Ablation of Gut Microbiota Alleviates DON-Induced Neurobehavioral Abnormalities and Brain Damage in Mice
Abstract
1. Introduction
2. Results
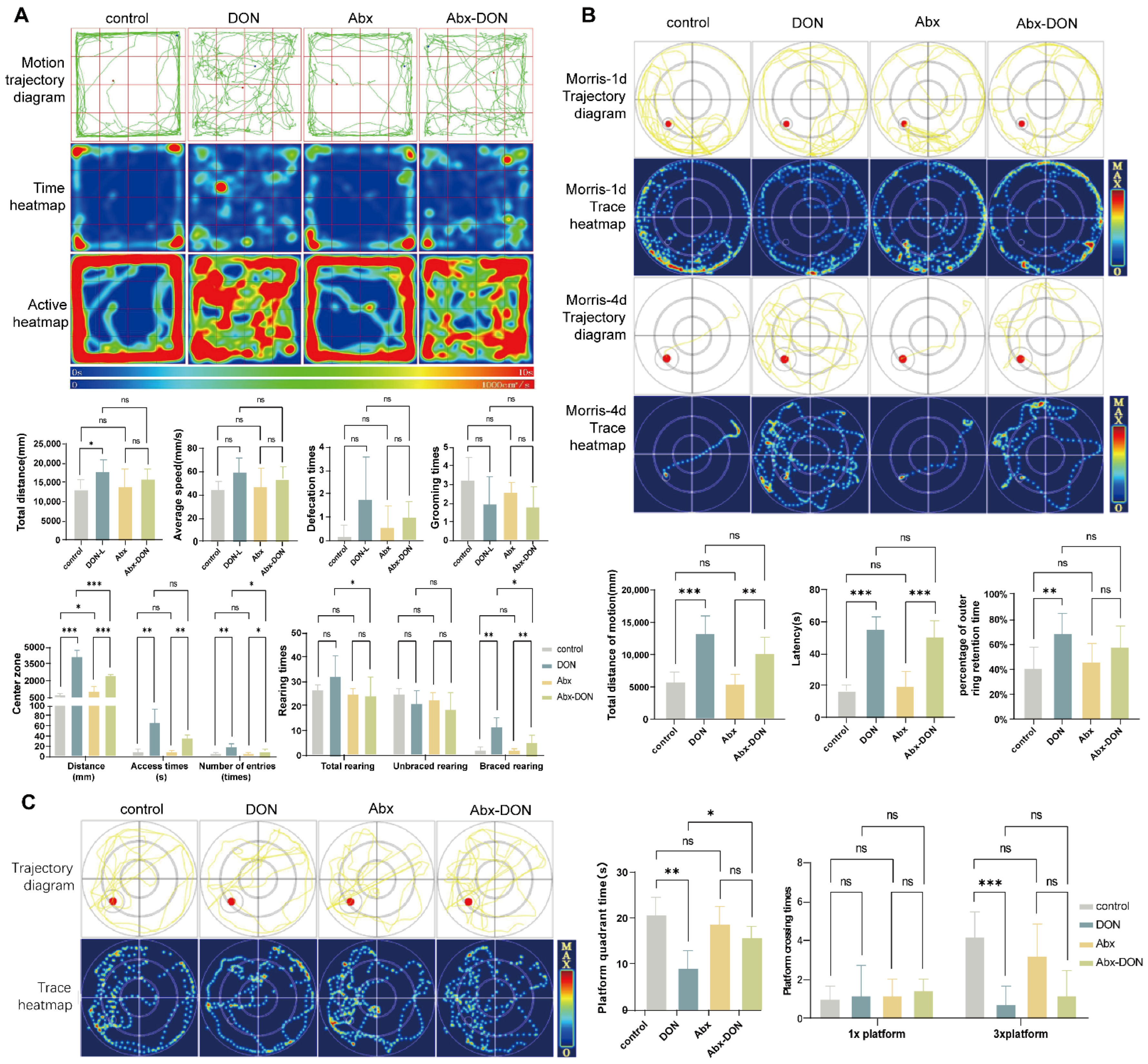
2.1. Effects of Ablation of Intestinal Microbiota on Behavioral Changes in DON Fed Mice
2.1.1. Results from Open Field Test
2.1.2. Results from Morris Test
- Hidden Platform results
- Spatial probe test results
2.2. Effects of Intestinal Ablation on Brain Specific Gravity
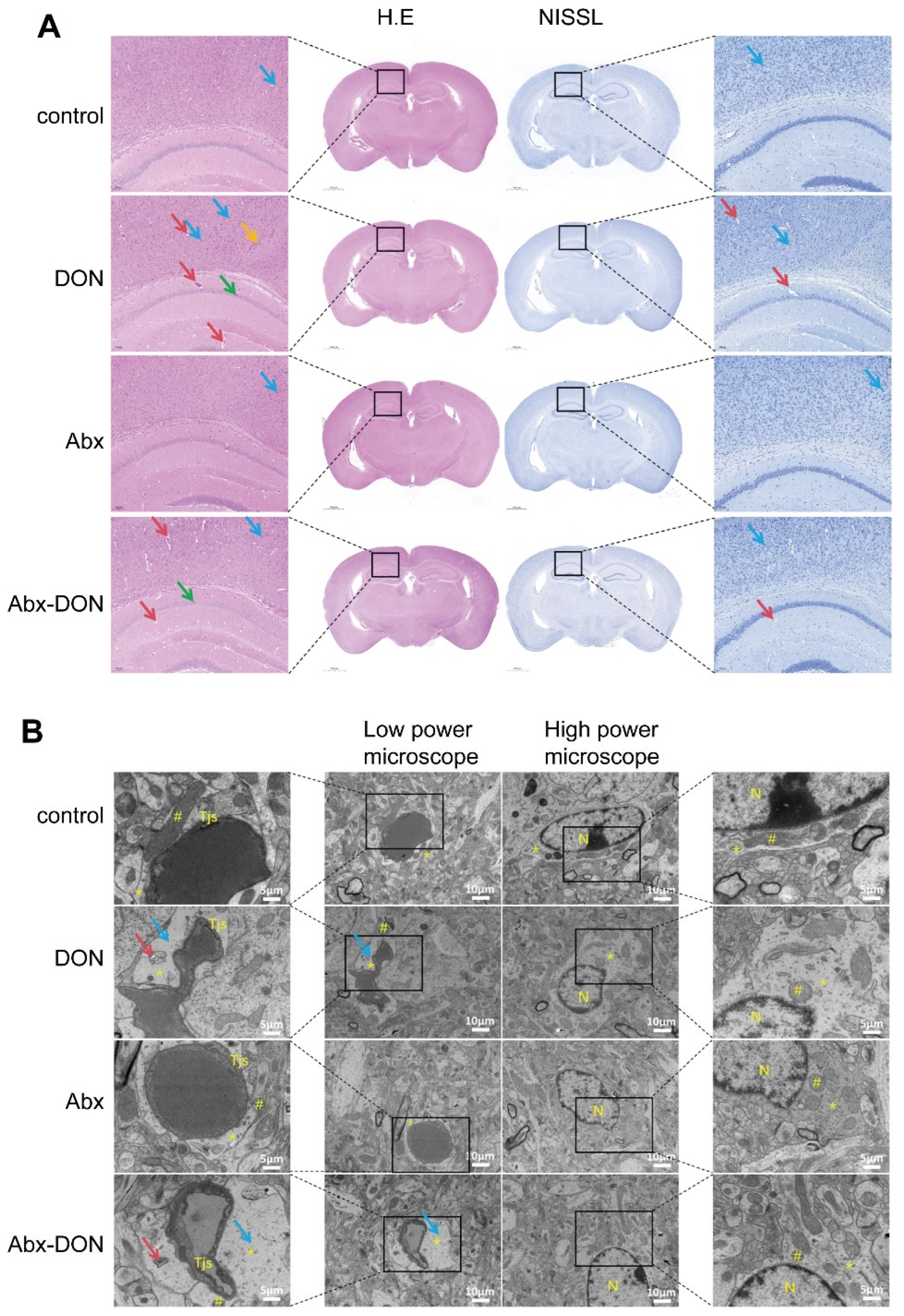
2.3. Effects of Interstinal Ablation on Pathology of Brain Tissue of Mice Fed DON
2.3.1. H&E Staining and Nissl Staining Results
2.3.2. Ablation of Intestinal Microbiota Alleviate Neuronal Ultrastructure Pathological Damage in DON Fed Mice
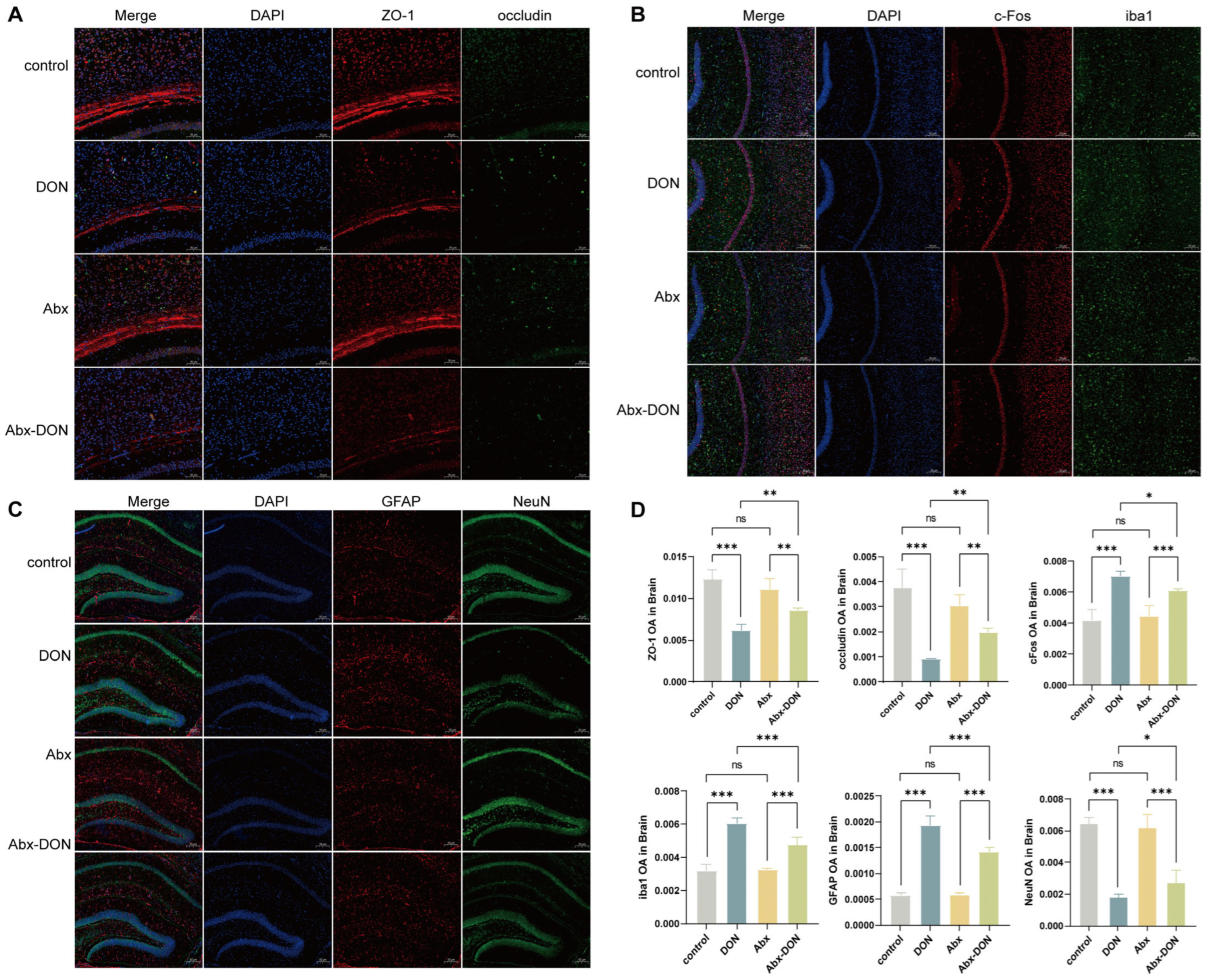
2.4. IF Detection of DON Expression in Brain Tight Junctions, Glial Cells, and Neurons
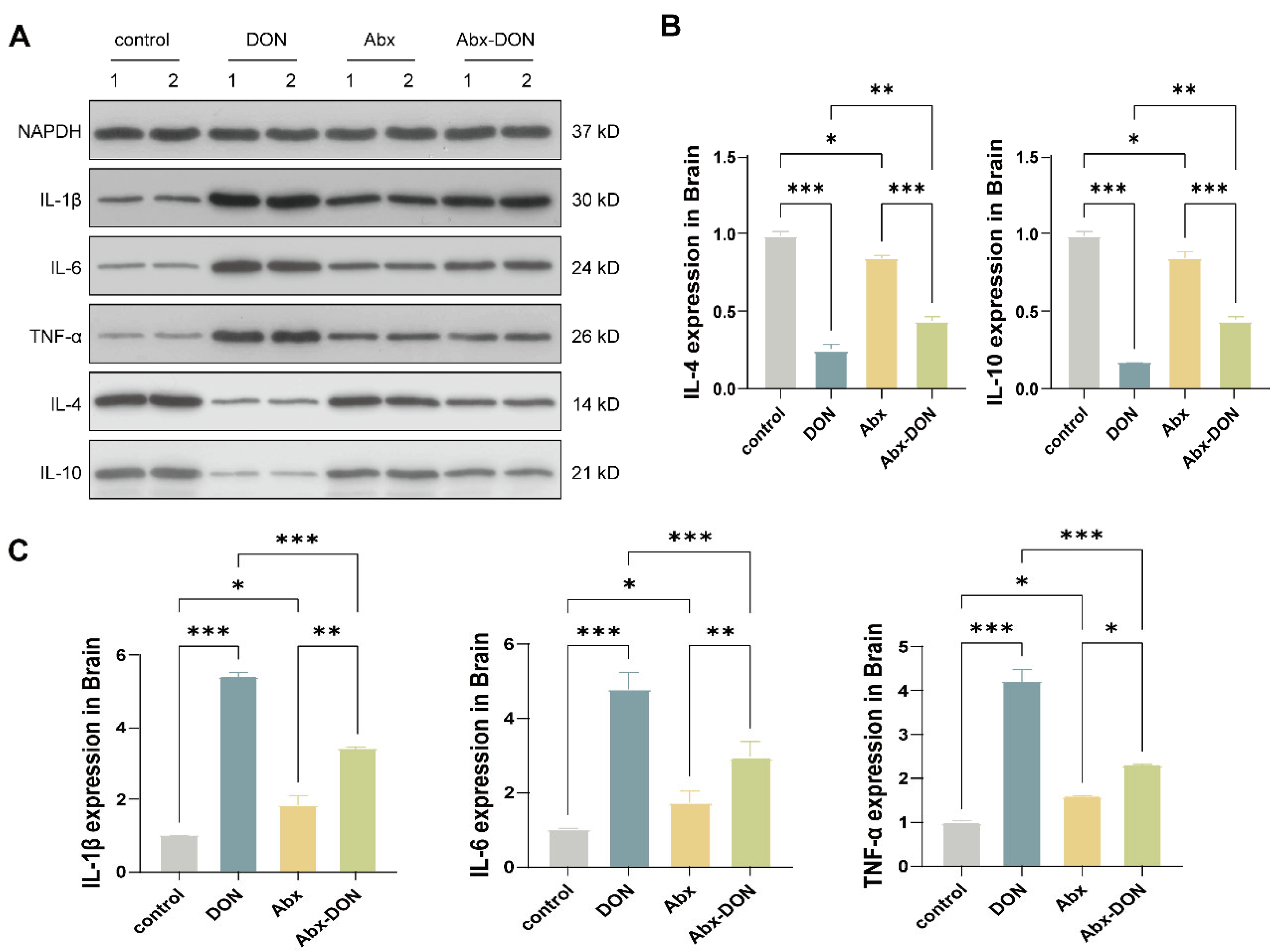
2.5. Western Blot Detection of the Effects of DON Expression on Inflammatory Cytokines in the Brain
2.6. RT qPCR Detection of the Expression Levels of Related Proteins at the mRNA Level
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Material and Design
5.2. Antibiotics Preparation and Treatment
5.3. DON Preparation and Administration
5.4. Behavioral Experiment
5.4.1. Open Field Test (OFT)
5.4.2. Morris
- Concealed platform test
- Spatial probe test
5.5. Brain Specific Gravity Measurement
5.6. Sample Collection and Preservation
5.7. Pathology of Brain Tissue
5.7.1. HE Staining of the Brain
5.7.2. Nissl Staining
5.7.3. Neuronal Ultrastructure Pathology
5.8. Immunofluorescence Detection of DON Localization and Expression in Brain Glial Cells, Neurons, and Tight Junctions
5.9. Western Blot Detection of the Effects of DON on the Expression of Inflammatory Cytokines in the Brain
5.10. RT qPCR Detection of the Effects of DON on the mRNA Expression Levels of Glial Cell Markers, Neuronal Markers, Tight Junctions, and Inflammatory Factors in the Brain
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueno, Y. The Toxicology of Mycotoxins. Crit. Rev. Toxicol. 1985, 14, 99–132. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium Mycotoxins: A Review of Global Implications for Animal Health, Welfare and Productivity. Anim. Feed. Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Kuča, K.; Dohnal, V.; Tian, Z. Deoxynivalenol: Signaling pathways and human exposure risk assessment--an update. Arch. Toxicol. 2014, 88, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of Deoxynivalenol and Its Acetylated and Modified Forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, Gut Microbiota and Immunotoxicity: A Potential Approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of Action, Human Exposure, and Toxicological Relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Tardivel, C.; Airault, C.; Djelloul, M.; Guillebaud, F.; Barbouche, R.; Troadec, J.-D.; Gaigé, S.; Dallaporta, M. The Food Born Mycotoxin Deoxynivalenol Induces Low-Grade Inflammation in Mice in the Absence of Observed-Adverse Effects. Toxicol. Lett. 2015, 232, 601–611. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Cao, L.; Zhu, L.; Zhang, Y.; Chu, X.; Zhu, D.; Rahman, S.U.; Peng, C.; Feng, S.; et al. Mechanism of Deoxynivalenol-Induced Neurotoxicity in Weaned Piglets Is Linked to Lipid Peroxidation, Dampened Neurotransmitter Levels, and Interference with Calcium Signaling. Ecotoxicol. Environ. Saf. 2020, 194, 110382. [Google Scholar] [CrossRef]
- Cao, L.; Jiang, Y.; Zhu, L.; Xu, W.; Chu, X.; Zhang, Y.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; et al. Deoxynivalenol Induces Caspase-8-Mediated Apoptosis through the Mitochondrial Pathway in Hippocampal Nerve Cells of Piglet. Toxins 2021, 13, 73. [Google Scholar] [CrossRef]
- Doi, K.; Uetsuka, K. Mechanisms of Mycotoxin-Induced Neurotoxicity through Oxidative Stress-Associated Pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.a.R. Review: Activation Patterns of Microglia and Their Identification in the Human Brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Schwartz, M.; Butovsky, O.; Brück, W.; Hanisch, U.-K. Microglial Phenotype: Is the Commitment Reversible? Trends Neurosci. 2006, 29, 68–74. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Interaction of Neurons and Astrocytes Underlies the Mechanism of Aβ-Induced Neurotoxicity. Biochem. Soc. Trans. 2014, 42, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, W.; Jiang, H.; Liu, D.; Liu, X.; Li, L.; Li, C.; Xiao, X.; Tang, S.; Li, D. Food-Origin Mycotoxin-Induced Neurotoxicity: Intend to Break the Rules of Neuroglia Cells. Oxidative Med. Cell. Longev. 2021, 2021, 9967334. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Kaur, C.; Ling, E.-A. Microglial Activation and Its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes Are Active Players in Cerebral Innate Immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Machado-Pereira, M.; Santos, T.; Bernardino, L.; Ferreira, R. Vascular Inter-Regulation of Inflammation: Molecular and Cellular Targets for CNS Therapy. J. Neurochem. 2017, 140, 692–702. [Google Scholar] [CrossRef]
- Fæste, C.K.; Solhaug, A.; Gaborit, M.; Pierre, F.; Massotte, D. Neurotoxic Potential of Deoxynivalenol in Murine Brain Cell Lines and Primary Hippocampal Cultures. Toxins 2022, 14, 48. [Google Scholar] [CrossRef]
- Rissato, D.F.; de Santi Rampazzo, A.P.; Borges, S.C.; Sousa, F.C.; Busso, C.; Buttow, N.C.; Natali, M.R.M. Chronic Ingestion of Deoxynivalenol-Contaminated Diet Dose-Dependently Decreases the Area of Myenteric Neurons and Gliocytes of Rats. Neurogastroenterol. Motil. 2020, 32, e13770. [Google Scholar] [CrossRef]
- Janabi, N.; Peudenier, S.; Héron, B.; Ng, K.H.; Tardieu, M. Establishment of Human Microglial Cell Lines after Transfection of Primary Cultures of Embryonic Microglial Cells with the SV40 Large T Antigen. Neurosci. Lett. 1995, 195, 105–108. [Google Scholar] [CrossRef]
- Behrens, M.; Hüwel, S.; Galla, H.-J.; Humpf, H.-U. Blood-Brain Barrier Effects of the Fusarium Mycotoxins Deoxynivalenol, 3 Acetyldeoxynivalenol, and Moniliformin and Their Transfer to the Brain. PLoS ONE 2015, 10, e0143640. [Google Scholar] [CrossRef] [PubMed]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut Microbiota Regulates Maturation of the Adult Enteric Nervous System via Enteric Serotonin Networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Pang, Y.; Wang, X.; Wu, Q.; Liu, H.; Liu, B.; Liu, G.; Ye, M.; Kong, W.; Jiang, C. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm. Sin. B 2019, 9, 702–710. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Gong, Z.; Dong, Z.; Yu, F.; Fu, Y.; Jiang, C.; Kong, W. Ablation of the gut microbiota alleviates high-methionine diet-induced hyperhomocysteinemia and glucose intolerance in mice. NPJ Sci. Food 2023, 7, 36. [Google Scholar] [CrossRef]
- Wekerle, H. Brain Inflammatory Cascade Controlled by Gut-Derived Molecules. Nature 2018, 557, 642–643. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Doenyas, C. Gut Microbiota, Inflammation, and Probiotics on Neural Development in Autism Spectrum Disorder. Neuroscience 2018, 374, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef] [PubMed]
- A Abdel Jaleel, G.; A Al-Awdan, S.; F Ahmed, R.; A H Ahmed-Farid, O.; Saleh, D.O. Melatonin Regulates Neurodegenerative Complications Associated with NAFLD via Enhanced Neurotransmission and Cellular Integrity: A Correlational Study. Metab. Brain Dis. 2020, 35, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Faeste, C.K.; Pierre, F.; Ivanova, L.; Sayyari, A.; Massotte, D. Behavioural and Metabolomic Changes from Chronic Dietary Exposure to Low-Level Deoxynivalenol Reveal Impact on Mouse Well-Being. Arch. Toxicol. 2019, 93, 2087–2102. [Google Scholar] [CrossRef]
- Bruszt, N.; Bali, Z.K.; Tadepalli, S.A.; Nagy, L.V.; Hernádi, I. Potentiation of Cognitive Enhancer Effects of Alzheimer’s Disease Medication Memantine by Alpha7 Nicotinic Acetylcholine Receptor Agonist PHA-543613 in the Morris Water Maze Task. Psychopharmacology 2021, 238, 3273–3281. [Google Scholar] [CrossRef]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris Water Maze Test for Learning and Memory Deficits in Alzheimer’s Disease Model Mice. J. Vis. Exp. 2011, 53, 2920. [Google Scholar] [CrossRef]
- Diao, J.; Xia, Y.; Jiang, X.; Qiu, J.; Cheng, S.; Su, J.; Duan, X.; Gao, M.; Qin, X.; Zhang, J.; et al. Silicon Dioxide Nanoparticles Induced Neurobehavioral Impairments by Disrupting Microbiota–Gut–Brain Axis. J. Nanobiotechnol. 2021, 19, 174. [Google Scholar] [CrossRef]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Shen, L.H.; Cui, H.M.; Xu, Z.W.; Hu, Y.C. Effect of the Fusarium Toxins, Zearalenone and Deoxynivalenol, on the Mouse Brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef]
- Nico, B.; Ribatti, D. Morphofunctional Aspects of the Blood-Brain Barrier. Curr. Drug Metab. 2012, 13, 50–60. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef]
- Van Hove, H.; Martens, L.; Scheyltjens, I.; De Vlaminck, K.; Pombo Antunes, A.R.; De Prijck, S.; Vandamme, N.; De Schepper, S.; Van Isterdael, G.; Scott, C.L.; et al. A Single-Cell Atlas of Mouse Brain Macrophages Reveals Unique Transcriptional Identities Shaped by Ontogeny and Tissue Environment. Nat. Neurosci. 2019, 22, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Gaigé, S.; Bonnet, M.S.; Tardivel, C.; Pinton, P.; Trouslard, J.; Jean, A.; Guzylack, L.; Troadec, J.-D.; Dallaporta, M. C-Fos Immunoreactivity in the Pig Brain Following Deoxynivalenol Intoxication: Focus on NUCB2/Nesfatin-1 Expressing Neurons. Neurotoxicology 2013, 34, 135–149. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathi, A.; Chaudhari, B.P.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Deoxynivalenol Induced Mouse Skin Cell Proliferation and Inflammation via MAPK Pathway. Toxicol. Appl. Pharmacol. 2014, 279, 186–197. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Pawłowska, M.; Barański, M.; Nowicka, K.; Zieliński, Z.; Bijoch, Ł.; Legutko, D.; Majka, P.; Bednarek, S.; Jermakow, N.; et al. Global Brain C-Fos Profiling Reveals Major Functional Brain Networks Rearrangements after Alcohol Reexposure. Neurobiol. Dis. 2023, 178, 106006. [Google Scholar] [CrossRef] [PubMed]
- Dunphy-Doherty, F.; O’Mahony, S.M.; Peterson, V.L.; O’Sullivan, O.; Crispie, F.; Cotter, P.D.; Wigmore, P.; King, M.V.; Cryan, J.F.; Fone, K.C.F. Post-Weaning Social Isolation of Rats Leads to Long-Term Disruption of the Gut Microbiota-Immune-Brain Axis. Brain Behav. Immun. 2018, 68, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Benzaria, A.; Taïeb, N.; Guo, X.-J.; Vidal, N.; Di Scala, C.; Varini, K.; Maresca, M. The Ribotoxin Deoxynivalenol Affects the Viability and Functions of Glial Cells. Glia 2011, 59, 1672–1683. [Google Scholar] [CrossRef]
- Aschner, M. Astrocytes as Mediators of Immune and Inflammatory Responses in the CNS. Neurotoxicology 1998, 19, 269–281. [Google Scholar]
- Streit, W.J.; Conde, J.R.; Fendrick, S.E.; Flanary, B.E.; Mariani, C.L. Role of Microglia in the Central Nervous System’s Immune Response. Neurol. Res. 2005, 27, 685–691. [Google Scholar] [CrossRef]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Mounien, L.; Trouslard, J.; et al. Central Inflammation and Sickness-like Behavior Induced by the Food Contaminant Deoxynivalenol: A PGE2-Independent Mechanism. Toxicol. Sci. 2011, 124, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. The Role of the Gut Microbiota in Development, Function and Disorders of the Central Nervous System and the Enteric Nervous System. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef] [PubMed]
- Larroya-García, A.; Navas-Carrillo, D.; Orenes-Piñero, E. Impact of Gut Microbiota on Neurological Diseases: Diet Composition and Novel Treatments. Crit. Rev. Food Sci. Nutr. 2019, 59, 3102–3116. [Google Scholar] [CrossRef]
- Tominaga, M.; Momonaka, Y.; Yokose, C.; Tadaishi, M.; Shimizu, M.; Yamane, T.; Oishi, Y.; Kobayashi-Hattori, K. Anorexic action of deoxynivalenol in hypothalamus and intestine. Toxicon Off. J. Int. Soc. Toxinology 2016, 118, 54–60. [Google Scholar] [CrossRef]
- Gaige, S.; Barbouche, R.; Barbot, M.; Boularand, S.; Dallaporta, M.; Abysique, A.; Troadec, J.D. Constitutively active microglial populations limit anorexia induced by the food contaminant deoxynivalenol. J. Neuroinflamm. 2022, 19, 280. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and Clinical Implications of the Brain-Gut-Enteric Microbiota Axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front. Microbiol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef]
- He, P.; Young, L.G.; Forsberg, C. Microbial Transformation of Deoxynivalenol (Vomitoxin). Appl. Environ. Microbiol. 1992, 58, 3857–3863. [Google Scholar] [CrossRef]
- Gong, S.; Yan, Z.; Liu, Z.; Niu, M.; Fang, H.; Li, N.; Huang, C.; Li, L.; Chen, G.; Luo, H.; et al. Intestinal Microbiota Mediates the Susceptibility to Polymicrobial Sepsis-Induced Liver Injury by Granisetron Generation in Mice. Hepatology 2019, 69, 1751–1767. [Google Scholar] [CrossRef]



| Gene Name | Primer | Sequence | Annealing Temp |
|---|---|---|---|
| IL-1β | F: TGGTGTGTGACGTTCCCATT R: TGTCGTTGCTTGGTTCTCCT | 16,176 | 60/60 |
| IL-6 | F: TGTCGTTGCTTGGTTCTCCT R: TGCAAGTGCATCATCGTTGTTC | 16,193 | 60/60 |
| TNF-α | F: TCTTCTCATTCCTGCTTGTGG R: ATGAGAGGGAGGCCATTTG | 21,926 | 60/60 |
| IL-4 | F:ACGGAGATGGATGTGCCAAAC R: AGCACCTTGGAAGCCCTACAGA | 16,189 | 60/63 |
| IL-10 | F: GCCAGAGCCACATGCTCCTA R: GCCAGAGCCACATGCTCCTA | 16,153 | 61/61 |
| ZO-1 | F: ACCAGATGTGGATTTACCCGTCA R: ACATCATTTCCACCAGCTAGTCG | 21,872 | 61/60 |
| Occludin | F: GGCAAGCGATCATACCCAGA R: GCTGCCTGAAGTCATCCACA | 18,260 | 60/60 |
| c-FOS | F: CGGGTTTCAACGCCGACTA R: TTGGCACTAGAGACGGACAGA | 14,281 | 59/61 |
| GFAP | F: CCTTCTGACACGGATTTGGT R: TAAGCTAGCCCTGGACATCG | 14,580 | 58/60 |
| NeuN | F: CCACCACTCTCTTGTCCGTT R: ATCAGCAGCGGCATAGACTC | 52,897 | 60/60 |
| iba1 | F: GGATCTGCCGTCCAAAC R: GCATTCGCTTCAAGGACAR | 11,629 | 54/53 |
| GAPDH | F: CGACTTCAACAGCAACTCCCACTCTTCC R: TGGGTGGTCCAGGGTTTCTTACTCCTT | 14,433 | 60/60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Okyere, S.K.; Guan, H.; Hua, Z.; Deng, Y.; Deng, H.; Deng, J. Ablation of Gut Microbiota Alleviates DON-Induced Neurobehavioral Abnormalities and Brain Damage in Mice. Toxins 2025, 17, 144. https://doi.org/10.3390/toxins17030144
Cui Y, Okyere SK, Guan H, Hua Z, Deng Y, Deng H, Deng J. Ablation of Gut Microbiota Alleviates DON-Induced Neurobehavioral Abnormalities and Brain Damage in Mice. Toxins. 2025; 17(3):144. https://doi.org/10.3390/toxins17030144
Chicago/Turabian StyleCui, Yujing, Samuel Kumi Okyere, Haoyue Guan, Zixuan Hua, Youtian Deng, Huidan Deng, and Junliang Deng. 2025. "Ablation of Gut Microbiota Alleviates DON-Induced Neurobehavioral Abnormalities and Brain Damage in Mice" Toxins 17, no. 3: 144. https://doi.org/10.3390/toxins17030144
APA StyleCui, Y., Okyere, S. K., Guan, H., Hua, Z., Deng, Y., Deng, H., & Deng, J. (2025). Ablation of Gut Microbiota Alleviates DON-Induced Neurobehavioral Abnormalities and Brain Damage in Mice. Toxins, 17(3), 144. https://doi.org/10.3390/toxins17030144





