Fumonisin B1 Exposure Causes Intestinal Tissue Damage by Triggering Oxidative Stress Pathways and Inducing Associated CYP Isoenzymes
Abstract
1. Introduction
2. Results
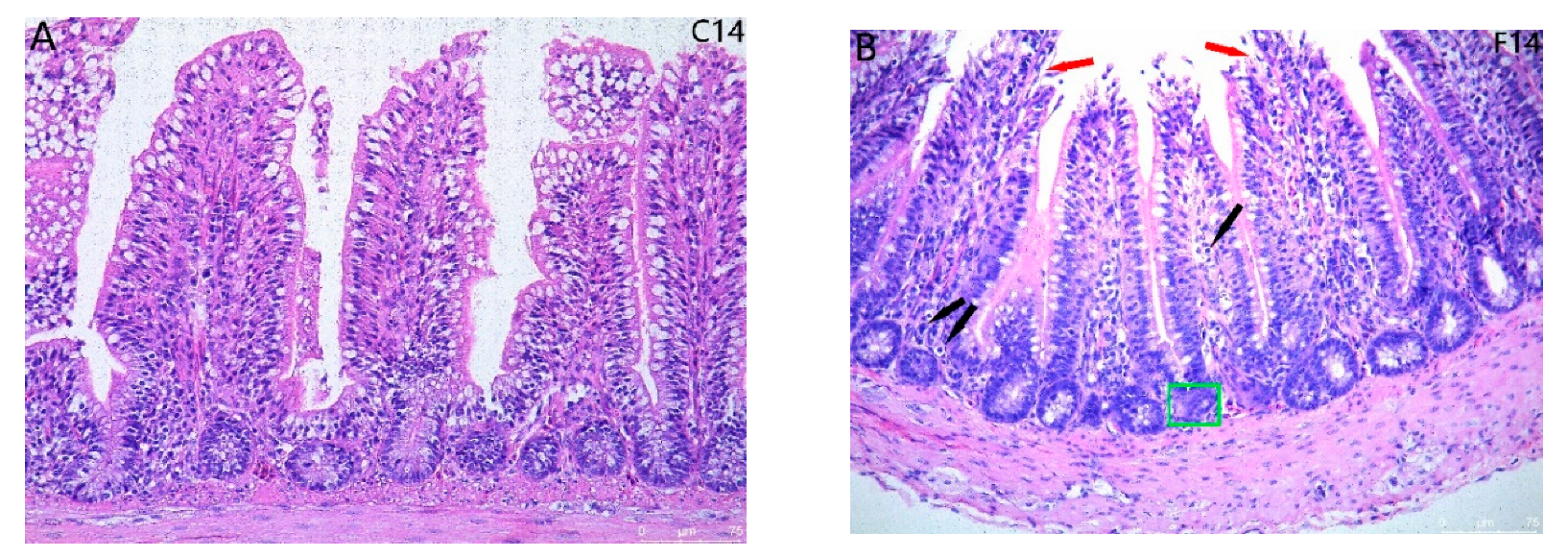
2.1. Histopathological Observation for Ileum Intestinal Tissue
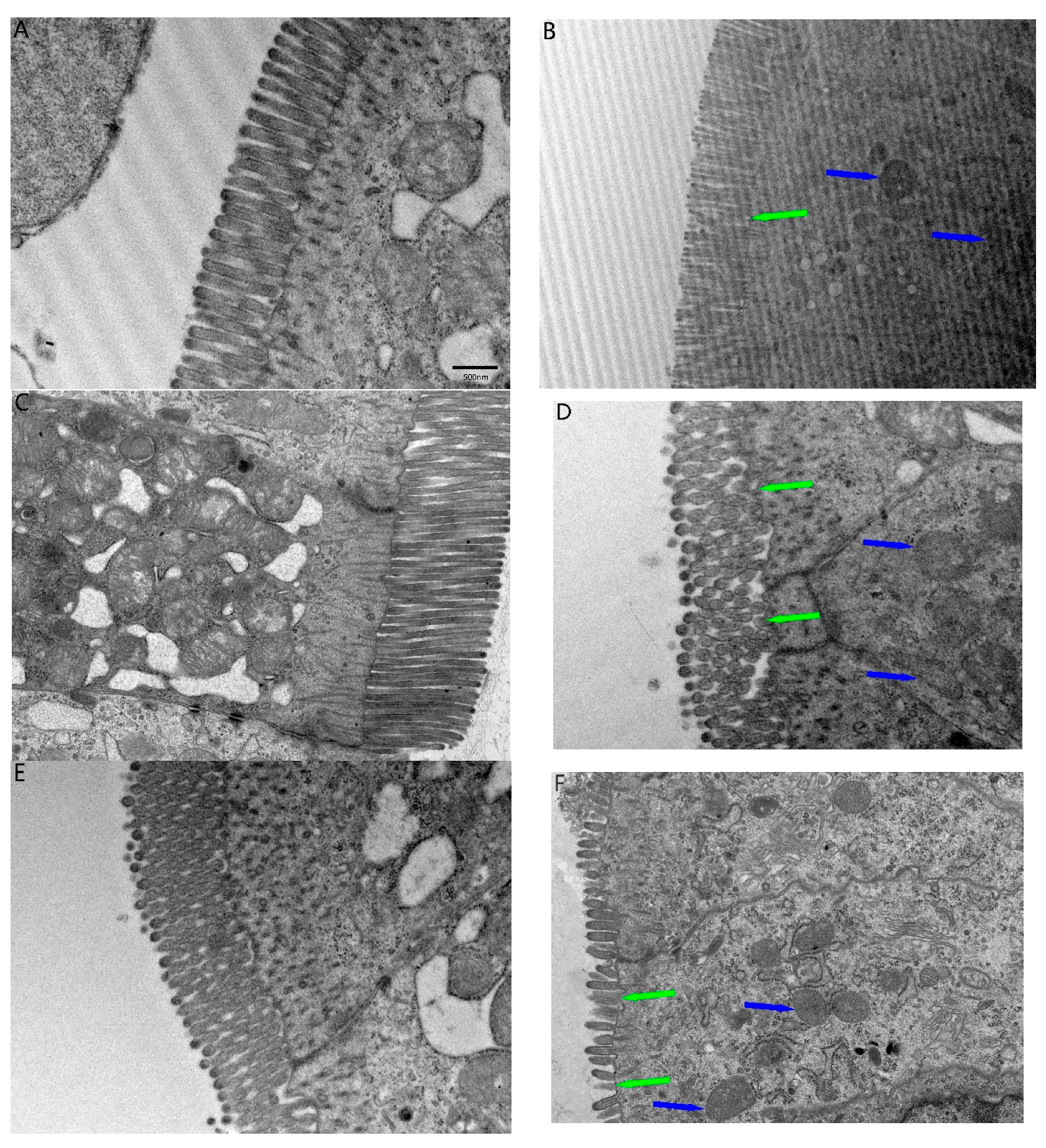
2.2. TEM Ultrastructural and Fibrous Changes in the Ileum
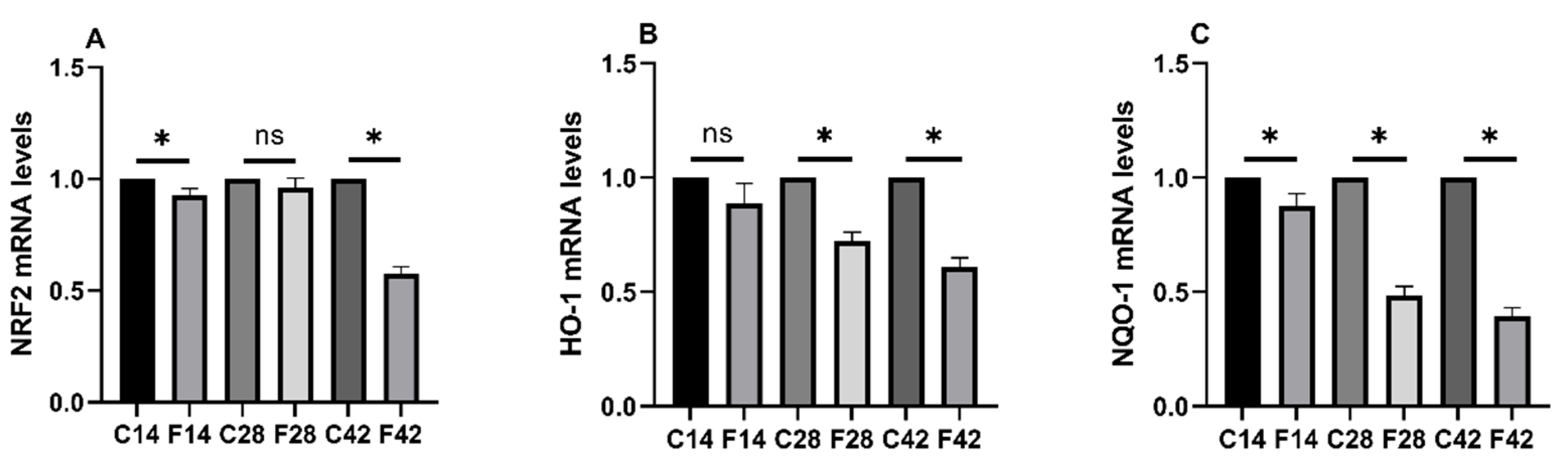
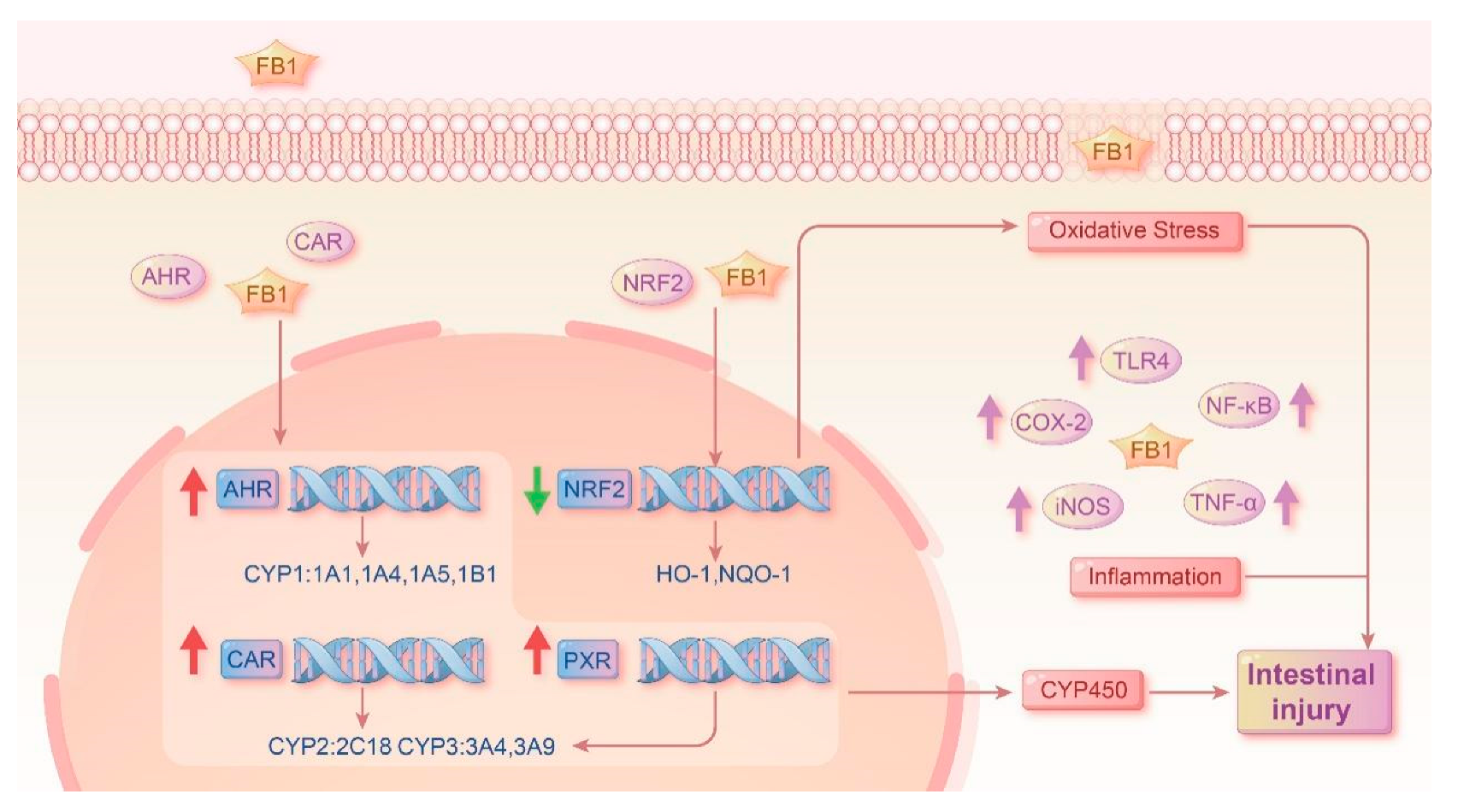
2.3. Functions of FB1 in Oxidative Stress-Associated mRNA Expression
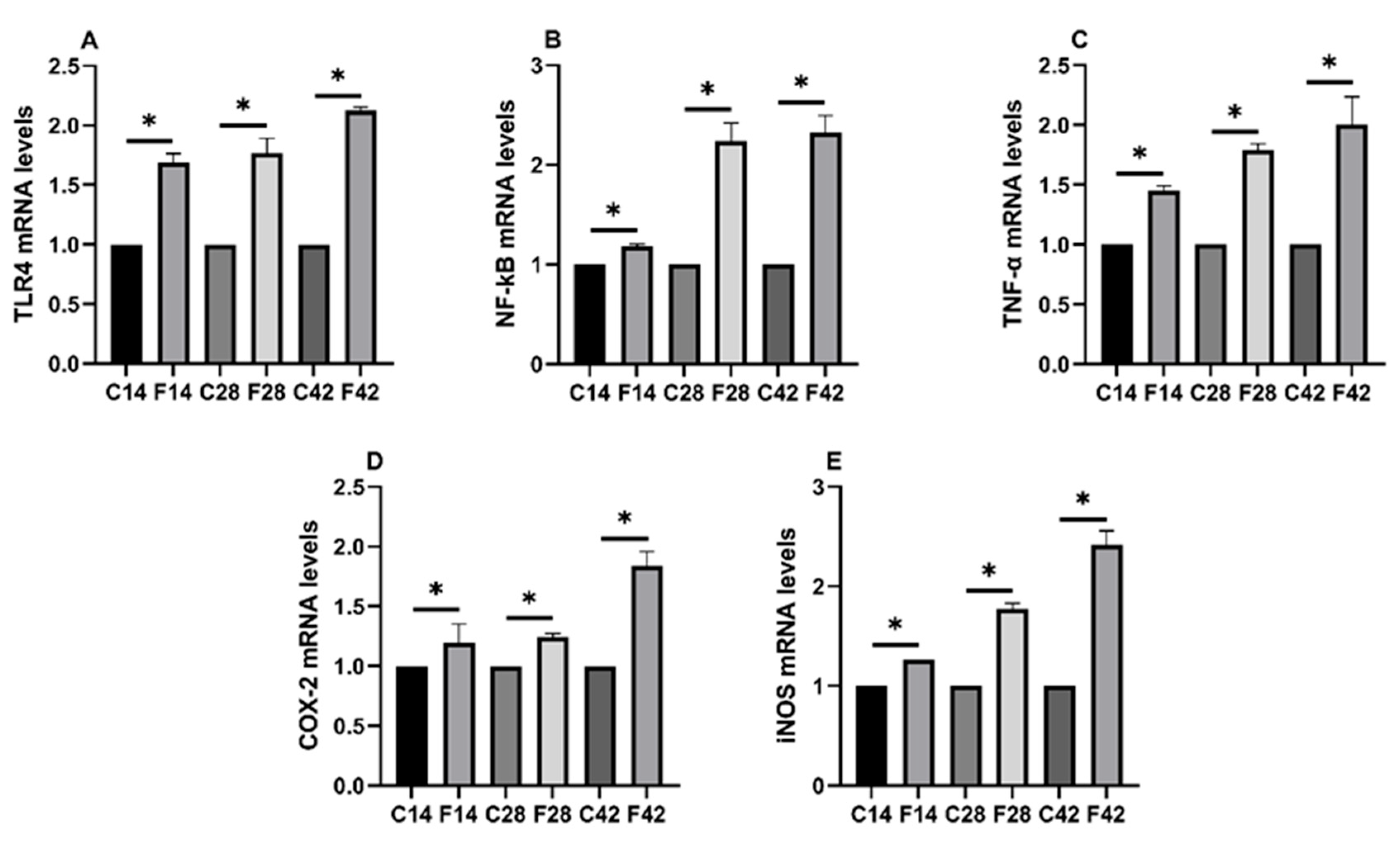
2.4. Functions of FB1 in Oxidative Stress and Inflammatory Signaling Pathways
2.5. Functions of FB1 in Heterologous Nuclear Receptors mRNA Expression and Related Subunits
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Treatments
5.2. Light Microscope Examination
5.3. Transmission Electron Microscope Examination
5.4. Real-Time Fluorescence Quantitative PCR Assay
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Jian, Q.; Chen, F.; Wang, Y.; Gong, L.; Duan, X.; Yang, B.; Jiang, Y. Influence of Butylated Hydroxyanisole on the Growth, Hyphal Morphology, and the Biosynthesis of Fumonisins in Fusarium proliferaturn. Front. Microbiol. 2016, 7, 1038. [Google Scholar]
- Frisvad, J.C.; Moller, L.L.H.; Larsen, T.O.; Kumar, R.; Arnau, J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl. Microbiol. Biotechnol. 2018, 102, 9481–9515. [Google Scholar] [CrossRef] [PubMed]
- Escriva, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Zhai, S.S.; Xia, Y.Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.W.; Zhang, H.F.; Zhang, M.H.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef]
- Proietti, M.; Perruzza, L.; Scribano, D.; Pellegrini, G.; D’Antuono, R.; Strati, F.; Raffaelli, M.; Gonzalez, S.F.; Thelen, M.; Hardt, W.D.; et al. ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat. Commun. 2019, 10, 250. [Google Scholar] [CrossRef]
- Worley, M.J. Immune evasion and persistence in enteric bacterial pathogens. Gut Microbes 2023, 15, 2163839. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Y.; Yan, D.Y.; Wang, Y.; Xu, X.; Zhao, Y.C.; Xiao, T.T. Protective Effects of Human Milk-Derived Exosomes on Intestinal Stem Cells Damaged by Oxidative Stress. Cell Transplant. 2020, 29, 963689720912690. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Dopavogui, L.; Polizzi, A.; Fougerat, A.; Gourbeyre, P.; Terciolo, C.; Klement, W.; Pinton, P.; Laffite, J.; Cossalter, A.M.; Bailly, J.D.; et al. Tissular Genomic Responses to Oral FB1 Exposure in Pigs. Toxins 2022, 14, 83. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Devriendt, B.; Gallois, M.; Verdonck, F.; Wache, Y.; Bimczok, D.; Oswald, I.P.; Goddeeris, B.M.; Cox, E. The food contaminant fumonisin B-1 reduces the maturation of porcine CD11R1(+) intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 2009, 40, 40. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, B.V.; Somashekaraiah, R.; Poornachandra Rao, K.; Deepa, N.; Dharanesha, N.K.; Girish, K.S.; Sreenivasa, M.Y. Lactobacillus plantarum MYS6 Ameliorates Fumonisin B1-Induced Hepatorenal Damage in Broilers. Front. Microbiol. 2017, 8, 2317. [Google Scholar] [CrossRef]
- Zou, Y.; Du, X.; Zheng, X.; Mao, S.; Xu, X.; Zhang, S.; Chen, X. Fumonisin B1 induces oxidative stress, inflammation and necroptosis in IPEC-J2 cells. Vet. Res. Commun. 2025, 49, 161. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.G.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Wang, H.J.; Chen, J.Y.; Liu, Y.; Wen, W.; Li, Y.H.; Huang, X.G. Lactobacillus delbrueckii Ameliorates Intestinal Integrity and Antioxidant Ability in Weaned Piglets after a Lipopolysaccharide Challenge. Oxidative Med. Cell. Longev. 2020, 2020, 6028606. [Google Scholar] [CrossRef]
- Albonico, M.; Schatz, L.F.; Caloni, F.; Cortinovis, C.; Spicer, L.J. Toxicological effects of fumonisin B-1 alone and in combination with other fusariotoxins on bovine granulosa cells. Toxicon 2016, 118, 47–53. [Google Scholar] [CrossRef]
- Chuturgoon, A.A.; Phulukdaree, A.; Moodley, D. Fumonisin B-1 modulates expression of human cytochrome P450 1b1 in human hepatoma (Hepg2) cells by repressing Mir-27b. Toxicol. Lett. 2014, 227, 50–55. [Google Scholar] [CrossRef] [PubMed]
- MartinezLarranaga, M.R.; Anadon, A.; Diaz, M.J.; Fernandez, R.; Sevil, B.; FernandezCruz, M.L.; Fernandez, M.C.; Martinez, M.A.; Anton, R. Induction of cytochrome P4501A1 and P4504A1 activities and peroxisomal proliferation by fumonisin B-1. Toxicol. Appl. Pharmacol. 1996, 141, 185–194. [Google Scholar] [CrossRef]
- Mary, V.S.; Valdehita, A.; Navas, J.M.; Rubinstein, H.R.; Fernandez-Cruz, M.L. Effects of aflatoxin B-1, fumonisin B-1 and their mixture on the aryl hydrocarbon receptor and cytochrome P450 1A induction. Food Chem. Toxicol. 2015, 75, 104–111. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Elshenawy, O.H.; Anwar-Mohamed, A.; Abdelhamid, G.; El-Kadi, A.O.S. Murine atrial HL-1 cell line is a reliable model to study drug metabolizing enzymes in the heart. Vasc. Pharmacol. 2013, 58, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Gaccioli, F.; Ramirez, V.I.; Jones, H.N.; Jansson, T.; Powell, T.L. Oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by toll-like receptor 4. J. Lipid Res. 2013, 54, 725–733. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| TLR4 | CATCCCAACCCAACCACAGTAGCA | TGAGCAGCACCAACGAGTAGTATAGC |
| NF-κB | AGCAGAACTGAGAATTTGCC | CTGAACACTATTGCGACCTG |
| TNF-α | GTGTTCTATGACCGCCCAGT | TGTTCCACGTCTTTCAGAGC |
| COX-2 | CCTATTACACAAGAAGCCTTCCACCAA | TCGCAGCAAGAATTTCTCCACAATCA |
| iNOS | TGTTATTAAGAACCAGCCCTC | CATCGGTATCTGCTTCTTGCT |
| Nrf2 | CTCGCTCCAGTCCCGCTCGTA | GTGACTTCCCAGCCCTTGTCC |
| HO-1 | ATGGAAACTTCGCAGCCACAC | CGTGACCAGCTTGAACTCGT |
| NQO-1 | TATGAGATGGAGACGGCGCA | GAAAACGCGGTCAAACCAGC |
| JNK3 | GCGAATGTCCTACCTGCTGTATCAA | CGAGTCACTACATAAGGCGTCATCAT |
| AHR | TTCAGGAAAGCAGAACAGCAA | TCACAACTAATACGAAGCCAT |
| CYP1A1 | TTGCGTGTTTATCAACCAGT | CTTTGTTCACTTCGGTCCCTT |
| CYP1A4 | ATGCTCGTTTCAGTGCCTTCGT | GTGTCAAAGCCTGCCCCAA |
| CYP1A5 | CTATGACAAGAACAGCATCCGAGACT | CCCCAAAGATGTCATTCACC |
| CAR | ACTTCACCTGCCCCTTTGCC | CCTTCCTCATCCCCACGTCCA |
| PXR | CCCTCAAGAGCTACATCGACCA | TGTTCTCCATCTTCAGCGTCT |
| CYP2C18 | AACCTCCATACGAAGCTGCAA | TGTGCCTTTGAAGACTTTCTCA |
| CYP3A4 | TCATAGTGTTGTTCCCCTT | GGTATCCTTCTTCCCGTTC |
| CYP3A9 | ATGCTCGTTTCAGTGCCTTCGT | GTGTCAAAGCCTGCCCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, C.; Hua, W.; Xian, R.; Liu, Y. Fumonisin B1 Exposure Causes Intestinal Tissue Damage by Triggering Oxidative Stress Pathways and Inducing Associated CYP Isoenzymes. Toxins 2025, 17, 239. https://doi.org/10.3390/toxins17050239
Cao C, Hua W, Xian R, Liu Y. Fumonisin B1 Exposure Causes Intestinal Tissue Damage by Triggering Oxidative Stress Pathways and Inducing Associated CYP Isoenzymes. Toxins. 2025; 17(5):239. https://doi.org/10.3390/toxins17050239
Chicago/Turabian StyleCao, Changyu, Weiping Hua, Runxi Xian, and Yang Liu. 2025. "Fumonisin B1 Exposure Causes Intestinal Tissue Damage by Triggering Oxidative Stress Pathways and Inducing Associated CYP Isoenzymes" Toxins 17, no. 5: 239. https://doi.org/10.3390/toxins17050239
APA StyleCao, C., Hua, W., Xian, R., & Liu, Y. (2025). Fumonisin B1 Exposure Causes Intestinal Tissue Damage by Triggering Oxidative Stress Pathways and Inducing Associated CYP Isoenzymes. Toxins, 17(5), 239. https://doi.org/10.3390/toxins17050239







