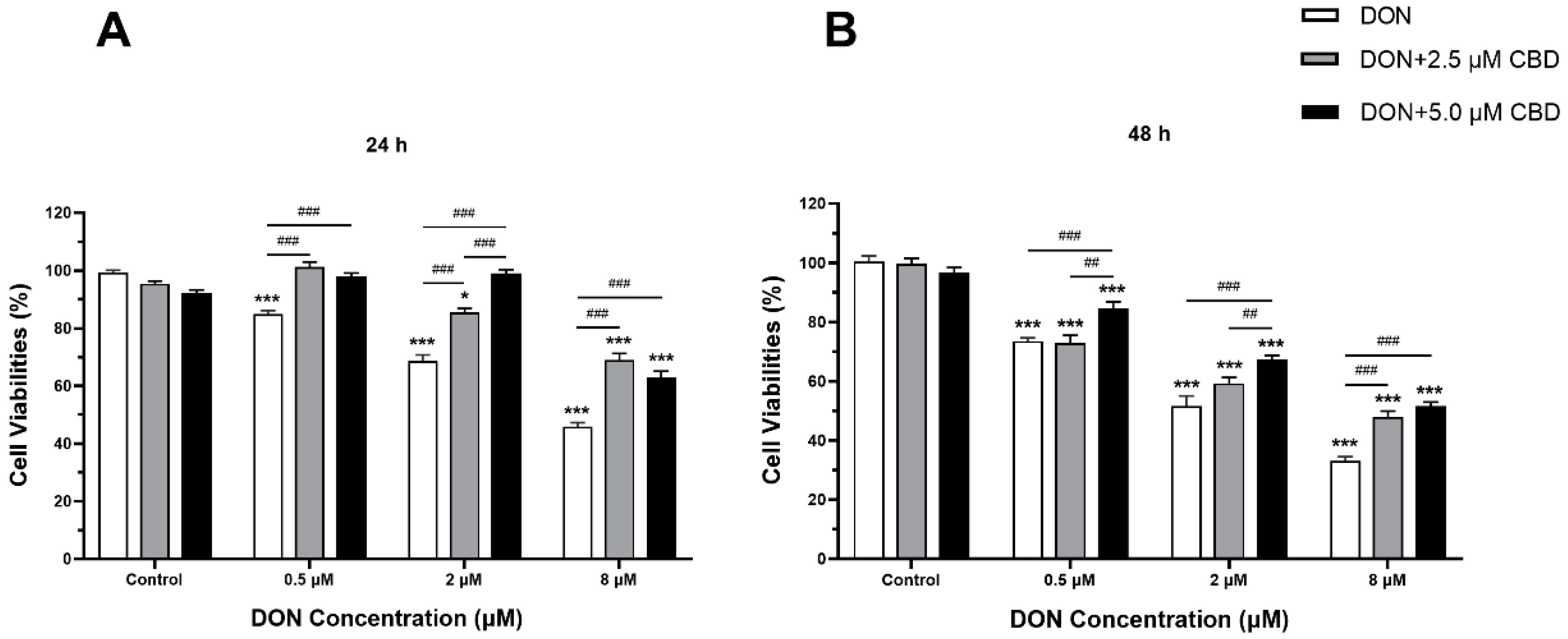
2.1. Low Concentrations of CBD, Unlike DON, Have No Effects on Cell Viability
2.2. CBD Mitigated the Cell Viability Alteration Induced by DON in IPEC-J2 Cells
2.3. CBD Exerts Divergent Effects on Apoptotic Regulation Depending on Cellular Differentiation State
2.4. CBD Modulates DON-Induced Inflammatory Responses in IPEC-J2 Cells
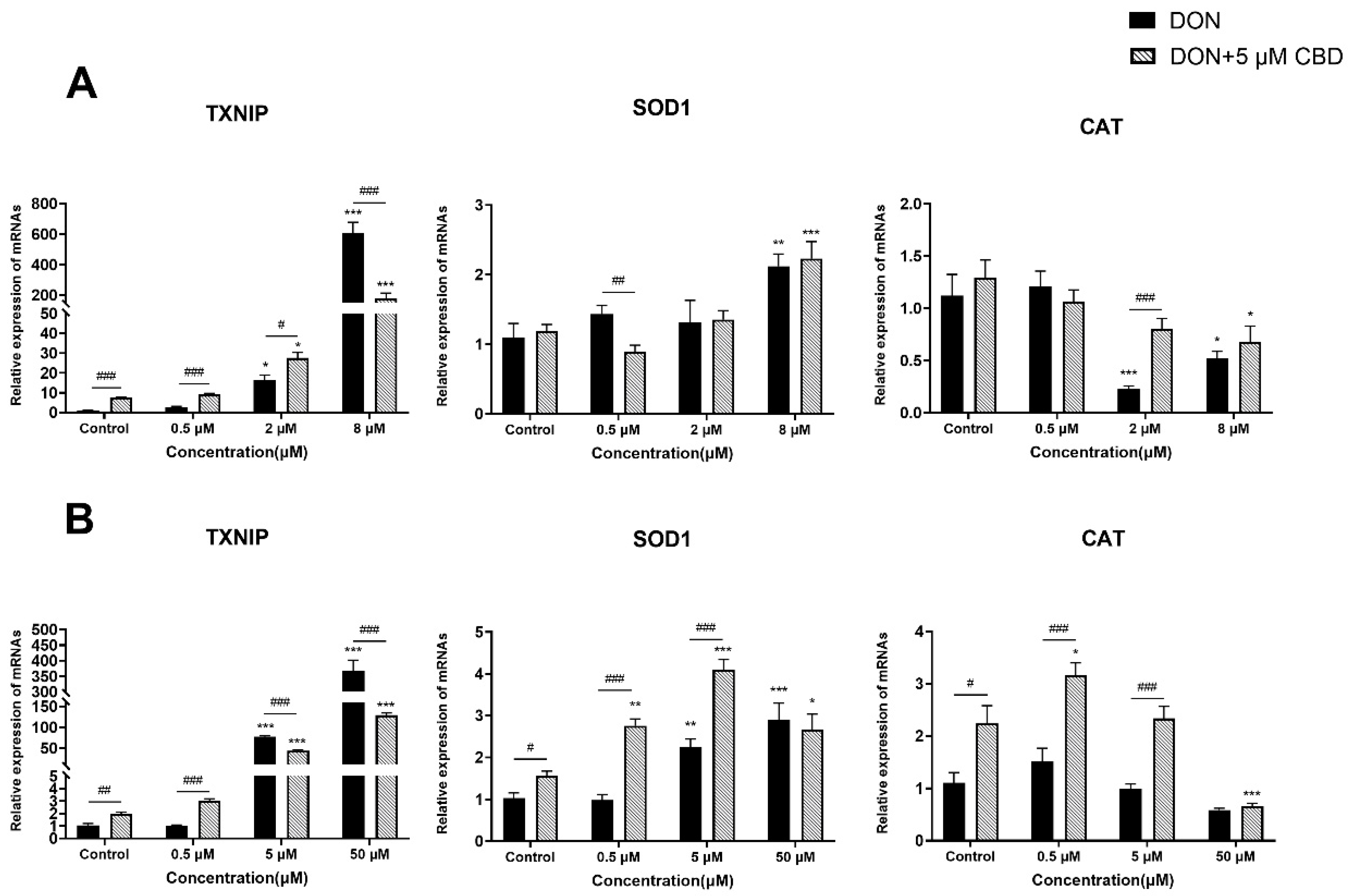
2.5. CBD Has a Limited Effect on DON-Induced Oxidative Stress
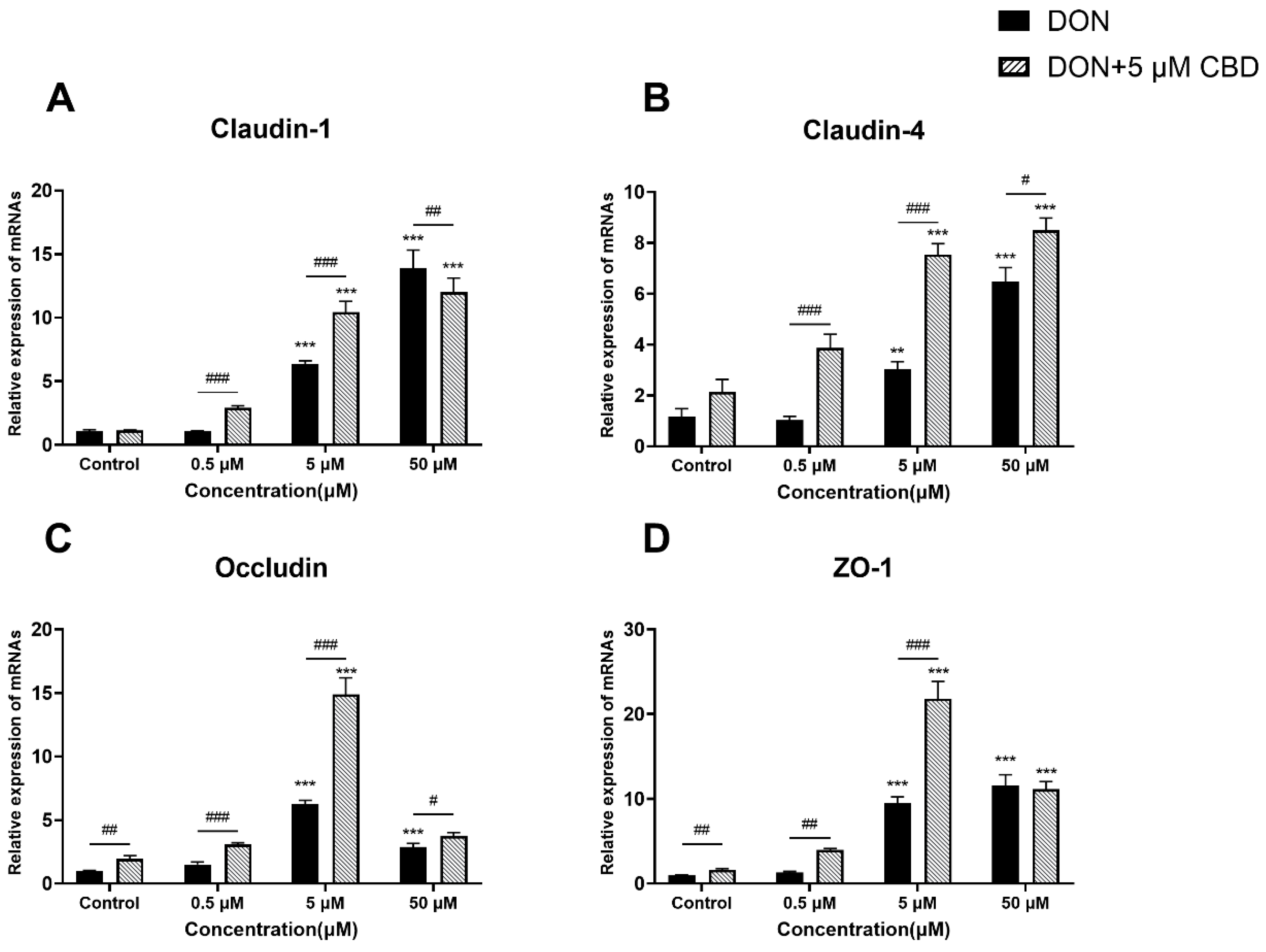
2.6. CBD Enhances Tight Junction Gene Expression Only Under Moderate DON Stress
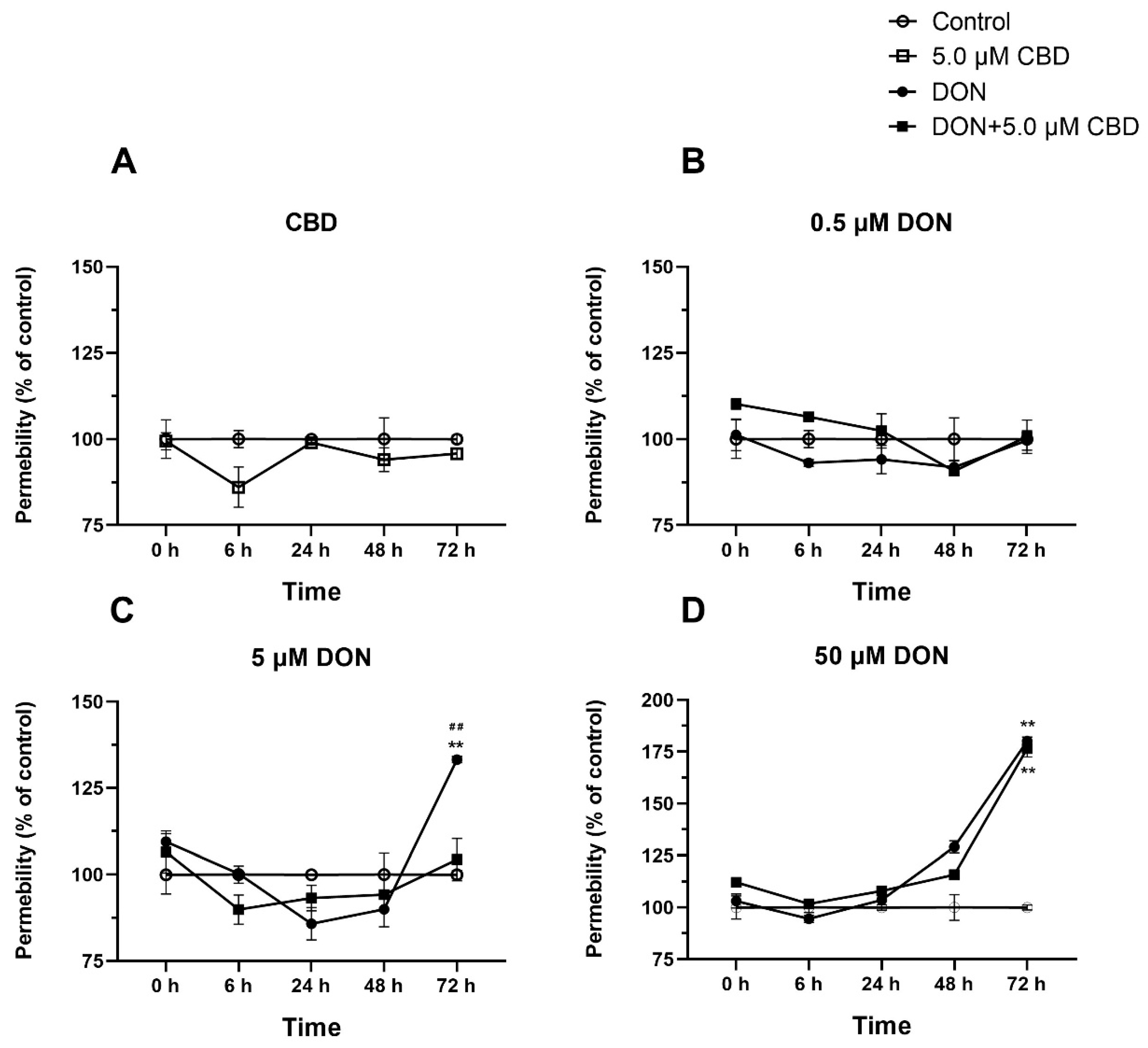
2.7. CBD Preserves Barrier Integrity Against Moderate but Not Severe DON Damage
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Phenol Red Permeability Assay
4.5. Total RNA Extraction and Real-Time qPCR (RT-qPCR)
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witczak, A.; Sikorski, Z. Toxins and Other Harmful Compounds in Foods; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”. Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, B.; Dhawan, K.; Vasundhara; Mishra, S.; Kumar, M.; Tripathi, A.D.; et al. Deoxynivalenol: An Overview on Occurrence, Chemistry, Biosynthesis, Health Effects and Its Detection, Management, and Control Strategies in Food and Feed. Microbiol. Res. 2022, 13, 292–314. [Google Scholar] [CrossRef]
- Pack, E.D.; Weiland, S.; Musser, R.; Schmale, D.G. Survey of zearalenone and type-B trichothecene mycotoxins in swine feed in the USA. Mycotoxin Res. 2021, 37, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Sumarah, M.W. The Deoxynivalenol Challenge. J. Agric. Food Chem. 2022, 70, 9619–9624. [Google Scholar] [CrossRef]
- Yue, J.; Guo, D.; Gao, X.; Wang, J.; Nepovimova, E.; Wu, W.; Kuca, K. Deoxynivalenol (Vomitoxin)-Induced Anorexia Is Induced by the Release of Intestinal Hormones in Mice. Toxins 2021, 13, 512. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Chaytor, A.C.; Hansen, J.A.; Van Heugten, E.; See, M.T.; Kim, S.W. Occurrence and decontamination of mycotoxins in swine feed. Asian-Australas. J. Anim. Sci. 2011, 24, 723–738. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Kang, T.H.; Shin, S.; Park, J.; Lee, B.R.; Lee, S.I. Pyroptosis-Mediated Damage Mechanism by Deoxynivalenol in Porcine Small Intestinal Epithelial Cells. Toxins 2023, 15, 300. [Google Scholar] [CrossRef]
- Hanyu, H.; Yokoi, Y.; Nakamura, K.; Ayabe, T.; Tanaka, K.; Uno, K.; Miyajima, K.; Saito, Y.; Iwatsuki, K.; Shimizu, M.; et al. Mycotoxin Deoxynivalenol Has Different Impacts on Intestinal Barrier and Stem Cells by Its Route of Exposure. Toxins 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, K.F.; Chen, F.J.; Chen, Y.H.; Yang, X.; Cai, Z.H.; Jiang, Y.B.; Wang, X.B.; Zhang, G.P.; Wang, F.Y. Deoxynivalenol triggers porcine intestinal tight junction disorder: Insights from mitochondrial dynamics and mitophagy. Ecotoxicol. Environ. Saf. 2022, 248, 114291. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Effects of Mycotoxins on the Intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Turner, P.C.; Co, V.A.; Wang, M.F.; Amiri, K.M.A.; El-Nezami, H. Schisandrin A protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 2019, 9, 19173. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Pinton, P.; Hupé, J.F.; Neves, M.; Lippi, Y.; Combes, S.; Castex, M.; Oswald, I.P. Saccharomyces cerevisiae Boulardii Reduces the Deoxynivalenol-Induced Alteration of the Intestinal Transcriptome. Toxins 2018, 10, 199. [Google Scholar] [CrossRef]
- Jing, G.; Wang, Y.; Wu, M.; Liu, W.; Xiong, S.; Yu, J.; Li, W.; Liu, W.; Jiang, Y. Photocatalytic Degradation and Pathway from Mycotoxins in Food: A Review. Food Rev. Int. 2024, 40, 276–292. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Arzimanoglou, A.; Brandl, U.; Cross, J.H.; Gil-Nagel, A.; Lagae, L.; Landmark, C.J.; Specchio, N.; Nabbout, R.; Thiele, E.A.; Gubbay, O.; et al. Epilepsy and cannabidiol: A guide to treatment. Epileptic Disord. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Ashton, C.H.; Moore, P.B.; Gallagher, P.; Young, A.H. Cannabinoids in bipolar affective disorder: A review and discussion of their therapeutic potential. J. Psychopharmacol. 2005, 19, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kent-Dennis, C.; Klotz, J.L. Immunomodulation by cannabidiol in bovine primary ruminal epithelial cells. BMC Vet. Res. 2023, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk And Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Zhu, L.; Xu, W.; Chu, X.; Zhang, Y.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; et al. Deoxynivalenol Induces Caspase-8-Mediated Apoptosis through the Mitochondrial Pathway in Hippocampal Nerve Cells of Piglet. Toxins 2021, 13, 73. [Google Scholar] [CrossRef]
- Bosier, B.; Muccioli, G.G.; Hermans, E.; Lambert, D.M. Functionally selective cannabinoid receptor signalling: Therapeutic implications and opportunities. Biochem. Pharmacol. 2010, 80, 1–12. [Google Scholar] [CrossRef]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qiao, L.; Dou, X.; Chang, J.; Zhang, Y.; Xu, C. Selenium nanoparticles alleviate deoxynivalenol-induced intestinal epithelial barrier dysfunction by regulating endoplasmic reticulum stress in IPEC-J2 cells. Toxicology 2023, 494, 153593. [Google Scholar] [CrossRef]
- Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Shen, Y.; Wu, S. Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells. Toxins 2022, 14, 275. [Google Scholar] [CrossRef]
- Stein, L.; Vollstaedt, M.L.; Amasheh, S. Cannabidiol Strengthening of Gastric Tight Junction Complexes Analyzed in an Improved Xenopus Oocyte Assay. Membranes 2024, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Jovov, B.; Wills, N.K.; Lewis, S.A. A spectroscopic method for assessing confluence of epithelial cell cultures. Am. J. Physiol. Cell Physiol. 1991, 261, C1196–C1203. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]



| Gene | Upstream Primer (5′→3′) | Downstream Primer (5′→3′) |
|---|---|---|
| NF-κB | CTCGCACAAGGAGACATGAA | ACTCAGCCGGAAGGCATTAT |
| IL-6 | ACCTGCTTGATGAGAATCACC | CTTCATCCACTCGTTCTGTGA |
| COX-2 | TGCGGGAACATAATAGAG | GTATCAGCCTGCTCGTCT |
| TXNIP | TTGGAGGAAAGACAGGAAAGA | AACAAAACCCCGAATCAAAG |
| SOD1 | GAGACCTGGGCAATGTGAC | GAGGGAATGTTTACTGGGTGA |
| CAT | GGCTTTTGGCTACTTTGAGG | AGGGTCACGAACTGTGTCAG |
| Bcl-2 | GGATAACGGAGGCTGGGATG | TTATGGCCCAGATAGGCACC |
| Bax | GCCCTTTTGCTTCAGGGTTTC | CAATGCGCTTGAGACACTCG |
| Claudin-1 | TTTCCTCAATACAGGAGGGAAGC | CCCTCTCCCCACATTCGAG |
| Claudin-4 | CTGCTTTGCTGCAACTGC | TCAACGGTAGCACCTTACACGTAG |
| Occludin | CTACTCGTCCAACGGGAAAG | ACGCCTCCAAGTTACCACTG |
| ZO-1 | AAGCCCTAAGTTCAATCACAATCT | ATCAAACTCAGGAGGCGGC |
| GAPDH | GGGCATGAACCATGAGAAGT | TGTGGTCATGAGTCCTTCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Decas, T.; Zhang, Y.; Alassane-Kpembi, I. Cannabidiol Mitigates Deoxynivalenol-Induced Intestinal Toxicity by Regulating Inflammation, Oxidative Stress, and Barrier Integrity. Toxins 2025, 17, 241. https://doi.org/10.3390/toxins17050241
Yang L, Decas T, Zhang Y, Alassane-Kpembi I. Cannabidiol Mitigates Deoxynivalenol-Induced Intestinal Toxicity by Regulating Inflammation, Oxidative Stress, and Barrier Integrity. Toxins. 2025; 17(5):241. https://doi.org/10.3390/toxins17050241
Chicago/Turabian StyleYang, Lingchen, Tristan Decas, Yuhang Zhang, and Imourana Alassane-Kpembi. 2025. "Cannabidiol Mitigates Deoxynivalenol-Induced Intestinal Toxicity by Regulating Inflammation, Oxidative Stress, and Barrier Integrity" Toxins 17, no. 5: 241. https://doi.org/10.3390/toxins17050241
APA StyleYang, L., Decas, T., Zhang, Y., & Alassane-Kpembi, I. (2025). Cannabidiol Mitigates Deoxynivalenol-Induced Intestinal Toxicity by Regulating Inflammation, Oxidative Stress, and Barrier Integrity. Toxins, 17(5), 241. https://doi.org/10.3390/toxins17050241






