Exaiptasia pallida Infection Model Reveals the Critical Role of Vibrio parahaemolyticus T3SS Virulence Factors in Its Pathogenicity for Sea Anemones
Abstract
1. Introduction
2. Results
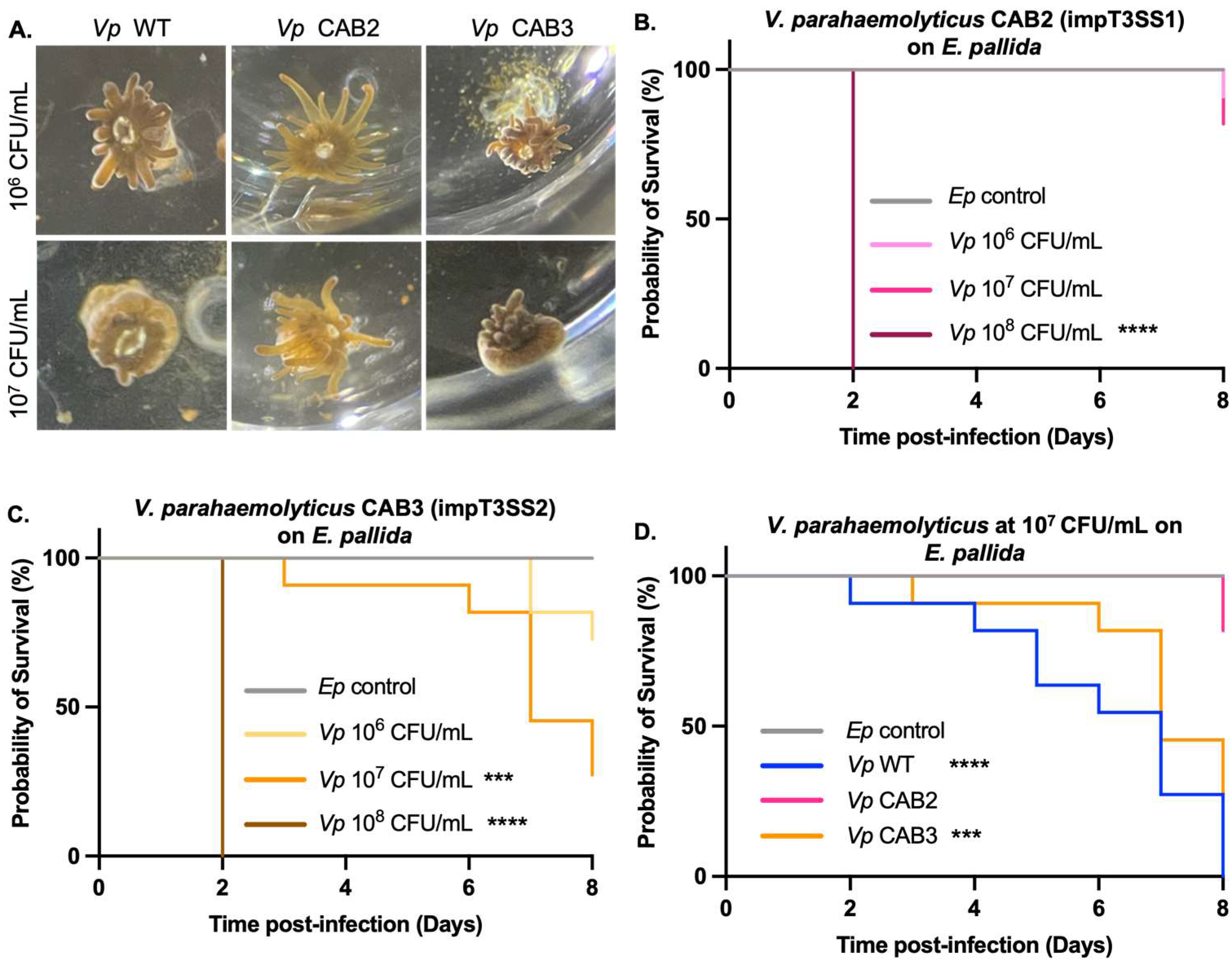
2.1. E. pallida Sensitivity to V. parahaemolyticus
2.2. T3SS1 Involvement in E. pallida’s Mortality
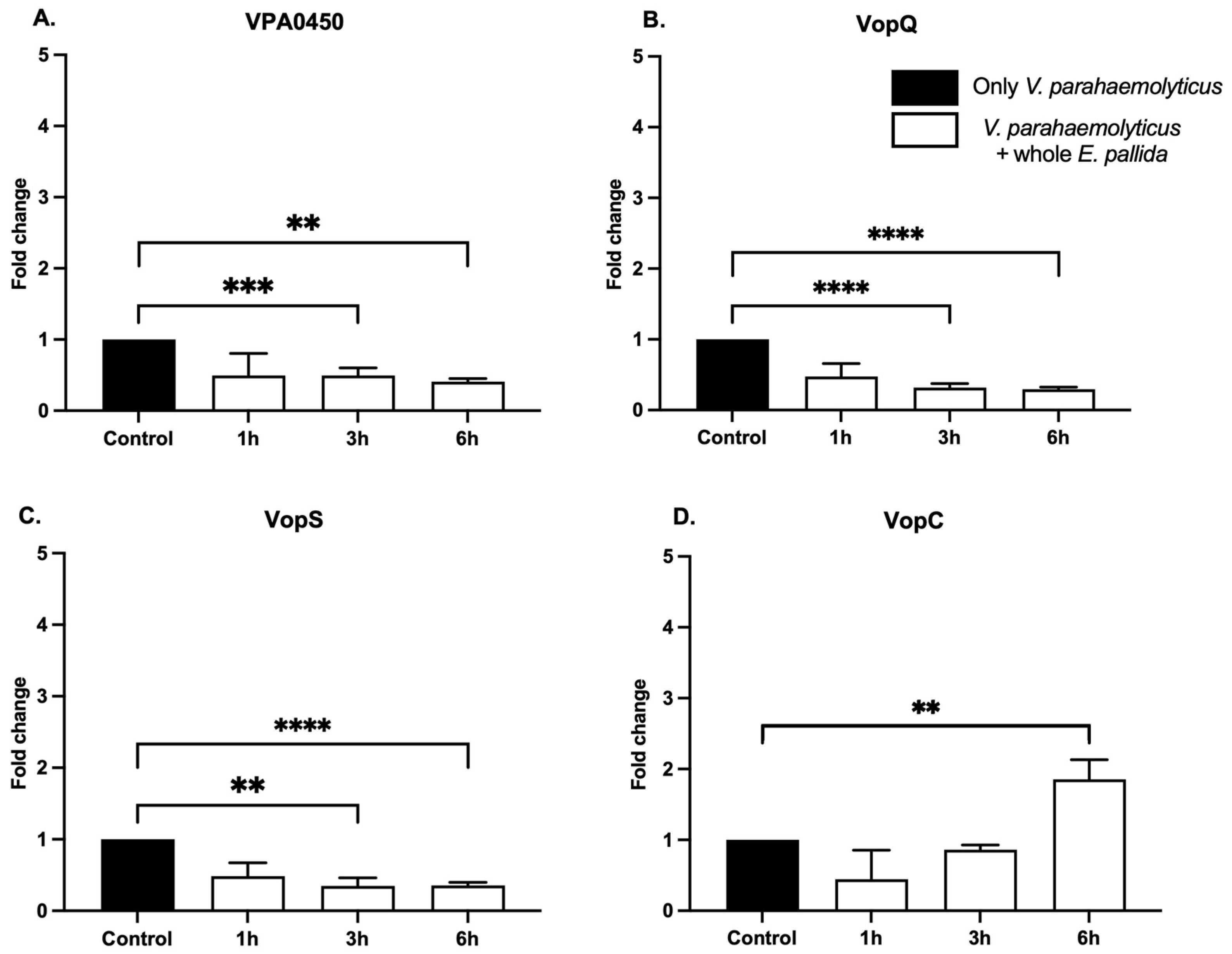
2.3. Expression of Bacterial Effectors in Presence of E. pallida
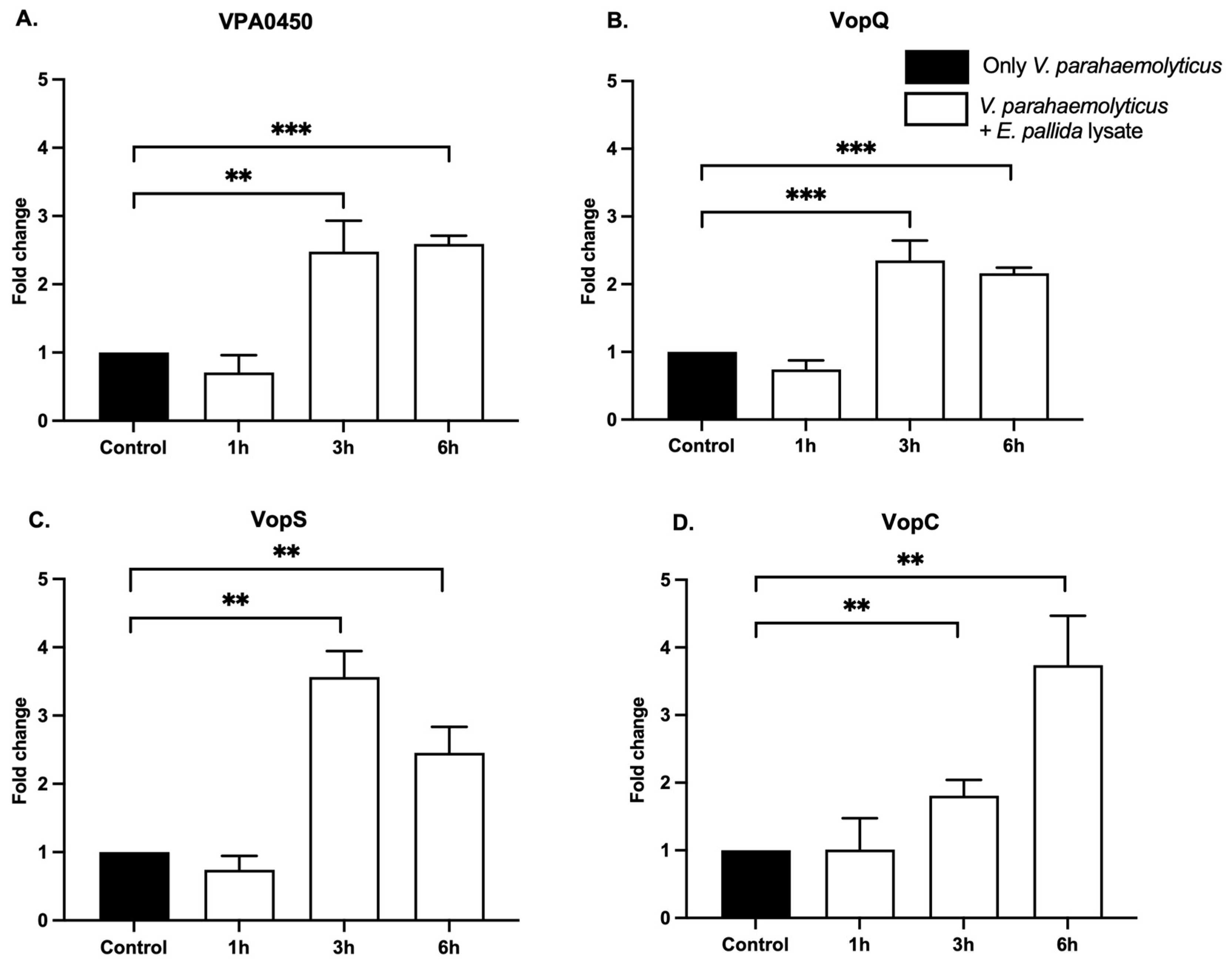
2.4. Effectors of Gene Expression Induction via Molecular Cues
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Exaiptasia pallida Anemone Husbandry
5.2. Bacterial Strain and Culture Preparation
5.3. Infection Experiments and Mortality Assays
5.4. Production of E. pallida Lysate
5.5. V. parahaemolyticus Virulence Stimulation
5.6. RNA Extraction
5.7. Reverse Transcription and Quantitative Polymerase Chain Reaction (RT-qPCR)
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velazquez-Roman, J.; León-Sicairos, N.; de Jesus Hernández-Díaz, L.; Canizalez-Roman, A. Pandemic Vibrio Parahaemolyticus O3:K6 on the American Continent. Front. Cell. Infect. Microbiol. 2014, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Vibrio Parahaemolyticus: A Review on the Pathogenesis, Prevalence, and Advance Molecular Identification Techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Baker-Austin, C. Vibrio Parahaemolyticus. Trends Microbiol. 2020, 28, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urtaza, J.; Baker-Austin, C.; Jones, J.L.; Newton, A.E.; Gonzalez-Aviles, G.D.; DePaola, A. Spread of Pacific Northwest Vibrio Parahaemolyticus Strain. N. Engl. J. Med. 2013, 369, 1573–1574. [Google Scholar] [CrossRef]
- Caburlotto, G.; Lleò, M.M.; Hilton, T.; Huq, A.; Colwell, R.R.; Kaper, J.B. Effect on Human Cells of Environmental Vibrio Parahaemolyticus Strains Carrying Type III Secretion System 2. Infect. Immun. 2010, 78, 3280–3287. [Google Scholar] [CrossRef]
- Fu, S.; Wang, L.; Tian, H.; Wei, D.; Liu, Y. Pathogenicity and Genomic Characterization of Vibrio Parahaemolyticus Strain PB1937 Causing Shrimp Acute Hepatopancreatic Necrosis Disease in China. Ann. Microbiol. 2018, 68, 175–184. [Google Scholar] [CrossRef]
- Ansar, N.M.S.; Ijong, F.G.; Tanod, W.A.; Cahyono, E.; Pumpente, O.I.; Palawe, J.F.P. Growth Characteristics of Vibrio Parahaemolyticus Isolated from Lobster (Panulirus sp.) Under Different Temperatures, pH, and NaCl Concentrations. Bioma Berk. Ilm. Biol. 2023, 25, 74–87. [Google Scholar] [CrossRef]
- Nithyanand, P.; Pandian, S.K. Phylogenetic Characterization of Culturable Bacterial Diversity Associated with the Mucus and Tissue of the Coral Acropora Digitifera from the Gulf of Mannar. FEMS Microbiol. Ecol. 2009, 69, 384–394. [Google Scholar] [CrossRef]
- Kemp, K.M.; Westrich, J.R.; Alabady, M.S.; Edwards, M.L.; Lipp, E.K. Abundance and Multilocus Sequence Analysis of Vibrio Bacteria Associated with Diseased Elkhorn Coral (Acropora Palmata) of the Florida Keys. Appl. Environ. Microbiol. 2018, 84, e01035-17. [Google Scholar] [CrossRef]
- CDC. About Vibrio Infection. Vibrio Infection (Vibriosis). Available online: https://www.cdc.gov/vibrio/about/index.html (accessed on 8 February 2025).
- Yang, Y.; Zhu, X.; Zhang, H.; Chen, Y.; Liu, Y.; Song, Y.; Ai, X. Vibrio Cholerae Was Found in Cultured Bullfrog. Epidemiol. Infect. 2022, 150, e30. [Google Scholar] [CrossRef]
- Meza, M.; Majrshi, H.; Tiong, H.K. Recovery of Pasteurization-Resistant Vibrio Parahaemolyticus from Seafoods Using a Modified, Two-Step Enrichment. Foods 2022, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Raszl, S.M.; Froelich, B.A.; Vieira, C.R.W.; Blackwood, A.D.; Noble, R.T. Vibrio Parahaemolyticus and Vibrio Vulnificus in South America: Water, Seafood and Human Infections. J. Appl. Microbiol. 2016, 121, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, P. Roles of Thermostable Direct Hemolysin (TDH) and TDH-Related Hemolysin (TRH) in Vibrio Parahaemolyticus. Front. Microbiol. 2015, 5, 805. [Google Scholar] [CrossRef]
- Cervino, J.M.; Hayes, R.L.; Polson, S.W.; Polson, S.C.; Goreau, T.J.; Martinez, R.J.; Smith, G.W. Relationship of Vibrio Species Infection and Elevated Temperatures to Yellow Blotch/Band Disease in Caribbean Corals. Appl. Environ. Microbiol. 2004, 70, 6855–6864. [Google Scholar] [CrossRef]
- Billaud, M.; Seneca, F.; Tambutté, E.; Czerucka, D. An Increase of Seawater Temperature Upregulates the Expression of Vibrio Parahaemolyticus Virulence Factors Implicated in Adhesion and Biofilm Formation. Front. Microbiol. 2022, 13, 840628. [Google Scholar] [CrossRef]
- Pootakham, W.; Mhuantong, W.; Yoocha, T.; Putchim, L.; Sonthirod, C.; Naktang, C.; Thongtham, N.; Tangphatsornruang, S. High Resolution Profiling of Coral-Associated Bacterial Communities Using Full-Length 16S rRNA Sequence Data from PacBio SMRT Sequencing System. Sci. Rep. 2017, 7, 2774. [Google Scholar] [CrossRef]
- Sussman, M.; Willis, B.L.; Victor, S.; Bourne, D.G. Coral Pathogens Identified for White Syndrome (WS) Epizootics in the Indo-Pacific. PLoS ONE 2008, 3, e2393. [Google Scholar] [CrossRef]
- Ziegler, M.; Seneca, F.O.; Yum, L.K.; Palumbi, S.R.; Voolstra, C.R. Bacterial Community Dynamics Are Linked to Patterns of Coral Heat Tolerance. Nat. Commun. 2017, 8, 14213. [Google Scholar] [CrossRef]
- Seneca, F.; Davtian, D.; Boyer, L.; Czerucka, D. Gene Expression Kinetics of Exaiptasia Pallida Innate Immune Response to Vibrio Parahaemolyticus Infection. BMC Genom. 2020, 21, 768. [Google Scholar] [CrossRef]
- Broberg, C.A.; Calder, T.J.; Orth, K. Vibrio Parahaemolyticus Cell Biology and Pathogenicity Determinants. Microbes Infect. 2011, 13, 992–1001. [Google Scholar] [CrossRef]
- Zhang, L.; Krachler, A.M.; Broberg, C.A.; Li, Y.; Mirzaei, H.; Gilpin, C.J.; Orth, K. Type III Effector VopC Mediates Invasion for Vibrio Species. Cell Rep. 2012, 1, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and Experimental Evidence of Vibrio Parahaemolyticus as the Causative Agent of Acute Hepatopancreatic Necrosis Disease of Cultured Shrimp (Litopenaeus Vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef]
- Praja, R.K.; Safnurbaiti, D.P. The Infection of Vibrio Parahaemolyticus in Shrimp and Human. Ocean. Biomed. J. 2018, 1, 44–58. [Google Scholar] [CrossRef]
- Broberg, C.A.; Zhang, L.; Gonzalez, H.; Laskowski-Arce, M.A.; Orth, K. A Vibrio Effector Protein Is an Inositol Phosphatase and Disrupts Host Cell Membrane Integrity. Science 2010, 329, 1660–1662. [Google Scholar] [CrossRef]
- Luong, P.; Kinch, L.N.; Brautigam, C.A.; Grishin, N.V.; Tomchick, D.R.; Orth, K. Kinetic and Structural Insights into the Mechanism of AMPylation by VopS Fic Domain. J. Biol. Chem. 2010, 285, 20155–20163. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.Q.; Shimohata, T.; Hatayama, S.; Tentaku, A.; Kido, J.; Bui, T.M.H.; Uebanso, T.; Mawatari, K.; Takahashi, A. Type III Secretion Effector VopQ of Vibrio Parahaemolyticus Modulates Central Carbon Metabolism in Epithelial Cells. mSphere 2020, 5, e00960-19. [Google Scholar] [CrossRef]
- Okada, K.; Na-Ubol, M.; Natakuathung, W.; Roobthaisong, A.; Maruyama, F.; Nakagawa, I.; Chantaroj, S.; Hamada, S. Comparative Genomic Characterization of a Thailand–Myanmar Isolate, MS6, of Vibrio Cholerae O1 El Tor, Which Is Phylogenetically Related to a “US Gulf Coast” Clone. PLoS ONE 2014, 9, e98120. [Google Scholar] [CrossRef]
- Yang, H.; de Souza Santos, M.; Lee, J.; Law, H.T.; Chimalapati, S.; Verdu, E.F.; Orth, K.; Vallance, B.A. A Novel Mouse Model of Enteric Vibrio Parahaemolyticus Infection Reveals That the Type III Secretion System 2 Effector VopC Plays a Key Role in Tissue Invasion and Gastroenteritis. mBio 2019, 10, e02608-19. [Google Scholar] [CrossRef]
- Garren, M.; Son, K.; Raina, J.-B.; Rusconi, R.; Menolascina, F.; Shapiro, O.H.; Tout, J.; Bourne, D.G.; Seymour, J.R.; Stocker, R. A Bacterial Pathogen Uses Dimethylsulfoniopropionate as a Cue to Target Heat-Stressed Corals. ISME J. 2014, 8, 999–1007. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Sun, X.-H.; Xu, X.-Y.; Zhao, Y.; Pan, Y.-J.; Hwang, C.-A.; Wu, V.C.H. Investigation of Reference Genes in Vibrio Parahaemolyticus for Gene Expression Analysis Using Quantitative RT-PCR. PLoS ONE 2015, 10, e0144362. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, A.; Bonnet, E.; Soone, A.; Boyer, L.; Seneca, F. Exaiptasia pallida Infection Model Reveals the Critical Role of Vibrio parahaemolyticus T3SS Virulence Factors in Its Pathogenicity for Sea Anemones. Toxins 2025, 17, 175. https://doi.org/10.3390/toxins17040175
Perrone A, Bonnet E, Soone A, Boyer L, Seneca F. Exaiptasia pallida Infection Model Reveals the Critical Role of Vibrio parahaemolyticus T3SS Virulence Factors in Its Pathogenicity for Sea Anemones. Toxins. 2025; 17(4):175. https://doi.org/10.3390/toxins17040175
Chicago/Turabian StylePerrone, Alexandre, Estelle Bonnet, Anna Soone, Laurent Boyer, and Francois Seneca. 2025. "Exaiptasia pallida Infection Model Reveals the Critical Role of Vibrio parahaemolyticus T3SS Virulence Factors in Its Pathogenicity for Sea Anemones" Toxins 17, no. 4: 175. https://doi.org/10.3390/toxins17040175
APA StylePerrone, A., Bonnet, E., Soone, A., Boyer, L., & Seneca, F. (2025). Exaiptasia pallida Infection Model Reveals the Critical Role of Vibrio parahaemolyticus T3SS Virulence Factors in Its Pathogenicity for Sea Anemones. Toxins, 17(4), 175. https://doi.org/10.3390/toxins17040175





