Consistent Killers: Conservation of Thrombin-Like Action on Fibrinogen by Bushmaster (Lachesis Species) Venoms Underpins Broad Antivenom Cross-Reactivities
Abstract
:1. Introduction
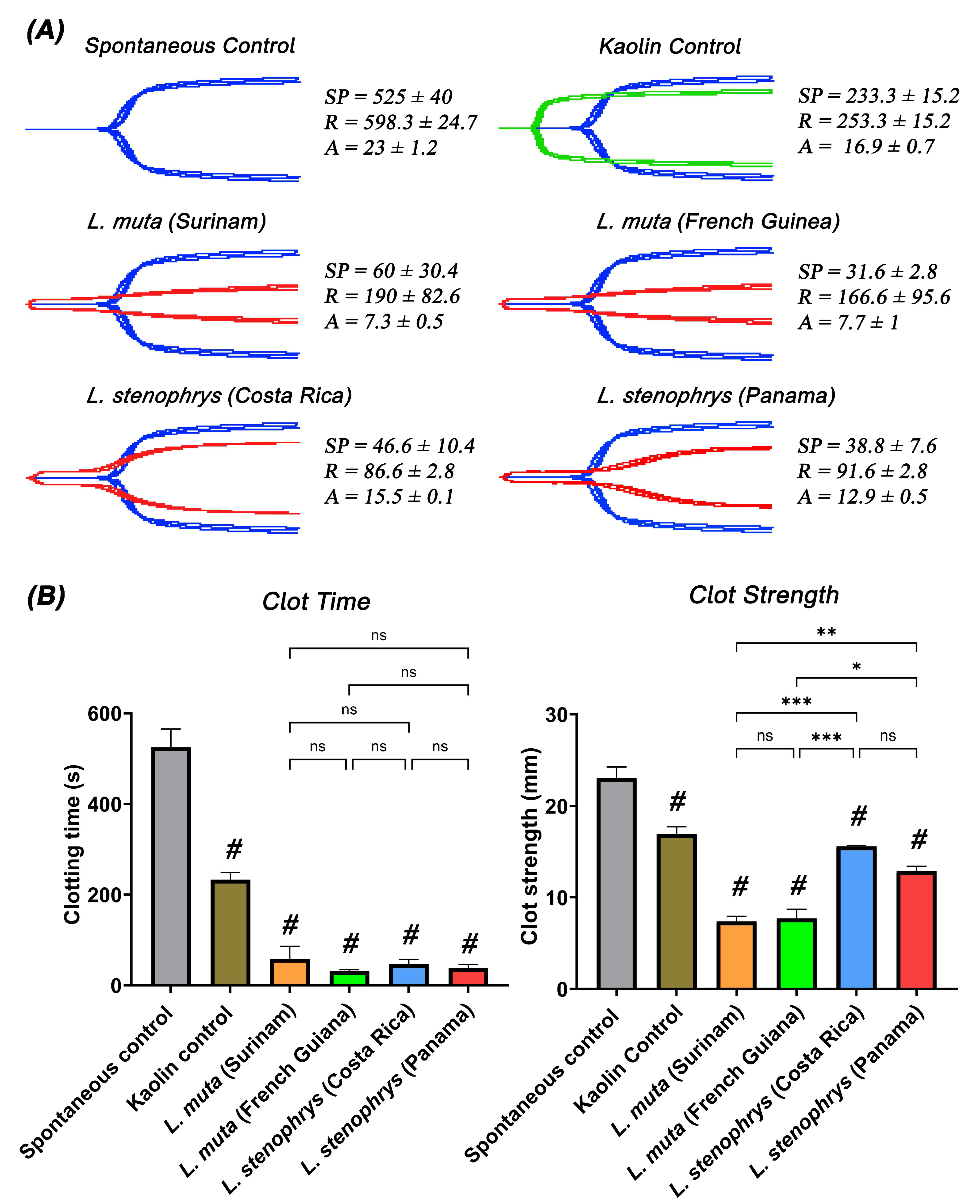
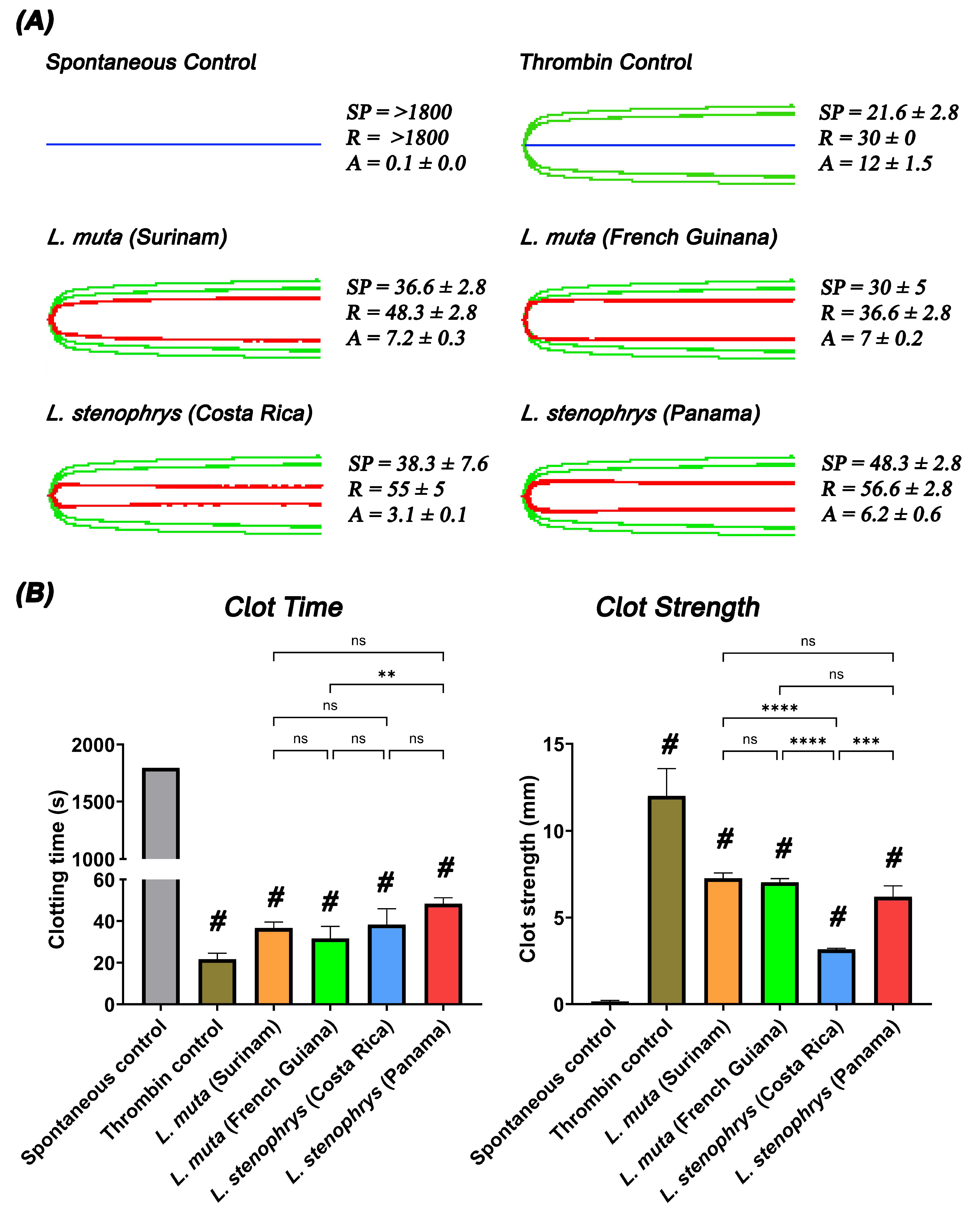
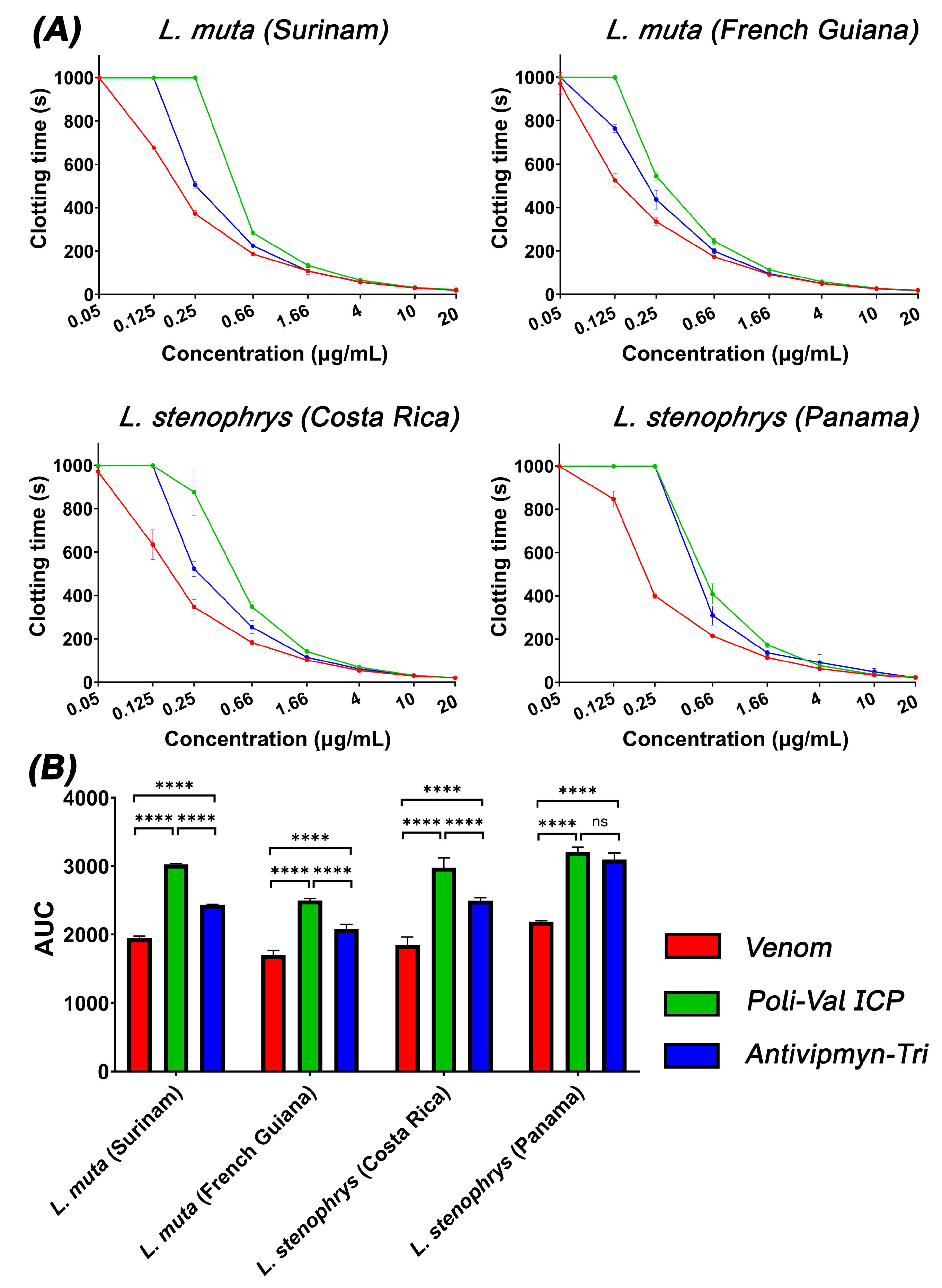
2. Results and Discussion
3. Methods
3.1. Venom, Antivenom, and Plasma
3.2. Coagulotoxicity Assays
3.3. Thromboelastography (TEG)
3.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chippaux, J.-P. Incidence and mortality due to snakebite in the Americas. PLoS Neglected Trop. Dis. 2017, 11, e0005662. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M. Snakebite envenoming in Latin America and the Caribbean. In Clinical Toxinology in Australia, Europe, and Americas; Springer: Dordrecht, The Netherlands, 2017; pp. 51–72. [Google Scholar]
- Madrigal, M.; Pla, D.; Sanz, L.; Barboza, E.; Arroyo-Portilla, C.; Corrêa-Netto, C.; Gutiérrez, J.M.; Alape-Girón, A.; Flores-Díaz, M.; Calvete, J.J. Cross-reactivity, antivenomics, and neutralization of toxic activities of Lachesis venoms by polyspecific and monospecific antivenoms. PLoS Neglected Trop. Dis. 2017, 11, e0005793. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Lomonte, B.; Pla, D.; Guerrero-Vargas, J.A.; Ayerbe-González, S.; Gutiérrez, J.M.; Sasa, M.; Calvete, J.J. Half a century of research on Bothrops asper venom variation: Biological and biomedical implications. Toxicon 2023, 221, 106983. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, S.F.V.; Peixoto, H.M.; Moura, N.; Monteiro, W.M.; de Oliveira, M.R.F. Snakebite envenomation in the Brazilian Amazon: A descriptive study. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 143–151. [Google Scholar] [CrossRef]
- Jorge, M.T.; Sano-Martins, I.S.; Tomy, S.C.; Castro, S.C.; Ferrari, R.A.; Ribeiro, L.A.; Warrell, D.A. Snakebite by the bushmaster (Lachesis muta) in Brazil: Case report and review of the literature. Toxicon 1997, 35, 545–554. [Google Scholar] [CrossRef]
- Garcês-Filho, A.Q.; Santos, H.H.; Aguiar, T.K.; Ramos, D.L.; Galan, L.E.; Dantas, D.S.; Cerni, F.A.; Carbonell, R.C.; Pucca, M.B. Severe Bushmaster Snakebite Envenoming: Case Report and Overview. Reports 2024, 7, 68. [Google Scholar] [CrossRef]
- Lima, P.H.S.d.; Haddad Junior, V. A snakebite caused by a bushmaster (Lachesis muta): Report of a confirmed case in State of Pernambuco, Brazil. Rev. Da Soc. Bras. De Med. Trop. 2015, 48, 636–637. [Google Scholar] [CrossRef]
- Sachett, J.d.A.G.; Marinho, A.P.S.; Santos, M.M.d.O.; Fan, H.W.; Bernarde, P.S.; Monteiro, W.M. When to think about a Lachesis muta envenomation in the Western Brazilian Amazon: Lessons from a case report. Rev. Da Soc. Bras. De Med. Trop. 2022, 55, e0027–2022. [Google Scholar] [CrossRef]
- Malaque, C.; Gutiérrez, J.M. Snakebite envenomation in central and South America. In Critical Care Toxicology; Springer: Cham, Switzerland, 2015; pp. 1–22. [Google Scholar]
- Pardal, P.P.d.O.; Souza, S.M.; Monteiro, M.R.d.C.d.C.; Fan, H.W.; Cardoso, J.L.C.; França, F.O.S.; Tomy, S.C.; Sano-Martins, I.S.; de Sousa-e-Silva, M.C.C.; Colombini, M. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef]
- Bolaños, R. Toxicity of Costa Rican snake venoms for the white mouse. Am. J. Trop. Med. Hyg. 1972, 21, 360–363. [Google Scholar] [CrossRef]
- Diniz-Sousa, R.; Moraes, J.d.N.; Rodrigues-da-Silva, T.M.; Oliveira, C.S.; Caldeira, C.A.d.S. A brief review on the natural history, venomics and the medical importance of bushmaster (Lachesis) pit viper snakes. Toxicon X 2020, 7, 100053. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Furtado, M.d.f.D.; Gonçalves, L.R.; Núñez, V.; García, M.E.; Osorio, R.G.; Romero, M.; Gutiérrez, J.M.a. Comparative study of the venoms of three subspecies of Lachesis muta (bushmaster) from Brazil, Colombia and Costa Rica. Toxicon 1998, 36, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.; Avila, C.; Camacho, Z.; Lomonte, B. Ontogenetic changes in the venom of the snake Lachesis muta stenophrys (bushmaster) from Costa Rica. Toxicon 1990, 28, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Escolano, J.; Ferretti, M.; Biscoglio, M.J.; Rivera, E.; Crescenti, E.J.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics of the South and Central American Bushmasters. Comparison of the toxin composition of Lachesis muta gathered from proteomic versus transcriptomic analysis. J. Proteom. 2008, 71, 46–60. [Google Scholar] [CrossRef]
- Pla, D.; Sanz, L.; Molina-Sánchez, P.; Zorita, V.; Madrigal, M.; Flores-Díaz, M.; Alape-Girón, A.; Núñez, V.; Andrés, V.; Gutiérrez, J.M. Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J. Proteom. 2013, 89, 112–123. [Google Scholar] [CrossRef]
- Madrigal, M.; Sanz, L.; Flores-Díaz, M.; Sasa, M.; Núñez, V.; Alape-Girón, A.; Calvete, J.J. Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of Lachesis stenophrys and comparative proteomics of the venoms of adult Lachesis melanocephala and Lachesis acrochorda. J. Proteom. 2012, 77, 280–297. [Google Scholar] [CrossRef]
- Magalhaes, A.; Ferreira, R.N.; Richardson, M.; Gontijo, S.; Yarleque, A.; Magalhaes, H.P.; Bloch, C.; Sanchez, E.F. Coagulant thrombin-like enzymes from the venoms of Brazilian and Peruvian bushmaster (Lachesis muta muta) snakes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 255–266. [Google Scholar] [CrossRef]
- Aragon-Ortiz, F.; Gubenšek, F. A thrombin-like enzyme from bushmaster (Lachesis muta stenophyrs) venom. Toxicon 1993, 31, 1435–1443. [Google Scholar] [CrossRef]
- Ogilvie, M.L.; Byl, J.W.; Gartner, T.K. Platelet Aggregation Is Stimulated by Lactose-lnhibitable Snake Venom Lectins. Thromb. Haemost. 1989, 62, 704–707. [Google Scholar] [CrossRef]
- Fuly, A.L.; de Miranda, A.L.P.; Zingali, R.B.; Guimarães, J.A. Purification and characterization of a phospholipase A2 isoenzyme isolated from Lachesis muta snake venom. Biochem. Pharmacol. 2002, 63, 1589–1597. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. Effects of snake venom proteins on blood platelets. Toxicon 1990, 28, 1387–1422. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Escalante, T.; Voisin, M.-B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.A.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M. Tissue localization and extracellular matrix degradation by PI, PII and PIII snake venom metalloproteinases: Clues on the mechanisms of venom-induced hemorrhage. PLoS Neglected Trop. Dis. 2015, 9, e0003731. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.F.; Costa, M.I.E.; Chavez-Olortegui, C.; Assakura, M.T.; Mandelbaum, F.R.; Diniz, C.R. Characterization of a hemorrhagic factor, LHF-I, isolated from the bushmaster snake (Lachesis muta muta) venom. Toxicon 1995, 33, 1653–1667. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Sampaio, V.S.; Salinas, J.L.; Queiroz, A.M.; da Silva, I.M.; Gomes, A.A.; Sachett, J.; Siqueira, A.M.; Ferreira, L.C.L.; Dos Santos, M.C. Older age and time to medical assistance are associated with severity and mortality of snakebites in the Brazilian Amazon: A case-control study. PLoS ONE 2015, 10, e0132237. [Google Scholar] [CrossRef]
- Angel-Camilo, K.L.; Guerrero-Vargas, J.A.; de Carvalho, E.F.; Lima-Silva, K.; de Siqueira, R.J.B.; Freitas, L.B.N.; de Sousa, J.A.C.; Mota, M.R.L.; Dos Santos, A.A.; da Costa Neves-Ferreira, A.G. Disorders on cardiovascular parameters in rats and in human blood cells caused by Lachesis acrochorda snake venom. Toxicon 2020, 184, 180–191. [Google Scholar] [CrossRef]
- Gutiérrez, J.M. Reducing the impact of snakebite envenoming in Latin America and the Caribbean: Achievements and challenges ahead. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 530–537. [Google Scholar] [CrossRef]
- Solano, G.; Gómez, A.; Corrales, G.; Chacón, D.; Estrada, R.; León, G. Contributions of the snake venoms of Bothrops asper, Crotalus simus and Lachesis stenophrys to the paraspecificity of the Central American polyspecific antivenom (PoliVal-ICP). Toxicon 2018, 144, 1–6. [Google Scholar] [CrossRef]
- Jones, L.; Lay, M.; Neri-Castro, E.; Zarzosa, V.; Hodgson, W.C.; Lewin, M.; Fry, B.G. Breaking muscle: Neurotoxic and myotoxic effects of Central American snake venoms and the relative efficacies of antivenom and varespladib. BMC Biol. 2024, 22, 243. [Google Scholar] [CrossRef]
- Jones, L.; Youngman, N.J.; Neri-Castro, E.; Guadarrama-Martínez, A.; Lewin, M.R.; Carter, R.; Frank, N.; Fry, B.G. Differential Antivenom and Small-Molecule Inhibition of Novel Coagulotoxic Variations in Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium American Viperid Snake Venoms. Toxins 2022, 14, 511. [Google Scholar] [CrossRef]
- Alfaro-Chinchilla, A.; Lomonte, B.; Zúniga, L.; Acevedo, M.; Neri-Castro, E.; Alagón, A.; Bonilla, F.; Diaz, C.; Sasa, M. Venom composition, toxicity and cross-neutralization by PoliVal-ICP antivenom, of Mesoamerican jumping pitvipers genus Metlapilcoatlus (Viperidae: Crotalinae). Trans. R. Soc. Trop. Med. Hyg. 2025, trae120. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Gutiérrez, J.M.; Sanchez, E.F.; Aldridge, M.; Fry, B.G. Pan-American lancehead pit-vipers: Coagulotoxic venom effects and antivenom neutralisation of Bothrops asper and B. atrox geographical variants. Toxins 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Otero-Patino, R.; Silva-Haad, J.J.; Barona Acevedo, M.J.; Toro Castano, M.F.; Quintana Castillo, J.C.; Diaz Cadavid, A.; Vasquez Velez, I.C.; Rodriguez Rivera, V.; Delgado Figueroa, C.I.; Fernandez Ceballos, M. Bothrops bites in Colombia: A multicenter study on the efficacy and safety of Antivipmyn-tri®, a polyvalent antivenom produced in Mexico. Iatreia 2007, 20, 244–262. [Google Scholar] [CrossRef]
- Fuchs, J.; Faber, K.; Tuchscherer, D.T.; Tsakiris, D.A.; Weiler, S.; Hofer, K.E. Bite by a juvenile Bothrops venezuelensis (Venezuelan lancehead) resulting in severe envenomation: A case report. Toxicon 2020, 180, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.; Bronner, J.; Akpunonu, P.; Plott, J.; Bailey, A.; Keyler, D. Crotalus durissus terrificus (viperidae; crotalinae) envenomation: Respiratory failure and treatment with antivipmyn TRI® antivenom. Toxicon 2019, 163, 32–35. [Google Scholar] [CrossRef]
- Larréché, S.; Bousquet, A.; Chevillard, L.; Gahoual, R.; Jourdi, G.; Dupart, A.-L.; Bachelot-Loza, C.; Gaussem, P.; Siguret, V.; Chippaux, J.-P. Bothrops atrox and Bothrops lanceolatus venoms in vitro Investigation: Composition, procoagulant effects, co-factor dependency, and correction using antivenoms. Toxins 2023, 15, 614. [Google Scholar] [CrossRef]
- Heckmann, X.; Lambert, V.; Mion, G.; Ehrhardt, A.; Marty, C.; Perotti, F.; Carod, J.-F.; Jolivet, A.; Boels, D.; Lehida Andi, I. Failure of a Mexican antivenom on recovery from snakebite-related coagulopathy in French Guiana. Clin. Toxicol. 2021, 59, 193–199. [Google Scholar] [CrossRef]
- Resiere, D.; Villalta, M.; Arias, A.S.; Kallel, H.; Nèviére, R.; Vidal, N.; Mehdaoui, H.; Gutiérrez, J.M. Snakebite envenoming in French Guiana: Assessment of the preclinical efficacy against the venom of Bothrops atrox of two polyspecific antivenoms. Toxicon 2020, 173, 1–4. [Google Scholar] [CrossRef]
- Cañas, C.A.; Castaño-Valencia, S.; Castro-Herrera, F. The Colombian bushmasters Lachesis acrochorda (García, 1896) and Lachesis muta (Linnaeus, 1766): Snake species, venoms, envenomation, and its management. Toxicon 2023, 230, 107152. [Google Scholar] [CrossRef]
- Orejuela, P.; Zavaleta, A.; Salas, M.; Marsh, N. Thrombin-like activity in snake venoms from Peruvian Bothrops and Lachesis genera. Toxicon 1991, 29, 1151–1154. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Tanaka-Azevedo, A.M.; Silveira, G.P.M.; Sant’Anna, S.S.; Grego, K.F.; Rodrigues, C.F.B.; Fry, B.G. Clinical and evolutionary implications of dynamic coagulotoxicity divergences in Bothrops (Lancehead Pit Viper) venoms. Toxins 2022, 14, 297. [Google Scholar] [CrossRef]
- Seneci, L.; Zdenek, C.N.; Rodrigues, C.F.; Chowdhury, A.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Fry, B. A Clot Twist: Potent Coagulotoxicity Found in Mexican Neotropical Rattlesnakes. Front. Immunol. 2021, 12, 552. [Google Scholar] [CrossRef]
- Campbell, J.A.; Lamar, W.W.; Brodie, E.D. The venomous Reptiles of the Western Hemisphere; Comstock Publishing Associates: Ithaca, NY, USA, 2004; Volume 2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.; Fry, B.G. Consistent Killers: Conservation of Thrombin-Like Action on Fibrinogen by Bushmaster (Lachesis Species) Venoms Underpins Broad Antivenom Cross-Reactivities. Toxins 2025, 17, 224. https://doi.org/10.3390/toxins17050224
Jones L, Fry BG. Consistent Killers: Conservation of Thrombin-Like Action on Fibrinogen by Bushmaster (Lachesis Species) Venoms Underpins Broad Antivenom Cross-Reactivities. Toxins. 2025; 17(5):224. https://doi.org/10.3390/toxins17050224
Chicago/Turabian StyleJones, Lee, and Bryan G. Fry. 2025. "Consistent Killers: Conservation of Thrombin-Like Action on Fibrinogen by Bushmaster (Lachesis Species) Venoms Underpins Broad Antivenom Cross-Reactivities" Toxins 17, no. 5: 224. https://doi.org/10.3390/toxins17050224
APA StyleJones, L., & Fry, B. G. (2025). Consistent Killers: Conservation of Thrombin-Like Action on Fibrinogen by Bushmaster (Lachesis Species) Venoms Underpins Broad Antivenom Cross-Reactivities. Toxins, 17(5), 224. https://doi.org/10.3390/toxins17050224






