Ivangustin Alleviates Deoxynivalenol-Induced Apoptosis by Regulating FOXO3a Translocation in Porcine Intestinal Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Identification of Effective Natural Product Candidates for the Mitigation of DON-Induced Cytotoxicity
2.2. Suppression of DON-Induced Apoptosis by IVAN
2.3. Effect of IVAN on DON-Regulated FOXO3a Expression
2.4. Inhibition of the DON-Mediated Translocation of FOXO3a into the Nucleus by IVAN
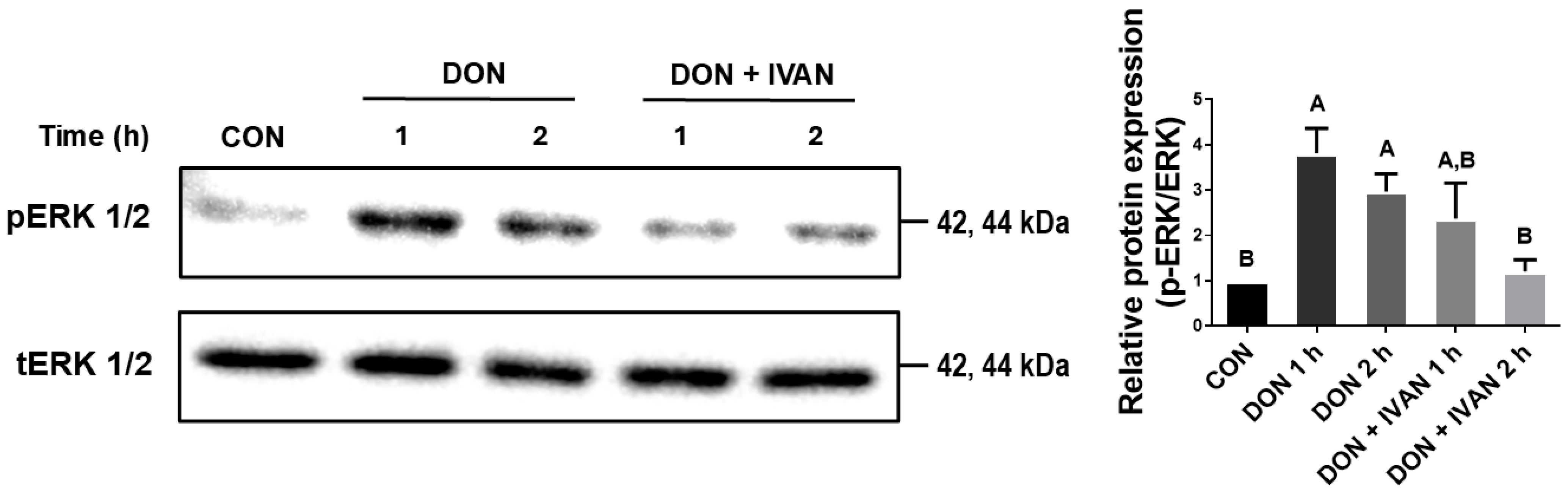
2.5. IVAN Inhibits DON-Mediated ERK1/2 Phosphorylation
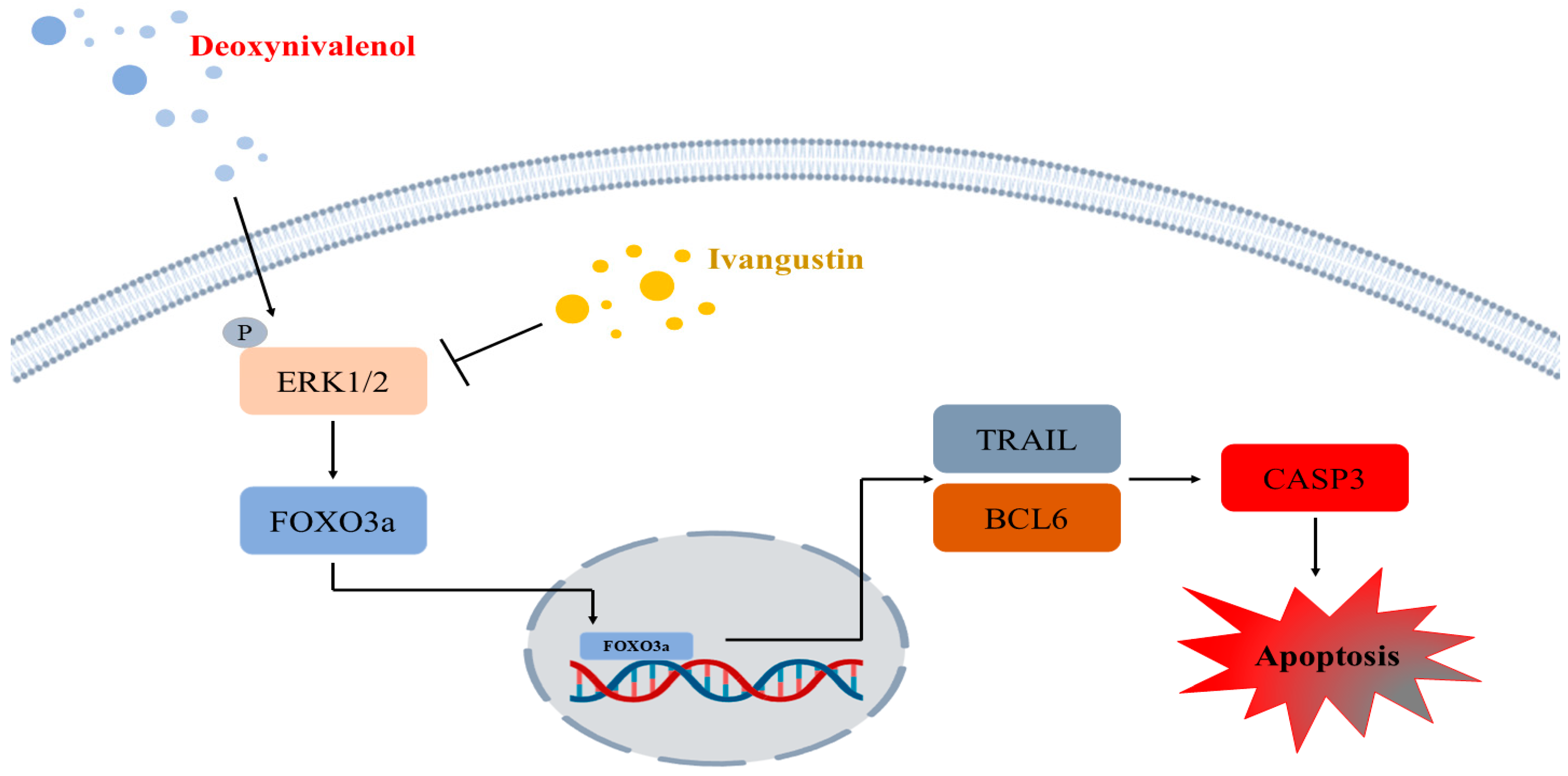
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and Treatment of Cells
5.2. Cell Viability
5.3. Annexin V and PI Staining
5.4. Extraction of Nuclear and Cytoplasmic Proteins
5.5. Real-Time Quantitative PCR and Western Blot Assay
5.6. Immunofluorescence Staining
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCL6 | B-cell lymphoma 6 |
| CASP3 | Caspase 3 |
| DON | Deoxynivalenol |
| FOXO | Forkhead box O |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| PI | Propidium iodide |
References
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk And Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Diesing, A.K.; Nossol, C.; Panther, P.; Walk, N.; Post, A.; Kluess, J.; Kreutzmann, P.; Danicke, S.; Rothkotter, H.J.; Kahlert, S. Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicol. Lett. 2011, 200, 8–18. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Mu, P.Q.; Lin, R.Q.; Wen, J.K.; Deng, Y.Q. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.E.; Song, J.; Lee, H.J.; Kim, M.; Kim, D.W.; Jung, H.J.; Kim, B.; Lee, Y.; Yu, D.; Kim, D.W.; et al. Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs. Toxins 2018, 10, 114. [Google Scholar] [CrossRef]
- Van de Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.X.; Zhang, L.; Shi, L.; Dai, J.F.; Han, Y.M.; Wu, Y.Y.; Khalil, M.M.; Sun, L.H. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Durmic, Z.; Blache, D. Bioactive plants and plant products: Effects on animal function, health and welfare. Anim. Feed Sci. Technol. 2012, 176, 150–162. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Liu, W.C.; Kim, I.H. Application of natural bioactive compounds in animal nutrition. Front. Vet. Sci. 2023, 10, 1204490. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Turner, P.C.; Co, V.A.; Wang, M.F.; Amiri, K.M.A.; El-Nezami, H. Schisandrin A protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 2019, 9, 19173. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Paszti, E.A.; Babiczky, A.; Szoladi, A.; Jerzsele, A.; Gere, E.P. Beneficial Effects of Rosmarinic Acid on IPEC-J2 Cells Exposed to the Combination of Deoxynivalenol and T-2 Toxin. Mediat. Inflamm. 2020, 2020, 8880651. [Google Scholar] [CrossRef]
- Salih, D.A.M.; Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008, 20, 126–136. [Google Scholar] [CrossRef]
- Lu, D.Y.; Liu, J.P.; Jiao, J.Q.; Long, B.; Li, Q.; Tan, W.Q.; Li, P.F. Transcription Factor Foxo3a Prevents Apoptosis by Regulating Calcium through the Apoptosis Repressor with Caspase Recruitment Domain. J. Biol. Chem. 2013, 288, 8491–8504. [Google Scholar] [CrossRef]
- Zhang, X.B.; Tang, N.M.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. BBA-Mol. Cell Res. 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Charununtakorn, S.T.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. Potential Roles of Humanin on Apoptosis in the Heart. Cardiovasc. Ther. 2016, 34, 107–114. [Google Scholar] [CrossRef]
- Rami, M.S. Apoptosis and pathological process. Likars’ka Spr. 2007, 8, 68–70. [Google Scholar]
- Gunther, C.; Neumann, H.; Neurath, M.F.; Becker, C. Apoptosis, necrosis and necroptosis: Cell death regulation in the intestinal epithelium. Gut 2013, 62, 1062–1071. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, I.H. Eckol Alleviates Intestinal Dysfunction during Suckling-to-Weaning Transition via Modulation of PDX1 and HBEGF. Int. J. Mol. Sci. 2020, 21, 4755. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Kang, R.F.; Li, R.N.; Dai, P.Y.; Li, Z.J.; Li, Y.S.; Li, C.M. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, W.; Gu, X.; Chang, C.; Wu, J. Repression of deoxynivalenol-triggered cytotoxicity and apoptosis by mannan/beta-glucans from yeast cell wall: Involvement of autophagy and PI3K-AKT-mTOR signaling pathway. Int. J. Biol. Macromol. 2020, 164, 1413–1421. [Google Scholar] [CrossRef]
- Gu, X.L.; Guo, W.Y.; Zhao, Y.J.; Liu, G.; Wu, J.N.; Chang, C. Deoxynivalenol-Induced Cytotoxicity and Apoptosis in IPEC-J2 Cells Through the Activation of Autophagy by Inhibiting PI3K-AKT-mTOR Signaling Pathway. ACS Omega 2019, 4, 18478–18486. [Google Scholar] [CrossRef]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. In Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of Deoxynivalenol and Its Acetylated Derivatives on the Intestine: Differential Effects on Morphology, Barrier Function, Tight Junction Proteins, and Mitogen-Activated Protein Kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Wang, J.M.; Jin, Y.C.; Wu, S.L.; Yu, H.; Zhao, Y.; Fang, H.T.; Shen, J.L.; Zhou, C.H.; Fu, Y.R.; Li, R.H.; et al. Deoxynivalenol induces oxidative stress, inflammatory response and apoptosis in bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1663–1674. [Google Scholar] [CrossRef]
- Kang, T.H.; Kang, K.S.; Lee, S.I. Deoxynivalenol Induces Apoptosis via FOXO3a-Signaling Pathway in Small-Intestinal Cells in Pig. Toxics 2022, 10, 539. [Google Scholar] [CrossRef]
- Reddy, P.R.K.; Elghandour, M.M.M.Y.; Salem, A.Z.M.; Yasaswini, D.; Reddy, P.P.R.; Reddy, A.N.; Hyder, I. Plant secondary metabolites as feed additives in calves for antimicrobial stewardship. Anim. Feed Sci. Technol. 2020, 264, 114469. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Pitino, R.; Simoni, M.; Mavrommatis, A.; De Marchi, M.; Righi, F.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Livestock Mammals’ Performances, Health, and Oxidative Status: A Review of the Literature in the Last 20 Years. Antioxidants 2021, 10, 1461. [Google Scholar] [CrossRef]
- Qi, X.X.; Chen, B.C.; Rao, J.J. Natural compounds of plant origin in the control of fungi and mycotoxins in foods. Curr. Opin. Food Sci. 2023, 52, 101054. [Google Scholar] [CrossRef]
- Yang, X.; Su, J.; He, Y.; Liu, H.; Li, H.; Zhang, W. Simultaneous determination of three sesquiterpene lactones from Herba Inula extract in rat plasma by LC/MS/MS and its application to pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 903, 40–45. [Google Scholar] [CrossRef]
- Tang, J.J.; He, Q.R.; Dong, S.; Guo, X.; Wang, Y.G.; Lei, B.L.; Tian, J.M.; Gao, J.M. Diversity Modification and Structure-Activity Relationships of Two Natural Products 1beta-hydroxy Alantolactone and Ivangustin as Potent Cytotoxic Agents. Sci. Rep. 2018, 8, 1722. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Sun, B.; Chen, H.; Wang, X.; Chen, T. Regorafenib induces Bim-mediated intrinsic apoptosis by blocking AKT-mediated FOXO3a nuclear export. Cell Death Discov. 2023, 9, 37. [Google Scholar] [CrossRef]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013, 5, 171ra117. [Google Scholar] [CrossRef]
- Das, T.P.; Suman, S.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis 2016, 7, e2111. [Google Scholar] [CrossRef]
- Fu, Z.; Tindall, D.J. FOXOs, cancer and regulation of apoptosis. Oncogene 2008, 27, 2312–2319. [Google Scholar] [CrossRef]




| Material Name | Chemical Formula | Molecular Weight | Cell Viability (%) |
|---|---|---|---|
| Procyanidin B3 | C30H26O12 | 578.52 | 76 |
| Bavachromanol | C20H20O5 | 340.37 | 74 |
| Ivangustin | C15H20O3 | 248.32 | 72 |
| Santamarine | C15H20O3 | 248.14 | 71 |
| (+)-Catechin | C15H14O6 | 292 | 70 |
| Coniferaldehyde | C10H10O3 | 178.06 | 70 |
| Lucidine primeveroside | C26H28O14 | 564.49 | 69 |
| 2-Stearo-1,3-dilinolein | C53H102O6 | 874.76 | 68 |
| Xanthalongin | C15H20O3 | 248.32 | 67 |
| Fargesin | C21H22O6 | 370.4 | 65 |
| 8,8′-Bieckol | C36H22O18 | 742 | 65 |
| Dauricumine | C19H24CINO6 | 397.87 | 65 |
| trans-(R)-Resveratrol | C14H12O3 | 228.08 | 65 |
| (+)-Catechin-7-O-β-D-apio furanoside | C20H22O10 | 422 | 64 |
| 6,9-Epi-8-O-acetylshanziside methyl ester | C19H28O12 | 448 | 63 |
| (2S)-4′,6-Dihydroxy-7-methoxyflavan | C16H16O4 | 272.29 | 63 |
| Astragaloside I | C45H72O16 | 868.48 | 63 |
| Chiratenol | C30H50O | 426.73 | 63 |
| Hispidin | C13H10O5 | 246.22 | 63 |
| 24-Methylenecycloartanol | C31H52O | 440.4 | 63 |
| Glucoaurantio-obtusin | C23H24O12 | 492.43 | 62 |
| Euphorbia Factor L2 | C38H42O9 | 642 | 62 |
| Spirobenzofuran | C15H18O4 | 262 | 62 |
| Bavacoumestan C | C20H16O7 | 368.34 | 62 |
| Kaempferol | C15H10O6 | 286.2 | 62 |
| Benzoylpaeoniflorin | C30H32O12 | 584.57 | 61 |
| 4′-Hydroxydehydrokawain | C14H12O4 | 244 | 61 |
| 6,6′-Bieckol | C36H22O18 | 742 | 61 |
| 2-Benzyl-2,3′,4′,6-tetrahydroxybenzo[b]furan-3(2H)-one | C15H12O6 | 288.26 | 61 |
| Germacrone epoxide | C15H22O2 | 234 | 60 |
| (2S)-4′, 6-Dihydroxy-7-methoxyflavanone | C16H14O5 | 286.27 | 60 |
| 3-epi-Oleanolic acid | C30H48O3 | 456.7 | 60 |
| 5,5′-Dihydroxy-7,8-dimethoxyflavanone-2-O-b-D-glucopyranoside | C23H26O12 | 494.14 | 60 |
| Apiopaeonoside | C20H28O12 | 460 | 60 |
| Astragaloside III | C41H68O14 | 784.46 | 60 |
| Cimifugin | C16H18O6 | 306.31 | 60 |
| 8-O-acetyl-harpagide | C17H26O11 | 406.39 | 59 |
| Sophoraflavanone G (vexibinol) | C25H28O6 | 424.18 | 59 |
| Germacrone | C15H22O | 218 | 59 |
| Brazilein | C16H12O5 | 284.26 | 59 |
| 2-(3-Hydroxy-2-oxoindolin-3-yl) acetic acid | C10H9NO4 | 207 | 59 |
| Kalopanaxsaponin C | C65H106O31 | 1382.67 | 58 |
| Hederacholichiside F | C65H106O31 | 1383.52 | 58 |
| Ferulic acid | C10H10O4 | 194.18 | 57 |
| Broussonin A | C16H18O3 | 258.31 | 57 |
| Formononetin | C16H12O4 | 268.26 | 57 |
| Senkyunolide A | C12H16O2 | 192.25 | 57 |
| Paeoniflorin | C23H28O11 | 480.46 | 56 |
| Atractylenolide III | C15H20O3 | 248.14 | 56 |
| β-Sitosterol | C29H50O | 414.71 | 55 |
| Gene | Description | Accession No. | Sequence (5′-3′) | |
|---|---|---|---|---|
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand | NM_001024696 | Forward | GCA GAC CTG TGT GTT GAT CC |
| Reverse | GGG ATC CCA GAA ACT GTC AT | |||
| BCL6 | B-cell CLL/lymphoma 6 | XM_005657112 | Forward | GTG TCC TAC GGT GCC TTT TT |
| Reverse | TGA CGC AGA ATG TGA TGA GA | |||
| FOXO3 | Fork headbox O3 | NM_001135959 | Forward | TCA GCC AGT CTA TGC AAA CC |
| Reverse | CCA TGA GTT CGC TAC GGA TA | |||
| CASP3 | Caspase 3 | NM_214131 | Forward | CTC AGG GAG ACC TTC ACA AC |
| Reverse | GCA CGC AAA TAA AAC TGC TC | |||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001206359 | Forward | ACA CCG AGC ATC TCC TGA CT |
| Reverse | GAC GAG GCA GGT CTC CCT AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.H.; Shin, S.S.; Kim, T.H.; Lee, S.I. Ivangustin Alleviates Deoxynivalenol-Induced Apoptosis by Regulating FOXO3a Translocation in Porcine Intestinal Epithelial Cells. Toxins 2025, 17, 174. https://doi.org/10.3390/toxins17040174
Kang TH, Shin SS, Kim TH, Lee SI. Ivangustin Alleviates Deoxynivalenol-Induced Apoptosis by Regulating FOXO3a Translocation in Porcine Intestinal Epithelial Cells. Toxins. 2025; 17(4):174. https://doi.org/10.3390/toxins17040174
Chicago/Turabian StyleKang, Tae Hong, Sang Su Shin, Tae Hyun Kim, and Sang In Lee. 2025. "Ivangustin Alleviates Deoxynivalenol-Induced Apoptosis by Regulating FOXO3a Translocation in Porcine Intestinal Epithelial Cells" Toxins 17, no. 4: 174. https://doi.org/10.3390/toxins17040174
APA StyleKang, T. H., Shin, S. S., Kim, T. H., & Lee, S. I. (2025). Ivangustin Alleviates Deoxynivalenol-Induced Apoptosis by Regulating FOXO3a Translocation in Porcine Intestinal Epithelial Cells. Toxins, 17(4), 174. https://doi.org/10.3390/toxins17040174




