Nafamostat Mesylate Regulates Glycosylation to Alleviate Aristolochic Acid Induced Kidney Injury
Abstract
1. Introduction
2. Results
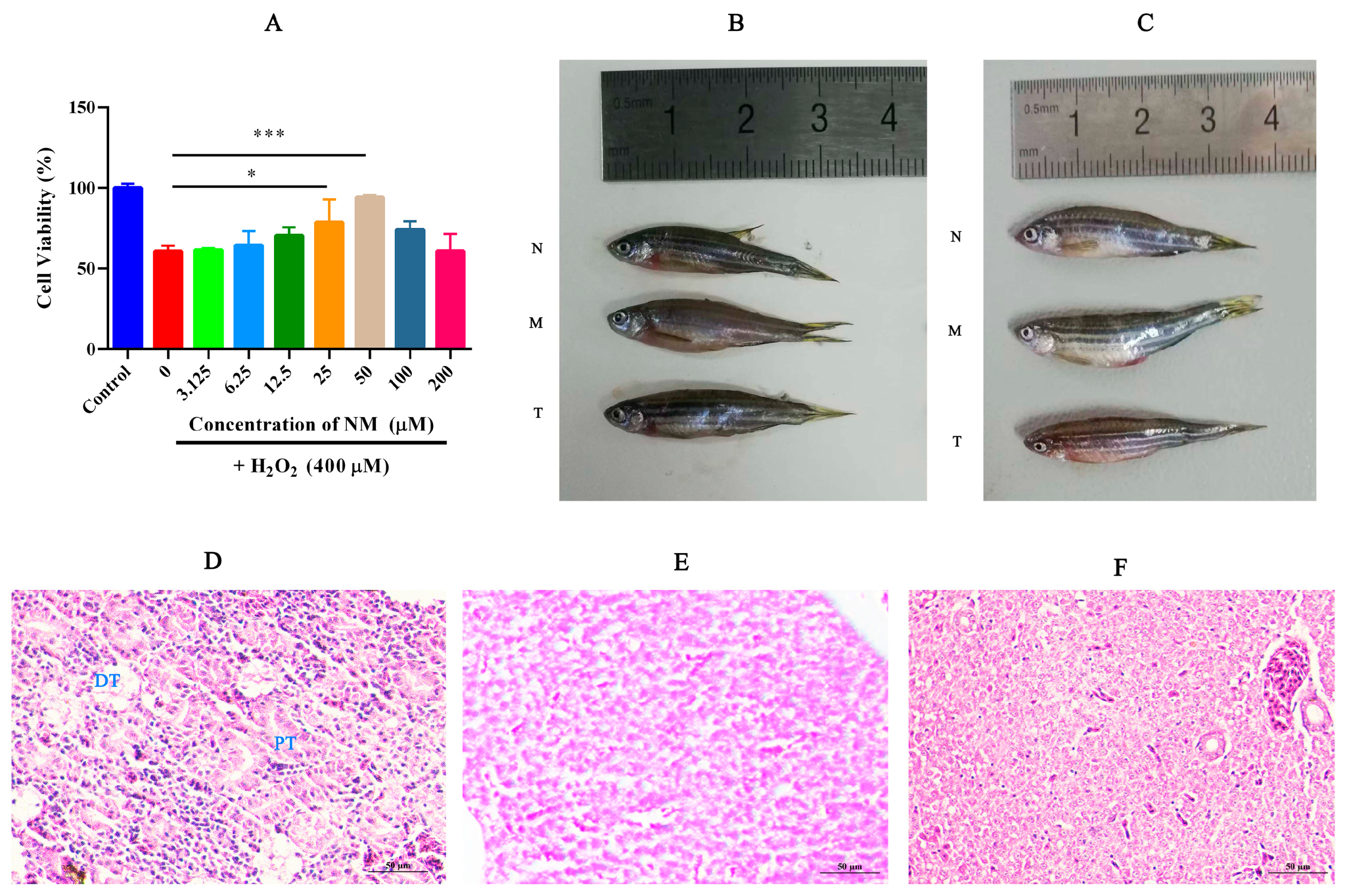
2.1. NM Alleviates Oxidative Damage in HK-2 Cells and Reduces Renal Tissue Injury in Zebrafish
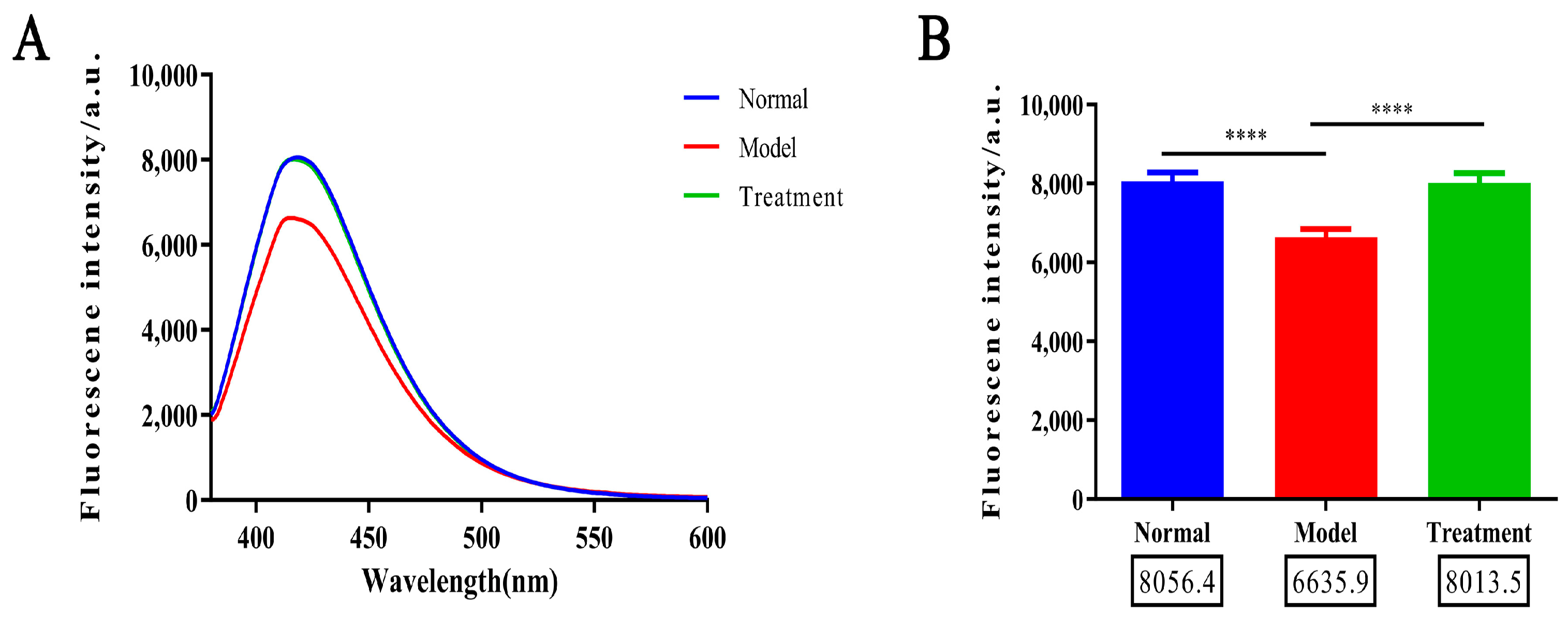
2.2. Glycosylation Degree Characterization
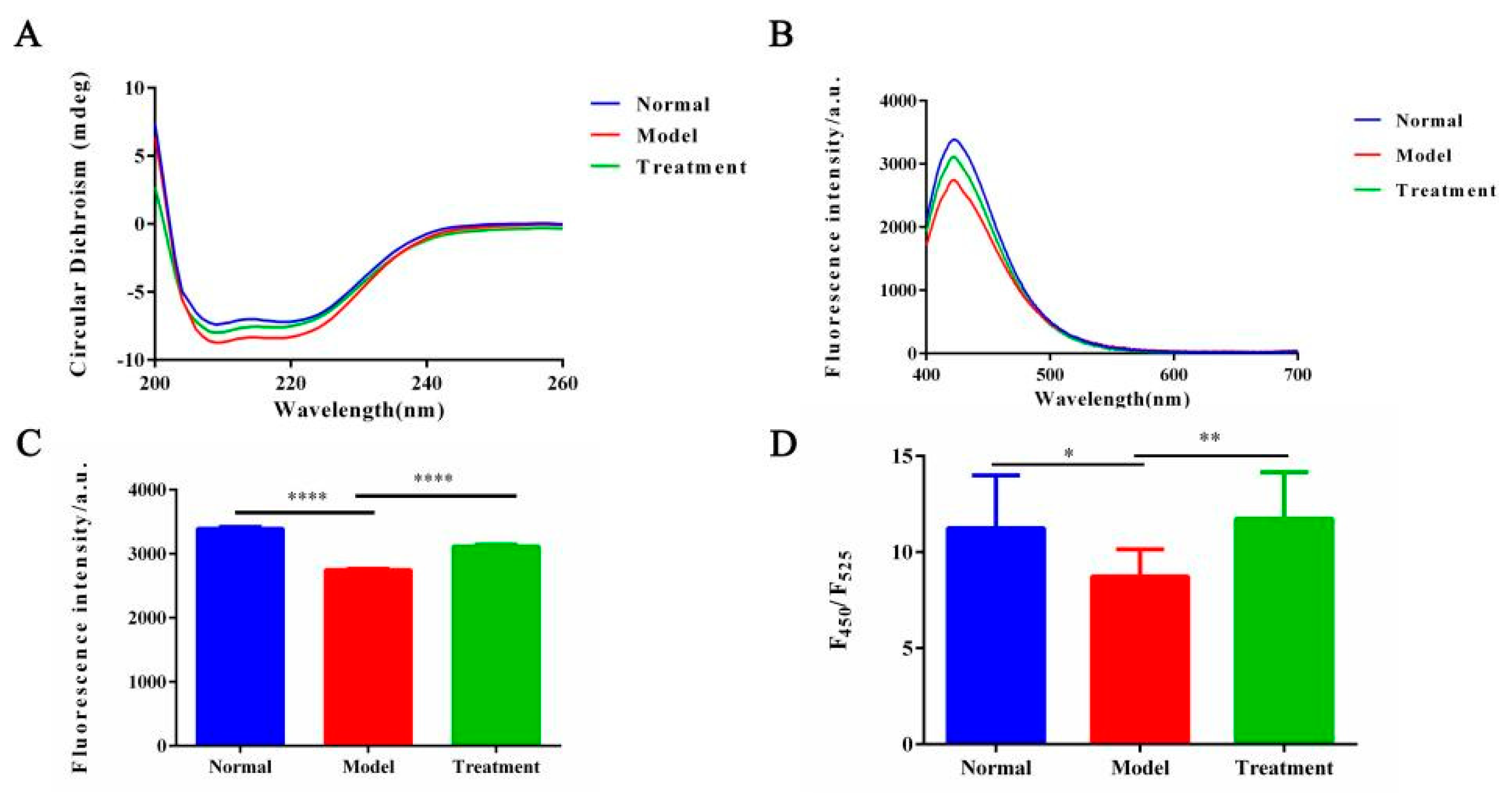
2.3. Effect of NM on Protein Conformation in the Glycosylation System
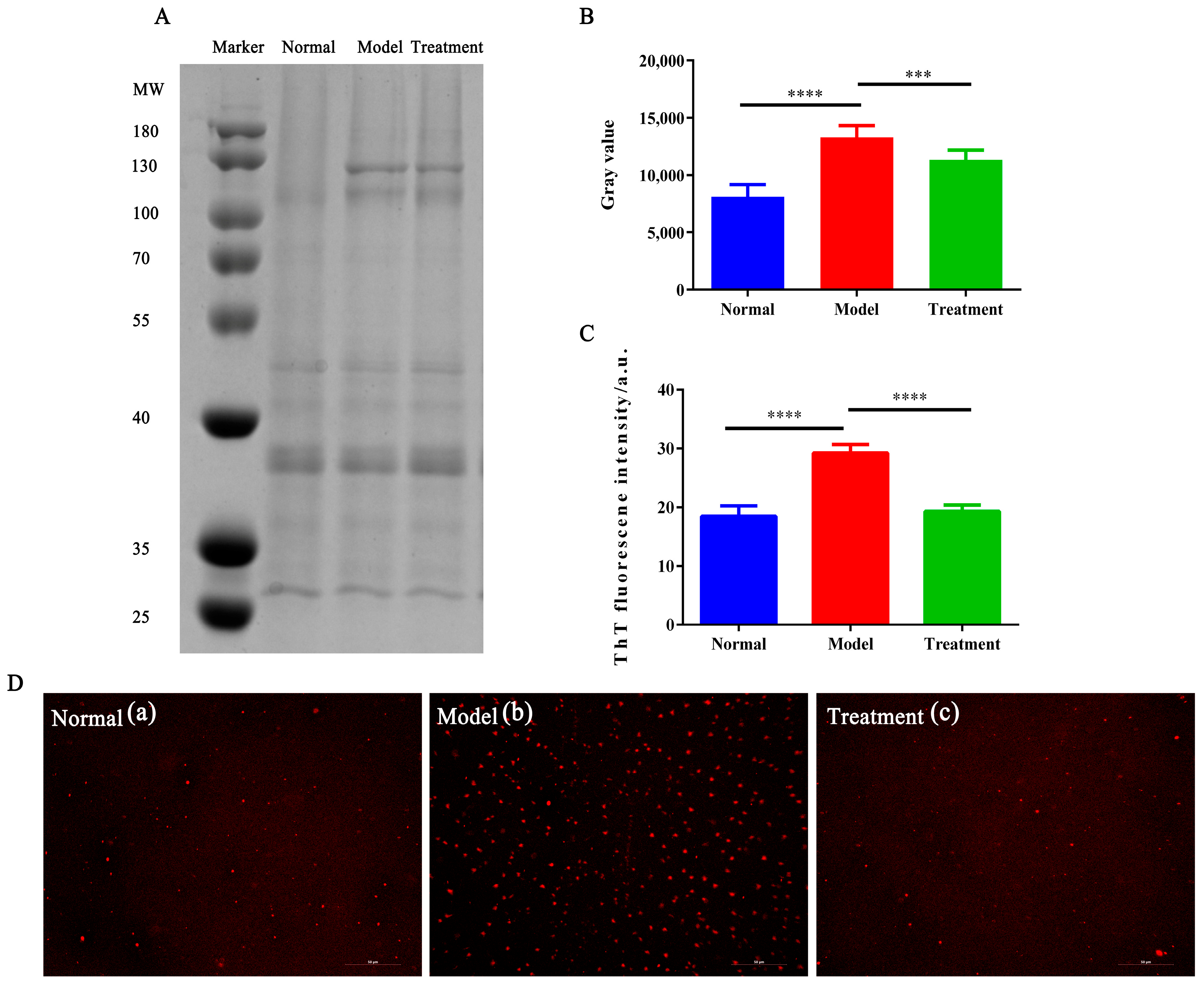
2.4. The Effect of NM on Glycosylation-Induced Protein Aggregation
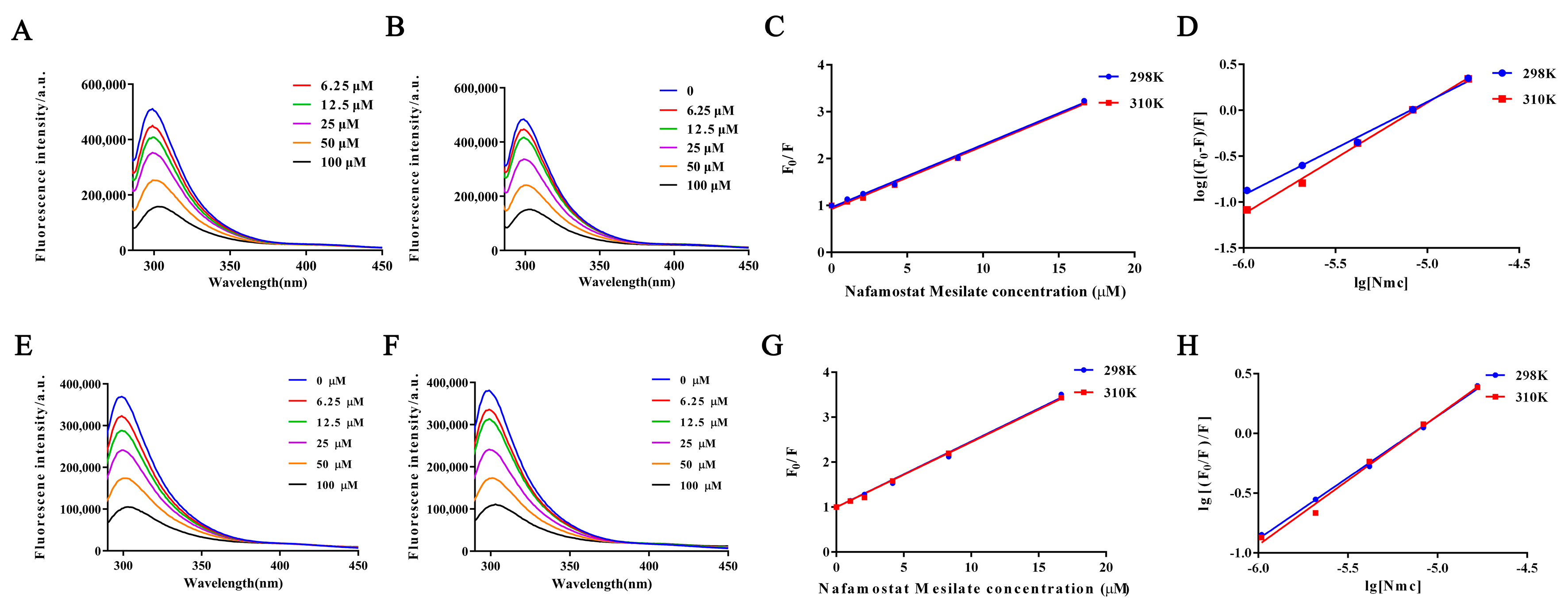
2.5. Fluorescence Quenching of Protein Interactions with NM
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Test Sample
4.3. Protective Effect of NM on Oxidative Damage in HK-2 Cells
4.4. Animal Husbandry and Model Establishment
4.5. Renal Tissue Histology and Protein Extraction
4.5.1. Zebrafish Anesthesia and Sample Collection
4.5.2. H&E Staining of Zebrafish Renal Tissue
4.5.3. Protein Extraction
4.6. AGEs Determination and Glycosylation Level Characterization
4.7. Changes in Protein Conformation After NM Intervention
4.7.1. Secondary Structure Analysis by Circular Dichroism (CD)
4.7.2. Tertiary Structure Analysis
4.8. Effect of NM on Glycosylation-Induced Protein Aggregation
4.8.1. SDS-PAGE to Detect Protein Aggregation Residues
4.8.2. ThT Fluorescent Probe to Investigate Amyloid Changes in Protein
4.8.3. Inverted Fluorescence Microscopy for Protein Aggregates
4.9. NM–Protein Interaction by Fluorescence Quenching
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar] [CrossRef]
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Stanski, N.L.; Rodrigues, C.E.; Strader, M.; Murray, P.T.; Endre, Z.H.; Bagshaw, S.M. Precision management of acute kidney injury in the intensive care unit: Current state of the art. Intensive Care Med. 2023, 49, 1049–1061. [Google Scholar] [CrossRef]
- Li, J.; Hou, F.; Lv, N.; Zhao, R.; Zhang, L.; Yue, C.; Nie, M.; Chen, L. From rare disorders of kidney tubules to acute renal injury: Progress and Prospective. Kidney Dis. 2024, 10, 153–166. [Google Scholar] [CrossRef]
- Bai, F.; Wang, C.; Wang, S.; Zhao, Y.; Feng, F.; Yu, K.; Liu, L.; Yang, X. DUSP5 deficiency suppresses the progression of acute kidney injury by enhancing autophagy through AMPK/ULK1 pathway. Transl. Res. 2024, 274, 1–9. [Google Scholar] [CrossRef]
- Moon, K.H.; Ko, I.K.; Yoo, J.J.; Atala, A. Kidney diseases and tissue engineering. Methods 2016, 99, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Birkelo, B.C.; Koyner, J.L.; Ostermann, M.; Bhatraju, P.K. The road to precision medicine for acute kidney injury. Crit. Care Med. 2024, 52, 1127–1137. [Google Scholar] [CrossRef]
- Ostermann, M.; Legrand, M.; Meersch, M.; Srisawat, N.; Zarbock, A.; Kellum, J.A. Biomarkers in acute kidney injury. Ann. Intensive Care 2024, 14, 145. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Griffin, K.A.; Lan, R.; Geng, H.; Saikumar, P.; Bidani, A.K. Acute kidney injury: A springboard for progression in chronic kidney disease. Am. J. Physiol.—Ren. Physiol. 2010, 5, 298. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef]
- Schetz, M.; Legrand, M. A nephrologist should be consulted in all cases of acute kidney injury in the ICU: We are not sure. Intensive Care Med. 2017, 43, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xing, J. Potential therapeutic applications of circular RNA in acute kidney injury. Biomed. Pharmacother. 2024, 174, 116502. [Google Scholar] [CrossRef]
- Kakizoe, Y.; Miyasato, Y.; Onoue, T.; Nakagawa, T.; Hayata, M.; Uchimura, K.; Morinaga, J.; Mizumoto, T.; Adachi, M.; Miyoshi, T.; et al. A serine protease inhibitor attenuates aldosterone-induced kidney injuries via the suppression of plasmin activity. J. Pharmacol. Sci. 2016, 132, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kakizoe, Y.; Mizumoto, T.; Nakagawa, T.; Hayata, M.; Izumi, Y.; Kuwabara, T.; Adachi, M.; Miyoshi, T.; Kitamura, K.; Mukoyama, M. SP074Serine Protease Inhibition Attenuates Salt-sensitive hypertension and kidney injury in a rat model of metabolic syndrome. Nephrol. Dial. Transplant. 2016, 1, 110. [Google Scholar] [CrossRef]
- Maehara, Y.; Oki, E.; Ota, M.; Harimoto, N.; Ando, K.; Nakanishi, R.; Kawazoe, T.; Fujimoto, Y.; Nonaka, K.; Kitao, H.; et al. Lineage of drug discovery research on fluorinated pyrimidines: Chronicle of the achievements accomplished by Professor Setsuro Fujii. Int. J. Clin. Oncol. 2023, 28, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Hitomi, Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim. Biophys. Acta 1981, 661, 342–345. [Google Scholar] [CrossRef]
- Hitomi, Y.; Ikari, N.; Fujii, S. Inhibitory effect of a new synthetic protease inhibitor (FUT-175) on the coagulation system. Pathophysiol. Haemost. Thromb. 1985, 15, 164–168. [Google Scholar] [CrossRef]
- Lee, B.; Cho, J.Y.; Han, H.-S. Effect of postoperative administration of nafamostat mesilate on posthepatectomy liver failure. Hepato-Pancreato-Biliary 2022, 24, 1569–1576. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Chen, T.; Fang, Y.; Shi, X.; Pang, T.; Zhang, L.; Liao, H. Nafamostat mesilate protects against acute cerebral ischemia via blood–brain barrier protection. Neuropharmacology 2016, 105, 398–410. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, T.; Zhao, X.; Pang, Y.; Li, W.; Fan, B.; Li, M.; Liu, X.; Ma, L.; Zhang, J.; et al. Delayed administration of nafamostat mesylate inhibits thrombin-mediated blood–spinal cord barrier breakdown during acute spinal cord injury in rats. Neuroinflammation 2022, 19, 189. [Google Scholar] [CrossRef]
- Mander, S.; You, D.-J.; Park, S.; Kim, D.H.; Yong, H.J.; Kim, D.-S.; Ahn, C.; Kim, Y.-H.; Seong, J.Y.; Hwang, J.-I. Nafamostat mesilate negatively regulates the metastasis of triple-negative breast cancer cells. Arch. Pharmacal Res. 2018, 41, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, J.; Wang, D. Nafamostat mesylate sensitizes ovarian cancer cells to carboplatin by promoting the ZNF24-mediated inhibition of WNT2B. J. Toxicol. Sci. 2024, 49, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Z.H. Efficacy and safety of Nafamostat mesylate in patients with endstage renal failure. World J. Clin. Cases 2024, 12, 68–75. [Google Scholar] [CrossRef]
- Fukuda, Y.; Okada, H.; Tomita, H.; Suzuki, K.; Mori, K.; Takada, C.; Kawasaki, Y.; Fukuda, H.; Minamiyama, T.; Nishio, A.; et al. Nafamostat mesylate decreases skin flap necrosis in a mouse model of type 2 diabetes by protecting the endothelial glycocalyx. Biochem. Biophys. Res. Commun. 2024, 710, 149843. [Google Scholar] [CrossRef]
- Na, K.-R.; Choi, H.; Jeong, J.; Lee, K.; Chang, Y.-K.; Choi, D. Nafamostat Mesilate attenuates ischemia-reperfusion–induced renal injury. Transplant. Proc. 2016, 48, 2192–2199. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Li, C.; Zhang, W.; Zhang, L.; An, L.; Pang, T.; Shi, X.; Liao, H. Nafamostat mesilate attenuates neuronal damage in a rat model of transient focal cerebral ischemia through thrombin inhibition. Sci. Rep. 2014, 4, 5531. [Google Scholar] [CrossRef]
- Cybulsky, A. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, M.; Jiang, W.; Dong, H.; Han, Y.; An, X.-F.; Zhang, J. Endoplasmic reticulum stress and its effects on renal tubular cells apoptosis in ischemic acute kidney injury. Ren. Fail. 2016, 38, 831–837. [Google Scholar] [CrossRef]
- Chen, K.; Shoulders, M.D. Protein Glycosylation Patterns Shaped By the IRE1-XBP1s Arm of the Unfolded Protein Response. Isr. J. Chem. 2024, 64, e202300162. [Google Scholar] [CrossRef]
- Macedo-da-Silva, J.; Santiago, V.F.; Rosa-Fernandes, L.; Marinho, C.R.F.; Palmisano, G. Protein glycosylation in extracellular vesicles: Structural characterization and biological functions. Mol. Immunol. 2021, 135, 226–246. [Google Scholar] [CrossRef]
- Singh, H.; Agrawal, D.K. Therapeutic potential of targeting the receptor for advanced glycation end products (RAGE) by small molecule inhibitors. Drug Dev. Res. 2022, 83, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Muthyalaiah, S.Y.; Jonnalagadda, B.; John, C.M.; Arockiasamy, S. Impact of Advanced Glycation End products (AGEs) and its receptor (RAGE) on cancer metabolic signaling pathways and its progression. Glycoconj. J. 2021, 38, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs-RAGE axis: Implications for inflammatory pathologies and therapeutic interventions—A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Beyze, A.; Larroque, C.; Le Quintrec, M. The role of antibody glycosylation in autoimmune and alloimmune kidney diseases. Nat. Rev. Nephrol. 2024, 20, 672–689. [Google Scholar] [CrossRef]
- Novak, J.; King, R.G.; Yother, J.; Renfrow, M.B.; Green, T.J. O-glycosylation of IgA1 and the pathogenesis of an autoimmune disease IgA nephropathy. Glycobiology 2024, 34, cwae060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-H.; Wang, Y.; Yang, J.; Zhang, D.-D.; Wang, Y.-L.; Li, S.-H.; Pan, Y.-N.; Zhang, H.-M.; Sun, Y. Comparative Analysis of Aristolochic Acids in Aristolochia Medicinal Herbs and Evaluation of Their Toxicities. Toxins 2022, 14, 879. [Google Scholar] [CrossRef]
- Li, W.-L.; Padanilam, B.J.; Kim, J. The Presence of Testis Determines Aristolochic Acid-Induced Nephrotoxicity in Mice. Toxins 2023, 15, 118. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, L.; Su, T.; Liu, G.; Yang, L. Overview of aristolochic acid nephropathy: An update. Kidney Res. Clin. Pract. 2023, 42, 579–590. [Google Scholar] [CrossRef]
- Calabrese, A.N.; Ault, J.R.; Radford, S.E.; Ashcroft, A.E. Using hydroxyl radical footprinting to explore the free energy landscape of protein folding. Methods 2015, 89, 38–44. [Google Scholar] [CrossRef]
- Rogers, D.M.; Jasim, S.B.; Dyer, N.T.; Auvray, F.; Réfrégiers, M.; Hirst, J.D. Electronic circular dichroism spectroscopy of proteins. Chem 2019, 5, 2751–2774. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.; Mo, S.; Zhang, Y.; Mao, X.; Chen, J.; Liu, Y.; Tong, W.-M.; Lu, Z.; Yu, S.; et al. TargetingN-glycosylation of 4F2hc mediated by glycosyltransferase B3GNT3 sensitizes ferroptosis of pancreatic ductal adenocarcinoma. Cell Death Differ. 2023, 30, 1988–2004. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Shuto, T.; Suzuki, S.; Sato, T.; Koga, T.; Suico, M.A.; Kusuhara, H.; Sugiyama, Y.; Cyr, D.M.; Kai, H. Posttranslational negative regulation of glycosylated and non-glycosylated BCRP expression by Derlin-1. Biochem. Biophys. Res. Commun. 2011, 404, 853–858. [Google Scholar] [CrossRef]
- Sharma, S.; Warsi, M.S.; Abidi, M.; Tufail, N.; Ahmad, R.; Siddiqui, S.A. Crotonaldehyde induced structural alterations in Low-Density Lipoprotein: Immunogenicity of the modified protein in experimental animals and auto-antibodies generation in various cancers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123332. [Google Scholar] [CrossRef]
- Veldman, M.; Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr. Res. 2008, 64, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Poureetezadi, S.J.; Wingert, R.A. Little fish, big catch: Zebrafish as a model for kidney disease. Kidney Int. 2016, 89, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Shaw, I.; Rider, S.; Mullins, J.; Hughes, J.; Péault, B. Pericytes in the renal vasculature: Roles in health and disease. Nat. Rev. Nephrol. 2018, 14, 521–534. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, M.J.; Kim, C.R.; Hwang, J.H.; Kim, D.H. Sp234effect of Nafamostat Mesilate as as an Anticoagulant During Continuous Renal Replacement Therapy in Critical Ill Patients. Nephrol. Dial. Transplant. 2015, 30, 455. [Google Scholar] [CrossRef]
- Kameda, S.; Fujii, T.; Ikeda, J.; Kageyama, A.; Takagi, T.; Miyayama, N.; Asano, K.; Endo, A.; Uezono, S. Unfractionated heparin versus nafamostat mesylate for anticoagulation during continuous kidney replacement therapy: An observational study. BMC Nephrol 2023, 24, 12. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, J.; Xia, H.; Dong, S.; Yan, S.; Zhuang, Y.; Chen, Y.; Peng, H. Nafamostat mesylate versus regional citrate anticoagulation for continuous renal replacement therapy in patients at high risk of bleeding: A retrospective single-center study. Eur. J. Med. Res. 2024, 29, 72. [Google Scholar] [CrossRef]
- Wang, X.; Giusti, A.; Ny, A.; de Witte, P.A. Nephrotoxic Effects in Zebrafish after Prolonged Exposure to Aristolochic Acid. Toxins 2020, 12, 217. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.-C.; Sun, G.-J.; Han, L.-W.; Wang, R.-C.; Peng, W.-B.; Sun, C.; Hsiao, C.-D.; Zhang, Y.; Hou, H.-R. Evaluation of nephrotoxic effects of aristolochic acid on zebrafish (Danio rerio) larvae. Hum. Exp. Toxicol. 2016, 35, 974–982. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal Injection into Adult Zebrafish. J. Vis. Exp. 2010, 42, e2126. [Google Scholar] [CrossRef]
- Von Krogh, K.; Higgins, J.; Saavedra Torres, Y.; Mocho, J.-P. Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose. Biology 2021, 10, 1133. [Google Scholar] [CrossRef]
- Valentim, A.M.; van Eeden, F.J.; Strähle, U.; Olsson, I.A.S. Euthanizing zebrafish legally in Europe: Are the approved methods of euthanizing zebrafish appropriate to research reality and animal welfare? EMBO Rep. 2016, 17, 1688–1689. [Google Scholar] [CrossRef]
- Gerlach, G.F.; Schrader, L.N.; Wingert, R.A. Dissection of the adult zebrafish kidney. J. Vis. Exp. 2011, 54, e2839. [Google Scholar] [CrossRef]
- Martins, T.; Diniz, E.; Félix, L.M.; Antunes, L. Evaluation of anaesthetic protocols for laboratory adult zebrafish (Danio rerio). PLoS ONE 2018, 13, 0197846. [Google Scholar] [CrossRef]
- Bozkurt, E.; Gul, H.I. Fluorescence quenching of novel pyrazoline derivative with aniline in different solvents. J. Photochem. Photobiol. A Chem. 2019, 383, 111996. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, Q.; Li, Y.; Liu, X.; Zhang, D.; Li, X. Study of the binding mechanism between hydroxytyrosol and bovine serum albumin using multispectral and molecular docking. Food Hydrocoll. 2021, 122, 107072. [Google Scholar] [CrossRef]
- Mizuno, H.; Fukuhara, G. Solution-State Hydrostatic Pressure Chemistry: Application to Molecular, Supramolecular, Polymer, and Biological Systems. Acc. Chem. Res. 2022, 55, 1748–1762. [Google Scholar] [CrossRef]
- Melien, R.; Garidel, P.; Hinderberger, D.; Blech, M. Thermodynamic Unfolding and Aggregation Fingerprints of Monoclonal Antibodies Using Thermal Profiling. Pharm. Res. 2020, 37, 78. [Google Scholar] [CrossRef]
- Monirinasab, H.; Zakariazadeh, M.; Kohestani, H.; Kouhestani, M.; Fathi, F. Study of β-lactam-based drug interaction with albumin protein using optical, sensing, and docking methods. J. Biol. Phys. 2022, 48, 177–194. [Google Scholar] [CrossRef] [PubMed]

| Group | T | KSV | Kb | Kq | △G | △H | △S |
|---|---|---|---|---|---|---|---|
| (K) | (L•mol−1) | (L•mol−1) | (L•mol−1•s −1) | (kJ•mol−1) | (kJ•mol−1) | (J•mol−1•K−1) | |
| Normal | 298 | 0.1304 | 145,077.48 | 1.30 × 1013 | −29.45 | 144,795.47 | 584.70 |
| 310 | 0.1279 | 1,393,477.63 | 1.28 × 1013 | −36.46 | 584.70 | ||
| Model | 298 | 0.1448 | 192,752.49 | 1.45 × 1013 | −30.15 | −40.53 | 237.17 |
| 310 | 0.1462 | 363,078.05 | 1.46 × 1013 | −32.99 | 237.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Liu, H.; Huo, X.; Chen, J.; Li, Y.; Huang, Y.; Yin, Z. Nafamostat Mesylate Regulates Glycosylation to Alleviate Aristolochic Acid Induced Kidney Injury. Toxins 2025, 17, 145. https://doi.org/10.3390/toxins17030145
Xie P, Liu H, Huo X, Chen J, Li Y, Huang Y, Yin Z. Nafamostat Mesylate Regulates Glycosylation to Alleviate Aristolochic Acid Induced Kidney Injury. Toxins. 2025; 17(3):145. https://doi.org/10.3390/toxins17030145
Chicago/Turabian StyleXie, Pei, Huijun Liu, Xingli Huo, Junlong Chen, Yu Li, Yu Huang, and Zongning Yin. 2025. "Nafamostat Mesylate Regulates Glycosylation to Alleviate Aristolochic Acid Induced Kidney Injury" Toxins 17, no. 3: 145. https://doi.org/10.3390/toxins17030145
APA StyleXie, P., Liu, H., Huo, X., Chen, J., Li, Y., Huang, Y., & Yin, Z. (2025). Nafamostat Mesylate Regulates Glycosylation to Alleviate Aristolochic Acid Induced Kidney Injury. Toxins, 17(3), 145. https://doi.org/10.3390/toxins17030145




