Learnings from Separate Aconitum Poisonings in British Columbia and Ontario, Canada in 2022
Abstract
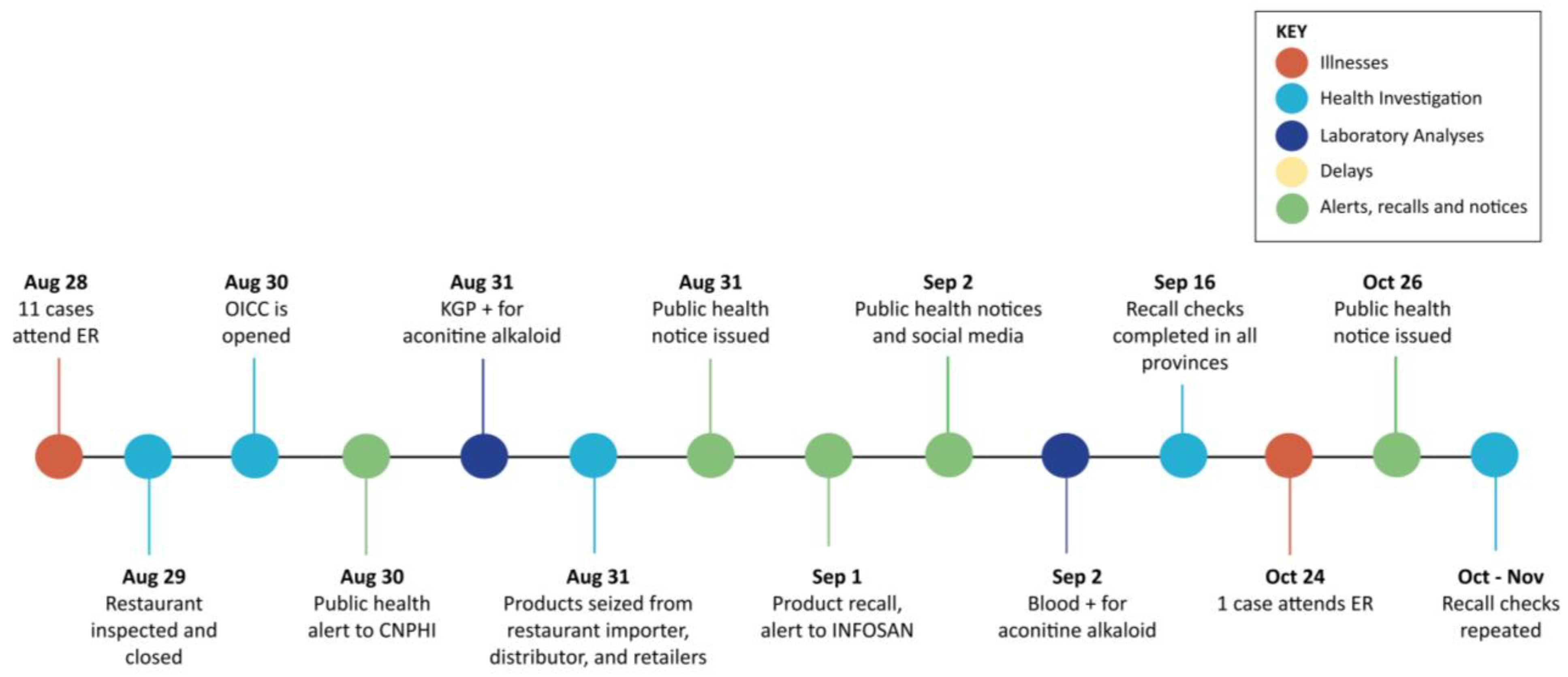
1. Introduction
2. Results
2.1. Case Descriptions
2.2. Blood Aconitine Levels
2.3. Aconitine Testing
2.4. Plant DNA Testing
2.5. Investigation Comparisons
2.5.1. Outbreak Identification
2.5.2. Premises Inspections and Investigations of Product Distribution
2.5.3. Communication Strategies and Interactions Among Agencies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Case Definition for Aconitine Poisoning
5.2. Aconitine Test Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawson, C.; McCabe, D.J.; Feldman, R. A Narrative Review of Aconite Poisoning and Management. J. Intensive Care Med. 2024, 8850666241245703. [Google Scholar] [CrossRef]
- Tai, C.-J.; El-Shazly, M.; Wu, T.-Y.; Lee, K.-T.; Csupor, D.; Hohmann, J.; Chang, F.-R.; Wu, Y.-C. Clinical Aspects of Aconitum Preparations. Planta Med. 2015, 81, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, T.; Huang, Z.; Wang, Y.; Chen, H.; Chen, Q.; Chen, L.; Ao, M. Systematic Evaluation of Toxicity of Aconite Based on Bibliometric Method. Evid. Based Complement. Altern. Med. 2021, 2021, 5514281. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Aconite Poisoning. Clin. Toxicol. 2009, 47, 279–285. [Google Scholar] [CrossRef]
- Chan, T.Y. Causes and Prevention of Herb-Induced Aconite Poisonings in Asia. Hum. Exp. Toxicol. 2011, 30, 2023–2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.L.; Ng, S.W.; Poon, W.T.; Lai, C.K.; Ngan, T.M.S.; Tse, M.L.; Chan, T.Y.K.; Chan, A.Y.W.; Mak, T.W.L. Aconite Poisoning over 5 Years. A Case Series in Hong Kong and Lessons Towards Herbal Safety. Drug Saf. 2012, 35, 575–587. [Google Scholar] [CrossRef]
- So, Y.; Fung, H.; Chung, K.; Hung, C.; Kam, C. A Case of Chuanwu and Fuzi Poisoning. Hong Kong J. Emerg. Med. 2000, 7, 230–233. [Google Scholar] [CrossRef]
- Chan, T.Y. Aconitum Alkaloid Poisoning Related to the Culinary Uses of Aconite Roots. Toxins 2014, 6, 2605–2611. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, H.; Nie, A.; Yang, K.; Xing, H.; Gao, Z.; Yang, L.; Wang, Z.; Zhang, L. Aconitine: A Review of Its Pharmacokinetics, Pharmacology, Toxicology and Detoxification. J. Ethnopharmacol. 2022, 293, 115270. [Google Scholar] [CrossRef]
- IPCS INCHEM Aconitum Napellus spp. (PIM 007). Available online: https://www.inchem.org/documents/pims/plant/aconitum.htm (accessed on 30 August 2023).
- Tai, Y.-T.; Lau, C.-P.; Young, K.; But, P.P.-H. Cardiotoxicity after Accidental Herb-Induced Aconite Poisoning. Lancet 1992, 340, 1254–1256. [Google Scholar] [CrossRef]
- Chan, T.Y. Aconitum Alkaloid Poisoning Because of Contamination of Herbs by Aconite Roots. Phytother. Res. 2016, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- San Francisco Department of Health. Two Cases of Accidental Aconite Poisoning from Medicinal Herbs Purchased in San Francisco Chinatown Shop; San Francisco Marin Medical Society: San Francisco, CA, USA, 2017. Available online: https://www.sfmms.org/news-events/sfmms-blog/sfph-health-alert-two-cases-of-accidental-aconite-poisoning-from-medicinal-herbs-purchased-in-san-francisco-chinatown-shop.aspx (accessed on 1 March 2025).
- Elvin-Lewis, M. Should We Be Concerned about Herbal Remedies. J. Ethnopharmacol. 2001, 75, 141–164. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Associated Press. Woman Dies After Drinking Herbal Tea from SF Chinatown Store; 2017; Available online: https://www.nbcbayarea.com/news/local/two-people-poisoned-after-drinking-herbal-tea-from-chinatown-store-in-san-francisco/47130/ (accessed on 6 March 2025).
- Zhou, C. Poisoning Associated with Consumption of a Homemade Medicinal Liquor—Chongqing, China, 2018. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 569–573. [Google Scholar] [CrossRef]
- Fraser Health Authority. Fraser Health Advises Public to Discard Sand Ginger Powder Purchased at Wing Hing Trading Co. Ltd. in Burnaby. Available online: https://www.fraserhealth.ca/news/2022/Mar/fraser-health-advises-public-to-discard-sand-ginger-powder-purchased-at-wing-hing-trading-co-ltd (accessed on 16 September 2024).
- York Region. York Region Public Health Investigating Potential Toxin in Food. Available online: https://www.york.ca/newsroom/public-notice/york-region-public-health-investigating-potential-toxin-food (accessed on 26 August 2024).
- Government of Canada; Health Canada. Mr. Right Brand Keampferia Galanga Powder (Sand Ginger Powder) Recalled Due to Aconitine Contamination. Available online: https://recalls-rappels.canada.ca/en/alert-recall/mr-right-brand-keampferia-galanga-powder-sand-ginger-powder-recalled-due-aconitine (accessed on 16 September 2024).
- Public Health Agency of Canada. Government of Canada Public Health Notice: Aconitine Toxin Found in Galanga Powder in Ontario with Possible Implications for Other Jurisdictions. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2022/aconitine-toxin-galanga-powder.html (accessed on 16 September 2024).
- Government of Canada; Health Canada. Organism—Aconitum Kusnezoffii. Available online: https://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredReq?id=5843 (accessed on 29 October 2024).
- York Region Food Recall Warning: Mr. Right Brand Keampferia Galanga Powder. Available online: https://www.york.ca/newsroom/news/food-recall-warning-mr-right-brand-keampferia-galanga-powder (accessed on 23 January 2025).
- B.C. Centre for Disease Control; Provincial Health Services Authority. British Columbia Foodborne Illness Outbreak Response Protocol: Guide to Multi-Partner Enteric Outbreak Response in British Columbia. 2024. 2024. Available online: http://www.bccdc.ca/resource-gallery/Documents/BC_FIORP_2024.pdf (accessed on 6 March 2025).
- Ministry of Health. Ontario’s Foodborne Illness Outbreak Response Protocol (ON-FIORP). 2023. Available online: https://www.ontario.ca/files/2023-12/moh-ophs-ref-foodborne-illness-outbreak-response-protocol-en-2023-12-15.pdf (accessed on 6 March 2025).
- Gafner, S.; Blumenthal, M.; Foster, S.; Cardellina, J.H.; Khan, I.A.; Upton, R. Botanical Ingredient Forensics: Detection of Attempts to Deceive Commonly Used Analytical Methods for Authenticating Herbal Dietary and Food Ingredients and Supplements. J. Nat. Prod. 2023, 86, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M. Food Frauds: Global Incidents and Misleading Situations. Trends Food Sci. Technol. 2021, 114, 424–442. [Google Scholar] [CrossRef]
- Observatory of Economic Complexity (OEC) Spices (HS: 0910) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/spices (accessed on 16 September 2024).
- Cadieux, B.; Goodridge, L.D.; Spink, J. Gap Analysis of the Canadian Food Fraud Regulatory Oversight and Recommendations for Improvement. Food Control 2019, 102, 46–55. [Google Scholar] [CrossRef]
- Barrere, V.; Everstine, K.; Théolier, J.; Godefroy, S. Food Fraud Vulnerability Assessment: Towards a Global Consensus on Procedures to Manage and Mitigate Food Fraud. Trends Food Sci. Technol. 2020, 100, 131–137. [Google Scholar] [CrossRef]
- Negi, A.; Pare, A.; Meenatchi, R. Emerging Techniques for Adulterant Authentication in Spices and Spice Products. Food Control 2021, 127, 108113. [Google Scholar] [CrossRef]
- Myers, R.; Vindiola, A.; Phillips, J.G.; Roman, S.; Neal-Kababick, J.; Ji, D.; Khan, I.; Reif, K.; Suzuki, M.; Walker, E.B.; et al. Methods Committee on Dietary Supplements. J. AOAC Int. 2009, 92, 35B–36B. [Google Scholar] [CrossRef]
- Wong, S.-K. Determination of Aconitum Alkaloids in Dietary Supplements and Raw Botanical Materials by Liquid Chromatography/UV Detection with Confirmation by Liquid Chromatography/Tandem Mass Spectrometry: Collaborative Study. J. AOAC Int. 2009, 92, 111–118. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency; Government of Canada. Non-Routine Determination of Aconitine in Spices by Liquid Chromatography-Tandem Mass Spectrometry, CVDR-M-18987177 (unpublished internal document). 2023.
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA Barcodes to Identify Flowering Plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. Lond. B Biol. Sci. 2003. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nishi, K. Genus Identification of Toxic Plant by Real-Time PCR. Int. J. Legal Med. 2011, 125, 211–217. [Google Scholar] [CrossRef] [PubMed]

| Case-Implicated Samples | Non-Case Samples | Controls | |||||
|---|---|---|---|---|---|---|---|
| Collection site | Emergency room | Retail | Customer | Retail | Retail | ||
| Brand/Lot | A/none | A/none | A/none | B | C | ||
| Number of samples | 1 | 1 | 10 | 1 | 1 | 1 | |
| Toxin values (ppm) a | |||||||
| Aconitine | 1034 b | 5900 | 6100 | 0.013–0.030 | <0.005 | <0.005 | 0.01 |
| Hypaconitine | 17 b | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Mesaconitine | NA b,c | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Package size (g) | 70 | 70 | 70 | 1000 | 55 | 55 | |
| Open or closed samples | Open | Closed | Closed | Open | Closed | Closed | |
| Case-Implicated Samples | Non-Case Samples | ||||
|---|---|---|---|---|---|
| Collection site | Restaurant | Distributor | |||
| Brand/Lot | D/X | D/Y | D/Z | D/Y | D/Z |
| Number of samples | 1 | 1 | 1 b | 1 b | 1 c |
| Toxin values (ppm) a | |||||
| Aconitine | 5500 | <0.005 | <0.005 | <0.005 | <0.005 |
| Hypaconitine | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Mesaconitine | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Package size (g) | 454 | 70 | 70 | 1000 | |
| Open or closed samples | Open | Closed | Closed | Open | |
| Number of Samples (n) | Plant ITS Genus Species (%) | Plant psbA | Plant rbcL | CT Value | Common Name of Plants Identified |
|---|---|---|---|---|---|
| BC case sample (Brand A, n = 1) | Aconitum karakolicum (100.00%) | A. stylosum (97.72%) | A. kusnezoffii (99.19%) | 13.34 | Monkshood (Aconitum) |
| ON case sample (Brand D, Lot X, n = 1) | A. talassicum (97.62%) | A. stylosum (95.95%) | A. kusnezoffii (100.00%) | 14.38 | Monkshood (Aconitum) |
| BC sample collected from customer (Brand A, n = 1) | Paeonia lactiflora (100%) | NA 1 | Litsea cubeba (100%) | 29.68 | Chinese peony (P.l), Chinese evergreen tree in family Lauraceae (L.c) |
| BC sample collected from retail (Brand A, n = 1) | A. karakolicum (100.00%) | A. stylosum (98.38%) | A. kusnezoffii (99.68%) | 13.23 | Monkshood (Aconitum) |
| BC sample collected from retail (Brand A, n = 1) | Taraxacum officinale (98.06%) | Oryza eichingeri (99.64%) | Oryza punctata (99.03%) | 30.11 | Common dandelion (T.o.), wild rice (O.e), red rice (O p.) |
| BC sample collected from retail (Brand A, n = 1) | NA | Oryza rufipogon (99.10%) | Oryza sativa (98.75%) | 28.94 | Brownbeard rice (O.r.), rice (O.s.) |
| BC sample collected from retail (Brand A, n = 1) | A. karakolicum (100.00%) | A. stylosum (96.19%) | Oryza punctata (98.55%) | 22.73 | Monkshood, red rice |
| BC sample collected from retail (Brand A, n = 1) | A. karakolicum (99.68%) | A. stylosum (98.11%) | Oryza sativa (98.75%) | 21.73 | Monkshood, rice |
| BC sample collected from retail (Brand A, n = 3) | NA | NA | Oryza glaberrima (99.52%, n=2, 99.31%) | 29.69, 28.73, 25.51 | African rice (O.g.) |
| BC sample collected from retail (Brand A, n = 1) | NA | Persea americana (98.10%) | Oryza punctata (99.36%) | 30.04 | Avocado (P.a.), red rice |
| BC sample collected from retail (Brand A, n = 1) | NA | A. flavum (97.81%) | Oryza punctata (99.52%) | 22.22 | Monkshood, red rice |
| BC sample collected from retail (Brand A, n = 1) | NA | Actinidia chinensis (98.41%) | Machilus japonica (93.48%) | 30.62 | Golden kiwifruit vine (A.c.), Chinese flowering vine (M.j.) |
| ON sample collected from restaurant (open Brand D, Lot Y, n = 1) | Anethum graveolens (98.07%) | Anethum foeniculum (99.13%) | Kaempferia elegans (95.16%) | NA | Dill (A.g.), fennel (A.f.), peacock ginger (K.e.) |
| ON sample collected from retail (closed Brand D, Lot Y, n = 1) | Anethum graveolens (97.15%) | Anethum foeniculum (99.02%) | NA | NA | Dill, fennel |
| ON samples collected from retail (closed Brand D, Lot Z, n = 2) | Cuminum cyminum (87.30%) | NA | NA | NA | Cumin |
| Control sample of sand ginger (Brand B, n = 1) | Cuminum cyminum (100%) | Cuminum cyminum (99.65%) | Cuminum cyminum (99.68%) | 31.13 | Cumin |
| Control sample of sand ginger (Brand C, n = 1) | NA | NA | NA | NA |
| Delays from Time of Illness | BC | ON |
|---|---|---|
| to site visit and product holds | 13 days | 11 h |
| to follow-up at retail and distributor levels | 13 days | 14.5 h |
| to importer traceback | 18 days | 1.5 days |
| to confirmatory toxin testing of case blood | ND | 5 days (RD + 7 days) |
| to confirmatory toxin testing of spice | 27 days | 3 days |
| to confirmatory Aconitum plant DNA | 53 days (RD + 12 days) | RP |
| to public health notice | 32 days | 3 days |
| Laboratory (Location) | Methods |
|---|---|
| BC Institute of Technology Natural Health and Food Products Research Group (Burnaby, BC) | KGP was tested for toxic alkaloids via LC/MS methods based on the AOAC International Official Method of Analysis for the determination of Aconitum alkaloids aconitine, mesaconitine and hypaconitine in dietary supplements and botanical materials [31,32]. Chromatographic separation was achieved on an Agilent 1290 Infinity II LC system coupled with an Agilent 6420 Triple Quad Spectrometer (Agilent Technologies Inc., Mississauga, ON, Canada) using a Waters Xterra RP18 column, 2.1 × 50 mm, 3.5 μm (Waters Ltd., Mississauga, ON, Canada). |
| Canadian Food Inspection Agency (federal) Toxin Laboratory (Saskatoon, Saskatchewan) | A representative sub-sample of KGP was extracted with an acidified mixture of methanol and water for 60 min at 60 °C while shaking. Extracts were cooled, centrifuged, diluted and analysed using a tandem mass spectrometer coupled with liquid chromatography (LC-MS/MS) (Sciex 5500 QTRAP, Framingham, MA, zUSA) [33]. Chromatographic separation was achieved using an Acquity BEH C18 column, 2.1 × 30 mm, 1.7 µm (Waters Ltd., Mississauga, ON, Canada). Selected reaction monitoring (SRM) transitions were acquired following positive-ionization electrospray: 646.3 > 586.3, 646.3 > 526.3, 646.3 > 368.3. |
| Canadian Food Inspection Agency (federal) Plant DNA Laboratory (Ottawa, ON) | To test KGP for Aconitum DNA, samples were assayed by DNA barcoding [34,35] at the internal transcribed spacer (ITS), the photosystem II protein D1 (psbA) and ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) loci to detect the presence of Aconitum plant DNA using bidirectional Sanger sequencing with a BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies Inc., Carlsbad, CA, USA) on an ABI 3730xl Genetic Analyzer (Life Technologies Inc., Carlsbad, CA, USA). An assay targeting a region of the ITS locus specific to toxic plants of the Aconitum genus [36] was also adapted to a TaqMan Real-time PCR assay (qPCR) and ran on a ViiA7 instrument (Life Technologies Inc., Carlsbad, CA, USA), using A. napellus as a positive control. TaqMan qPCR primers and probe sequences were the following (5′ 3′): F-ACGGTCGGCACAAATGTT, R-CGACGCGTCTTGATGTCTTT, PRB-FAM/CGGTCAGTG/ZEN/GTGGTTGTATTTCTCATCC. |
| Centre of Forensic Sciences (Toronto, ON) | Clinical blood samples were tested for toxic alkaloids. Submitted blood samples underwent sample preparation and were analysed using a Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS) method to screen for various over-the-counter, recreational and prescription drugs, poisons and metabolites, including acotinine. A Waters Acquity UPLC I-Class System, with an Acquity UPLC HSS C18 1.8 µm 2.1 × 150 mm column, was used for chromatographic separation coupled with a Xevo G2-S QToF for identification. Aconitine was reported as detected once the minimum acceptance criteria for mass error (ppm), retention time error and expected fragment counts were met. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntyre, L.; Georgopoulos, S.; Simone, D.; Newhouse, E.; Fernandes, J.; McVea, D.A.; Fok, A.; McIntyre, A.-M.; Shurmer, B.; Gagnon, M.-C.; et al. Learnings from Separate Aconitum Poisonings in British Columbia and Ontario, Canada in 2022. Toxins 2025, 17, 125. https://doi.org/10.3390/toxins17030125
McIntyre L, Georgopoulos S, Simone D, Newhouse E, Fernandes J, McVea DA, Fok A, McIntyre A-M, Shurmer B, Gagnon M-C, et al. Learnings from Separate Aconitum Poisonings in British Columbia and Ontario, Canada in 2022. Toxins. 2025; 17(3):125. https://doi.org/10.3390/toxins17030125
Chicago/Turabian StyleMcIntyre, Lorraine, Stefanie Georgopoulos, Dorianna Simone, Emily Newhouse, JoAnne Fernandes, David A. McVea, Arnold Fok, Ania-Maria McIntyre, Bryn Shurmer, Marie-Claude Gagnon, and et al. 2025. "Learnings from Separate Aconitum Poisonings in British Columbia and Ontario, Canada in 2022" Toxins 17, no. 3: 125. https://doi.org/10.3390/toxins17030125
APA StyleMcIntyre, L., Georgopoulos, S., Simone, D., Newhouse, E., Fernandes, J., McVea, D. A., Fok, A., McIntyre, A.-M., Shurmer, B., Gagnon, M.-C., Chan, M., Chiaravalloti, M., Saha Turna, N., Kent, D., Leong, D., Paphitis, K., Lee, C., & the Outbreak Investigation Teams. (2025). Learnings from Separate Aconitum Poisonings in British Columbia and Ontario, Canada in 2022. Toxins, 17(3), 125. https://doi.org/10.3390/toxins17030125





