The Proteolytic Activation, Toxic Effects, and Midgut Histopathology of the Bacillus thuringiensis Cry1Ia Protoxin in Rhynchophorus ferrugineus (Coleoptera: Curculionidae)
Abstract
1. Introduction
2. Results
2.1. Establishment of a Laboratory Colony of RPW
2.2. Obtainment of Cry1Ia Protoxin
2.3. Incubation of Cry1Ia Protoxin with RPW Midgut Proteases
2.4. Toxicity of Cry1Ia Protoxin to RPW Larvae
2.5. Intoxication with Cry1Ia Protoxin Damaged the Gut Epithelium of RPW Larvae
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Cloning of Cry1Ia Gene
4.3. Expression and Purification of 6xHis-Cry1Ia Protoxin
4.4. Western Blotting
4.5. Obtainment of Cry1Ac Protoxin
4.6. Midgut Juice Isolation and in Vitro Processing of Protoxins
4.7. Insect Bioassays
4.8. Histopathological Studies
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ávalos, J.A.; Martí-Campoy, A.; Soto, A. Study of the flying ability of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) adults using a computer-monitored flight mill. Bull. Entom. Res. 2014, 104, 462–470. [Google Scholar] [CrossRef]
- Rochat, D.; Dembilio, O.; Jaques, J.A.; Suma, P.; Pergola, A.L.; Hamidi, R.; Kontodimas, D.; Soroker, V. Rhynchophorus ferrugineus: Taxonomy, distribution, biology, and life cycle. In Handbook of Major Palm Pests: Biology and Management; Soroker, V., Colazza, S., Eds.; John Wiley and Sons: Oxford, UK, 2017; pp. 69–104. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization). Rhynchophorus ferrugineus. EPPO Datasheets on Pests Recommended for Regulation. Available online: https://gd.eppo.int (accessed on 20 December 2024).
- Faleiro, J. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Naveed, H.; Andoh, V.; Islam, W.; Chen, L.; Chen, K. Sustainable pest management in date palm ecosystems: Unveiling the ecological dynamics of Red Palm Weevil (Coleoptera: Curculionidae) infestations. Insects 2023, 14, 859. [Google Scholar] [CrossRef]
- European Commission. The Insect Killing our Palm Trees. In EU Efforts to Stop the Red Palm Weevil; Office for Official Publications of the European Communities: Luxembourg, 2011; pp. 1–32. ISBN 978-92-79-21268-0. [Google Scholar]
- Manachini, B.; Billeci, N.; Palla, F. Exotic insect pests: The impact of the Red Palm Weevil on natural and cultural heritage in Palermo (Italy). J. Cult. Herit. 2013, 14, e177–e182. [Google Scholar] [CrossRef]
- Castillo, A.; van den Bergh, J.C.; Savin, I.; Monteys, V. Cost-benefit analysis of conservation policy: The red palm weevil in Catalonia, Spain. Ecol. Econ. 2020, 167, 106453. [Google Scholar] [CrossRef]
- DRAPC (Direção Regional de Agricultura e Pescas do Centro). Plano de Ação para o controlo de Rhynchophorus ferrugineus (Olivier), ver. 01. Available online: https://www.drapc.gov.pt/base/documentos/plano_accao_r%20_ferrugineus%20_2013_dgav.pdf (accessed on 2 October 2024).
- Boavida, C.; Franca, M.F. Rhynchophorus ferrugineus (Olivier, 1790) (coleoptera: Curculionoidea: Dryophthoridae), an exotic weevil recently introduced into Portugal. Boletín Soc. Entomológica Aragonesa 2008, 42, 425–426. [Google Scholar]
- Pete, N. Rhynchophorus ferrugineus in Europe survey results. In Proceedings of the International Conference on Red Palm Weevil, Valencia, Spain, 29–31 March 2010; p. 37. [Google Scholar]
- Ramos, A.P.; Caetano, M.F.; Rocha, M.; Lima, S.B. Doenças e pragas que condicionam o uso de palmeiras em espaços verdes. Revista APH 2013, 112, 37–40. [Google Scholar]
- Fernandes, D. Pragas no município do Porto: Monitorização e proposta de gestão de três espécies de insetos. Master’s Thesis, Lisbon University, Lisboa, Portugal, 2016. Available online: https://repositorio.ul.pt/bitstream/10451/25300/1/ulfc120675_tm_Diana_Fernandes.pdf (accessed on 1 September 2024).
- Llácer, E.; Jacas, J.A. Efficacy of phosphine as a fumigant against Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in palms. Span. J. Agric. Res. 2010, 8, 775–779. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Qayyum, M.A.; Ghazanfar, M.U.; Al-Sadi, A.M.; Bedford, G.O.; Kwon, Y.J. Resistance to commonly used insecticides and phosphine fumigant in red palm weevil, Rhynchophorus ferrugineus (Olivier) in Pakistan. PLoS ONE 2018, 13, e0192628. [Google Scholar] [CrossRef]
- Hussain, A.; Haq, M.R.U.; Al-Jabr, A.M.; Al-Ayied, H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013, 11, 456–463. [Google Scholar]
- Na, S.-M.; Im, G.-I.; Lee, W.-S.; Kim, D.-G. Assessment of attractant combinations for the management of red palm weevils (Rhynchophorus ferrugineus) in the United Arab Emirates. Insects 2024, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Rehman, G.; Mamoon-ur-Rashid, M. Evaluation of entomopathogenic nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Insects 2022, 13, 733. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.M.; Almasoud, M.; Alsulaiman, Y.M.; Baeshen, R.S.; Elshazly, H.; Kadi, R.H.; Hassan, M.M.; Shawer, R. Efficiency of Bacillus thuringiensis and Bacillus cereus against Rhynchophorus ferrugineus. Insects 2022, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Ment, D.; Levy, N.; Allouche, A.; Davidovitz, M.; Yaacobi, G. Efficacy of entomopathogenic fungi as prevention against early life stages of the Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in laboratory and greenhouse trials. Insects 2023, 14, 918. [Google Scholar] [CrossRef]
- Wakil, W.; Boukouvala, M.C.; Kavallieratos, N.G.; Filintas, C.S.; Eleftheriadou, N.; Ghazanfar, M.U.; Yasin, M.; Qayyum, M.A.; Avery, P.B. Current status of biology–biotechnic, agronomic, and biological control of Rhynchophorus ferrugineus: A review. Insects 2024, 15, 955. [Google Scholar] [CrossRef]
- Husain, M.; Rasool, K.G.; Sutanto, K.D.; Omer, A.O.; Tufail, M.; Aldawood, A.S. Laboratory evaluation of indigenous and commercial entomopathogenic nematodes against Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects 2024, 15, 290. [Google Scholar] [CrossRef]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Azizoglu, U.; Salehi Jouzani, G.; Sansinenea, E.; Sanchis-Borja, V. Biotechnological advances in Bacillus thuringiensis and its toxins: Recent updates. Rev. Environ. Sci. Biotechnol. 2023, 22, 319–348. [Google Scholar] [CrossRef]
- Kumar, P.; Kamle, M.; Borah, R.; Mahato, D.K.; Sharma, B. Bacillus thuringiensis as microbial biopesticide: Uses and application for sustainable agriculture. Egypt. J. Biol. Pest Control 2021, 31, 95. [Google Scholar] [CrossRef]
- Knowles, B.H.; Ellar, D.J. Colloid-osmotic lysis is a general feature of Bacillus thuringiensis delta-endotoxins. J. Cell Sci. 1987, 88, 197–206. [Google Scholar]
- Liu, L.; Bulla, L.A., Jr. Cell death signaling in Anopheles gambiae initiated by Bacillus thuringiensis Cry4B toxin involves Na+/K+ ATPase. Exp. Biol. Med. 2023, 248, 1191–1205. [Google Scholar] [CrossRef]
- Shahina, F.; Salma, J.; Mehreen, G.; Bhatti, M.; Tabassum, K. Rearing of Rhynchophorus ferrugineus in laboratory and field conditions for carrying out various efficacy studies using EPNs. Pak. J. Nematol. 2009, 27, 219–228. [Google Scholar]
- Guo, Y.; Sun, Y.; Liao, Q.; Carballar-Lejarazú, R.; Sheng, L.; Wang, S.; Zhou, J.; Zhang, F.; Wu, S. Proteolytic activation of Bacillus thuringiensis Cry3Aa toxin in the Red Palm Weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2021, 114, 2406–2411. [Google Scholar] [CrossRef]
- Qureshi, A.; Keen, E.; Brown, G.; Cator, L. The size of larval rearing container modulates the effects of diet amount and larval density on larval development in Aedes aegypti. PLoS ONE 2023, 18, e0280736. [Google Scholar] [CrossRef]
- Al-Ayedh, H. Evaluating a semi-synthetic diet for rearing the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2011, 31, 20–28. [Google Scholar] [CrossRef]
- Aldawood, A.S.; Rasool, K.G.; Sukirno, S.; Husain, M.; Sutanto, K.D.; Alduailij, M.A. Semi-artificial diet developed for the successful rearing of red palm weevil: Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) in the laboratory. J. King Saud Univ. Sci. 2022, 34, 102272. [Google Scholar] [CrossRef]
- Domínguez-Arrizabalaga, M.; Villanueva, M.; Escriche, B.; Ancín-Azpilicueta, C.; Caballero, P. Insecticidal activity of Bacillus thuringiensis proteins against Coleopteran Pests. Toxins 2020, 12, 430. [Google Scholar] [CrossRef]
- Walters, F.S.; Stacy, C.M.; Lee, M.K.; Palekar, N.; Chen, J.S. An engineered chymotrypsin/cathepsin G site in domain I renders Bacillus thuringiensis Cry3A active against Western corn rootworm larvae. Appl. Environ. Microbiol. 2008, 74, 367–374. [Google Scholar] [CrossRef]
- Guo, C.H.; Zhao, S.T.; Ma, Y.; Hu, J.J.; Han, X.J.; Chen, J.; Lu, M.Z. Bacillus thuringiensis Cry3Aa fused to a cellulase-binding peptide shows increased toxicity against the longhorned beetle. Appl. Microbiol. Biotechnol. 2011, 93, 1249–1256. [Google Scholar] [CrossRef]
- Tailor, R.; Tippett, J.; Gibb, G.; Pells, S.; Pike, D.; Jordan, L.; Ely, S. Identification and characterization of a novel Bacillus thuringiensis delta-endotoxin entomocidal to coleopteran and lepidopteran larvae. Mol. Microbiol. 1992, 6, 1211–1217. [Google Scholar] [CrossRef]
- Grossi-de-Sa, M.F.; Quezado de Magalhaes, M.; Silva, M.S.; Silva, S.M.; Dias, S.C.; Nakasu, E.Y.; Brunetta, P.S.; Oliveira, G.R.; Neto, O.B.; Sampaio de Oliveira, R.; et al. Susceptibility of Anthonomus grandis (cotton boll weevil) and Spodoptera frugiperda (fall armyworm) to a cry1ia-type toxin from a Brazilian Bacillus thuringiensis strain. J. Biochem. Mol. Biol. 2007, 40, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.S.; Aguiar, R.W.; Martins, N.F.; Melatti, V.M.; Falcão, R.; Gomes, A.C.; Ribeiro, B.M.; Monnerat, R.G. Recombinant Cry1Ia protein is highly toxic to cotton boll weevil (Anthonomus grandis Boheman) and fall armyworm (Spodoptera frugiperda). J. Appl. Microbiol. 2008, 104, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Ross, P.A. Rates and patterns of laboratory adaptation in (mostly) insects. J. Econ. Entomol. 2018, 111, 501–509. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.A.; Negri, B.F.; Hernández-Martínez, P.; Basso, M.F.; Escriche, B. Mpp23Aa/Xpp37Aa insecticidal proteins from Bacillus thuringiensis (Bacillales: Bacillaceae) are highly toxic to Anthonomus grandis (Coleoptera: Curculionidae) larvae. Toxins 2023, 15, 55. [Google Scholar] [CrossRef]
- Contreras, E.; Rausell, C.; Real, M.D. Proteome response of Tribolium castaneum larvae to Bacillus thuringiensis toxin producing strains. PLoS ONE 2013, 8, e0055330. [Google Scholar] [CrossRef]
- Ekobu, M.; Solera, M.; Kyamanywa, S.; Mwanga, R.O.; Odongo, B.; Ghislain, M.; Moar, W.J. Toxicity of seven Bacillus thuringiensis Cry proteins against Cylas puncticollis and Cylas brunneus (Coleoptera: Brentidae) using a novel artificial diet. J. Econ. Entomol. 2010, 103, 1493–1502. [Google Scholar] [CrossRef]
- Hernández-Martínez, P.; Khorramnejad, A.; Prentice, K.; Andrés-Garrido, A.; Vera-Velasco, N.M.; Smagghe, G.; Escriche, B. The independent biological activity of Bacillus thuringiensis Cry23Aa protein against Cylas puncticollis. Front. Microbiol. 2020, 11, 1734. [Google Scholar] [CrossRef]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. 2001, 47, 47–61. [Google Scholar] [CrossRef]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef]
- Bergamasco, V.B.; Mendes, D.R.; Fernandes, O.A.; Desidério, J.A.; Lemos, M.V. Bacillus thuringiensis Cry1Ia10 and Vip3Aa protein interactions and their toxicity in Spodoptera spp. (Lepidoptera). J. Invertebr. Pathol. 2013, 112, 152–158. [Google Scholar] [CrossRef]
- Lightwood, D.J.; Ellar, D.J.; Jarrett, P. Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac δ-endotoxin. Appl. Environ. Microbiol. 2000, 66, 5174–5181. [Google Scholar] [CrossRef] [PubMed]
- Ayra-Pardo, C.; Davis, P.; Ellar, D.J. The mutation R(423)S in the Bacillus thuringiensis hybrid toxin CryAAC slightly increases toxicity for Mamestra brassicae L. J. Invertebr. Pathol. 2007, 95, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B. Protease interactions with Bacillus thuringiensis insecticidal toxins. Arch. Insect Biochem. Physiol. 1999, 42, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Oppert, B.; Higgins, R.A.; Huang, F.; Zhu, K.Y.; Buschman, L.L. Comparative analysis of proteinase activities of Bacillus thuringiensis-resistant and -susceptible Ostrinia nubilalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 2004, 34, 753–762. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Ge, A.Z.; Shivarova, N.I.; Dean, D.H. Location of the Bombyx mori specificity domain on a Bacillus thuringiensis delta-endotoxin protein. Proc. Natl. Acad. Sci. USA 1989, 86, 4037–4041. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 5 August 2023).
- Venables, W.N.; Ripley, B.D. MASS: Support Functions and Datasets for Venables and Ripley’s MASS. R Package Version 7.3-60. 2023. Available online: https://CRAN.R-project.org/package=MASS (accessed on 5 August 2023).
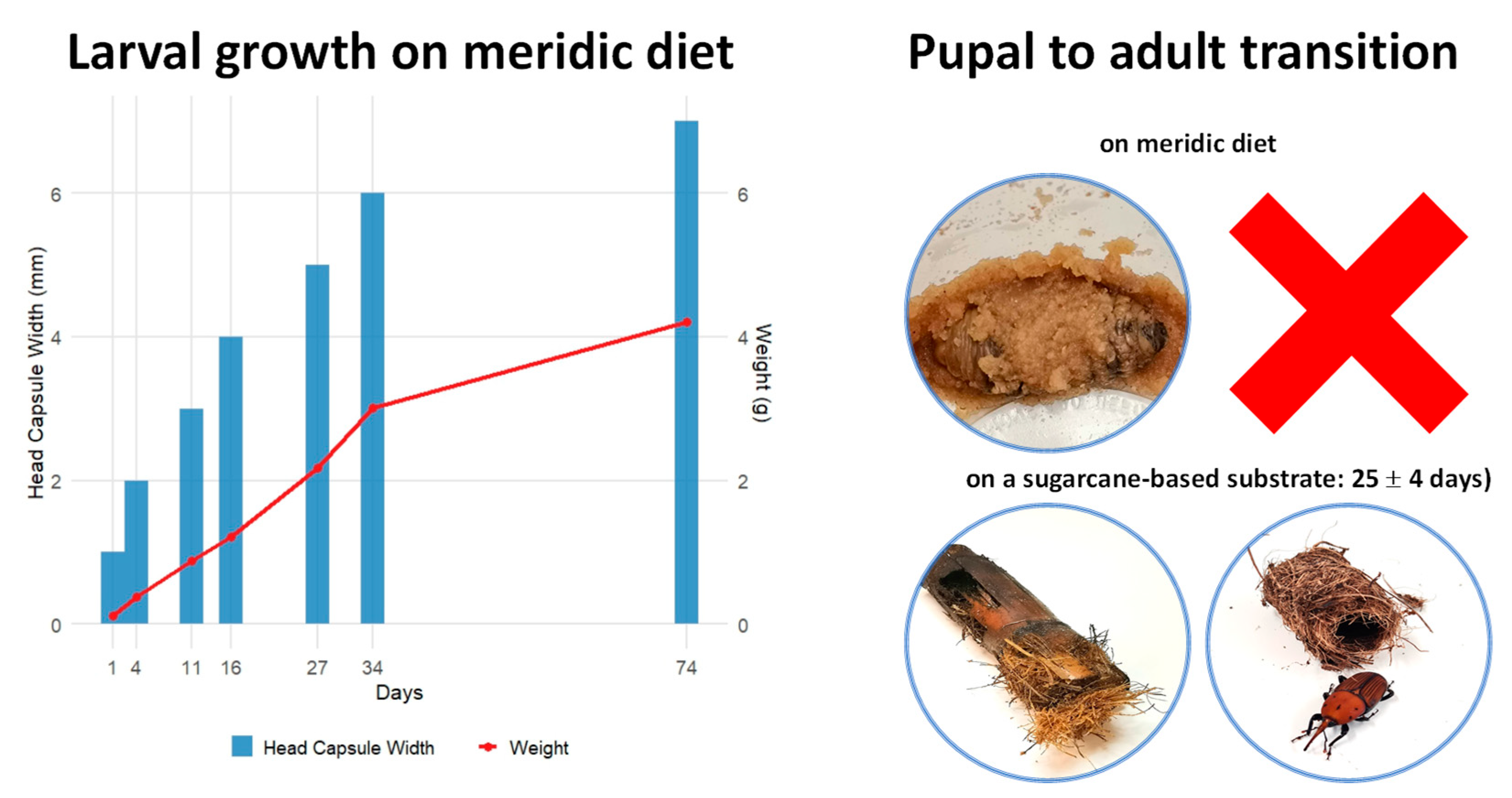




| Ingredient | Quantity |
|---|---|
| Distilled water | 900 mL |
| Agar | 15.0 g |
| Wheat germ | 45.0 g |
| Maize flour | 45.0 g |
| Brewer’s yeast | 7.0 g |
| Vitamin mix | 6.0 g |
| Ascorbic acid | 4.0 g |
| Methylparaben | 3.3 g |
| Fabco-I | 2.5 g |
| Sorbic acid | 1.6 g |
| Apple smoothie | 100 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayra-Pardo, C.; Ramaré, V.; Couto, A.; Almeida, M.; Martins, R.; Sousa, J.A.; Santos, M.J. The Proteolytic Activation, Toxic Effects, and Midgut Histopathology of the Bacillus thuringiensis Cry1Ia Protoxin in Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Toxins 2025, 17, 84. https://doi.org/10.3390/toxins17020084
Ayra-Pardo C, Ramaré V, Couto A, Almeida M, Martins R, Sousa JA, Santos MJ. The Proteolytic Activation, Toxic Effects, and Midgut Histopathology of the Bacillus thuringiensis Cry1Ia Protoxin in Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Toxins. 2025; 17(2):84. https://doi.org/10.3390/toxins17020084
Chicago/Turabian StyleAyra-Pardo, Camilo, Victor Ramaré, Ana Couto, Mariana Almeida, Ricardo Martins, José Américo Sousa, and Maria João Santos. 2025. "The Proteolytic Activation, Toxic Effects, and Midgut Histopathology of the Bacillus thuringiensis Cry1Ia Protoxin in Rhynchophorus ferrugineus (Coleoptera: Curculionidae)" Toxins 17, no. 2: 84. https://doi.org/10.3390/toxins17020084
APA StyleAyra-Pardo, C., Ramaré, V., Couto, A., Almeida, M., Martins, R., Sousa, J. A., & Santos, M. J. (2025). The Proteolytic Activation, Toxic Effects, and Midgut Histopathology of the Bacillus thuringiensis Cry1Ia Protoxin in Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Toxins, 17(2), 84. https://doi.org/10.3390/toxins17020084







