Metabolomic Profiling of Human Urine Related to Mycotoxin Exposure
Abstract
1. Introduction
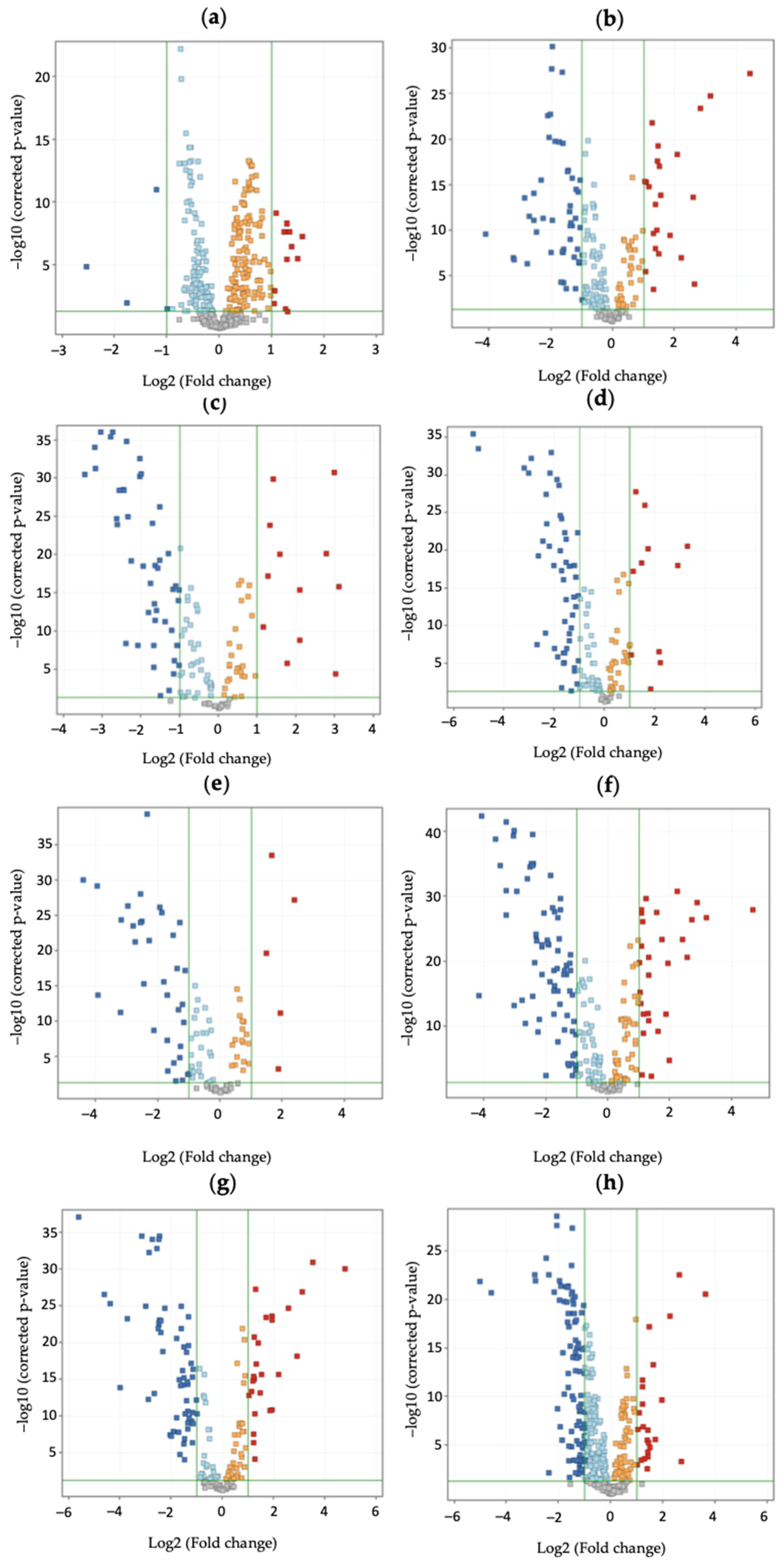
2. Results
3. Discussion
3.1. Lipid Metabolism
3.2. Linoleic Acid Metabolism
3.3. Glycine, Serine, and Threonine Metabolism
3.4. Arginine and Proline Metabolism
3.5. Strengths and Limitations
4. Conclusions
5. Materials and Methods
5.1. Study Population and Design
5.2. Sample Extraction Essays
5.3. LC-Q-TOF-MS Analysis
5.4. Data Processing
5.5. Statistical Analysis
5.6. Metabolite Identification and Metabolite Pathway Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFs | Aflatoxins |
| AFB1 | Aflatoxin B1 |
| AFBO | Aflatoxin B1-8,9-epoxide |
| BEA | Beauvericin |
| CHEBI | Chemical Entities of Biological Interest |
| DON | Deoxynivalenol |
| ENNs | Enniatins |
| ENNA | Enniatin A |
| ENNB | Enniatin B |
| ESI | Electrospray ionization |
| HMDB | Human Metabolome Database |
| HPLC | High performance liquid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC | Liquid chromatography |
| OTα | Ochratoxin alpha |
| PAT | Patulin |
| PUFA | Polyunsaturated fatty acid |
| QCs | Quality control samples |
| QSAR | Quantitative structure–activity relationship |
| Q-TOF | Quadrupole time of flight |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| UHD | Ultra-High Definition |
References
- Habschied, K.; Kanizai Saric, G.; Krstanovic, V.; Mastanjevic, K. Mycotoxins—Biomonitoring and Human Exposure. Toxins 2021, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Ramos, A.J.; Sanchis, V.; Cano-Sancho, G. An overview of mycotoxin biomarker application in exposome-health studies. Curr. Opin. Food Sci. 2021, 39, 31–35. [Google Scholar] [CrossRef]
- Serasinghe, N.; Vepsäläinen, H.; Lehto, R.; Abdollahi, A.M.; Erkkola, M.; Roos, E.; Ray, C. Associations between socioeconomic status, home food availability, parental role-modeling, and children’s fruit and vegetable consumption: A mediation analysis. BMC Public Health 2023, 23, 1037. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.K.; Stamos, G.; Mitchell, A.J.; Gonoud, R.; Horgan, A.M.; Nomura, O.; Young, A.; Nigg, J.T.; Gustafsson, H.C.; Sullivan, E.L. The association between food desert severity, socioeconomic status, and metabolic state during pregnancy in a prospective longitudinal cohort. Sci. Rep. 2023, 13, 7197. [Google Scholar] [CrossRef]
- Dasí-Navarro, N.; Lozano, M.; Llop, S.; Vioque, J.; Peiró, J.; Esplugues, A.; Manyes, L.; Vila-Donat, P. Associated factors with mycotoxin exposure in Spanish population. Environ. Res. 2024, 242, 117618. [Google Scholar] [CrossRef]
- Cimbalo, A.; Frangiamone, M.; Juan, C.; Font, G.; Lozano, M.; Manyes, L. Proteomics evaluation of enniatins acute toxicity in rat liver. Food Chem. Toxicol 2021, 151, 112130. [Google Scholar] [CrossRef]
- Hasuda, A.L.; Bracarense, A.P.F.R.L. Toxicity of the emerging mycotoxins beauvericin and enniatins: A mini-review. Toxicon 2024, 239, 107534. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Alonso-Garrido, M.; Tedeschi, P.; Maietti, A.; Font, G.; Marchetti, N.; Manyes, L. Mitochondrial transcriptional study of the effect of aflatoxins, enniatins and carotenoids in vitro in a blood brain barrier model. Food Chem. Toxicol. 2020, 137, 111077. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Yaoxing Chen, Y. Neurotoxic mechanisms of mycotoxins: Focus on aflatoxin B1 and T-2 toxin. Environ. Pollut. 2024, 356, 124359. [Google Scholar] [CrossRef]
- Pérez-Fuentes, N.; Alvariño, R.; Alfonso, A.; González-Jartín, J.; Gegunde, S.; Vieytes, M.R.; Luis, M.; Botana, L.M. Enniatins A1 and B1 alter calcium homeostasis of neuronal cells leading to apoptotic death. Food Chem. Toxicol. 2022, 168, 113361. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Llompart, M.; Zanni, R.; Manyes, L.; Meca, G. Elucidating the mechanism of action of mycotoxins through machine learning-driven QSAR models: Focus on lipid peroxidation. Food Chem. Toxicol. 2023, 182, 114120. [Google Scholar] [CrossRef]
- Jorvekar, S.B.; Jala, A.; Rai, A.; Jangili, S.; Adla, D.; Borkar, G.; Das, A.; Kakati, K.; Das, K.; Sarma, A.; et al. Urinary Metabolomics Identified Metabolic Perturbations Associated with Gutka, a Smokeless Form of Tobacco. Chem. Res. Toxicol. 2023, 36, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, D.M.; Mendes, M.P.R.; Marciano, L.P.d.A.; Costa, L.F.; Macedo Sakakibara, I.M.; Silvério, A.C.P.; Paiva, M.J.N.; André, L.C. An Exploratory Study of the Metabolite Profiling from Pesticides Exposed Workers. Metabolites 2023, 13, 596. [Google Scholar] [CrossRef]
- Pal, S.; Rendedula, D.; Nagendla, N.K.; Kaliyaperumal, M.; Mudiam, M.K.R.; Ansari, K.M. Serum and urine metabolomics analysis reveals the role of altered metabolites in patulin-induced nephrotoxicity. Food Res. Int. 2022, 156, 111177. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kinnebrew, G.; Hsu, P.-C.; Weng, D.Y.; Song, M.-A.; Reisinger, S.A.; McElroy, J.P.; Keller-Hamilton, B.; Ferketich, A.K.; Freudenheim, J.L.; et al. Untargeted Metabolomics and Body Mass in Adolescents: A Cross-Sectional and Longitudinal Analysis. Metabolites 2023, 13, 899. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Upadhyay, D.; Sharma, U.; Jagannathan, N.; Singh, S.B.; Ganju, L. Urine metabolite profiling of Indian Antarctic Expedition members: NMR spectroscopy-based metabolomic investigation. Heliyon 2021, 7, e07114. [Google Scholar] [CrossRef]
- Jala, A.; Dutta, R.; Josyula, J.V.N.; Mutheneni, S.R.; Borkar, R.M. Environmental phenol exposure associates with urine metabolome alteration in young Northeast Indian females. Chemosphere 2023, 317, 137830. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Hu, P.; Zhang, Y.; Liu, Y.; Yang, Q.; Xu, L.; Gong, Z.; Yang, J.; Sun, W.; et al. Impact of enniatins and beauvericin on lipid metabolism: Insights from a 3D HepaRG spheroid model. Environ. Int. 2024, 191, 108969. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, L.; Liu, Q.; Wang, P.; Chen, H.; Wang, C. Different metabolites induced by deoxynivalenol in the serum and urine of weaned rabbits detected using LC–MS-based metabolomics. Comp. Biochem. Physiol. Part C 2021, 250, 109184. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.Y.; Lee, S.Y.; Park, S.B.; Chun, H.S. Simultaneous determination of 17 regulated and non-regulated Fusarium mycotoxins co-occurring in foodstuffs by UPLC-MS/MS with solid-phase extraction. Food Chem. 2024, 438, 137624. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Ramos, J.A.; Marín-Sáez, J.; Sanchis, V.; Gámiz-Gracia, L.; García-Campaña, A.M.; Hernández-Mesa, M.; Cano-Sancho, G. Simultaneous detection of mycotoxins and pesticides in human urine samples: A 24-h diet intervention study comparing conventional and organic diets in Spain. Food Chem. Toxicol. 2024, 188, 114650. [Google Scholar] [CrossRef] [PubMed]
- De Sá, S.V.M.; Faria, M.A.; Fernandes, J.O.; Cunha, S.C. Investigating the individual and mixture cytotoxicity of co-occurring aflatoxin B1, enniatin B, and sterigmatocystin on gastric, intestinal, hepatic, and renal cellular models. Food Chem. Toxicol. 2024, 188, 114640. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, T.; Liao, G.; Gu, G.; Hou, R.; Qiu, J. Lipidomic profiling study on neurobehavior toxicity in zebrafish treated with aflatoxin B. Sci. Total Environ. 2023, 898, 165553. [Google Scholar] [CrossRef]
- Pérez-Fuentes, N.; Alvariño, R.; Alfonso, A.; González-Jartín, J.; Gegunde, S.; Vieytes, M.R.; Luis, M.; Botana, L.M. Single and combined effects of regulated and emerging mycotoxins on viability and mitochondrial function of SH-SY5Y cells. Food Chem. Toxicol. 2021, 154, 112308. [Google Scholar] [CrossRef] [PubMed]
- Søderstrøm, S.; Lie, K.K.; Lundebye, A.-K.; Søfteland, L. Beauvericin (BEA) and enniatin B (ENNB)-induced impairment of mitochondria and lysosomes—Potential sources of intracellular reactive iron triggering ferroptosis in Atlantic salmon primary hepatocytes. Food Chem. Toxicol. 2022, 161, 112819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Song, J. Important roles of linoleic acid and α-linolenic acid in regulating cognitive impairment and neuropsychiatric issues in metabolic-related dementia. Life Sci. 2024, 337, 122356. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.P. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cáncer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Rogalsky, D.K.; Martindale, R.G. Nutritional and Metabolic Therapy. In Pharmacology and Physiology for Anesthesia, 2nd ed.; Hemmings, H.C., Jr., Egan, T.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 657–670. [Google Scholar] [CrossRef]
- Oberkersch, R.E.; Santoro, M.M. Role of amino acid metabolism in angiogenesis. Vasc. Pharmacol. 2019, 112, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, M.; Chang, M.; Hou, X.; Wang, F.; Song, W.; Wang, Y.; Feng, K.; Yuan, Y.; Yue, T. Integrated transcriptomics and metabolomics reveal the mechanism of intestinal damage upon acute patulin exposure in mice. Ecotoxicol. Environ. Saf. 2024, 276, 116270. [Google Scholar] [CrossRef]
- Li, M.; Fang, Q.; Xiu, L.; Yu, L.; Peng, S.; Wu, X.; Chen, X.; Niu, X.; Wang, G.; Kong, Y. The molecular mechanisms of alpha-lipoic acid on ameliorating aflatoxin B1-induced liver toxicity and physiological dysfunction in northern snakehead (Channa argus). Aquat. Toxicol. 2023, 257, 106466. [Google Scholar] [CrossRef] [PubMed]
- Guxens, M.; Ballester, F.; Espada, M.; Fernández, M.F.; Grimalt, J.O.; Ibarluzea, J.; Olea, N.; Rebagliato, M.; Tardón, A.; Torrent, M.; et al. Cohort profile: The INMA—INfancia y Medio ambiente—(environment and childhood) Project. Int. J. Epidemiol. 2012, 41, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Dasí-Navarro, N.; Lozano, M.; Llop, S.; Esplugues, A.; Cimbalo, A.; Font, G.; Manyes, L.; Mañes, J.; Vila-Donat, P. Development and validation of LC-Q-TOF-MS methodology to determine mycotoxin biomarkers in human urine. Toxins 2022, 14, 651. [Google Scholar] [CrossRef]
- Obradović, D.; Fedorova, E.; Bogojević, A.; Shpigun, O.; Buryak, A.; Lazović, S. A comparative study of the predictive performance of different descriptor calculation tools: Molecular-based elution order modeling and interpretation of retention mechanism for isomeric compounds from METLIN database. J. Chromatogr. A. 2024, 1719, 464731. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Hastings, J.; Owen, G.; Dekker, A.; Ennis, M.; Kale, N.; Muthukrishnan, V.; Turner, S.; Swainston, N.; Mendes, P.; Steinbeck, C. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016, 44, D1214–D1219. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.; MacDonald, P.; Wishart, D.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
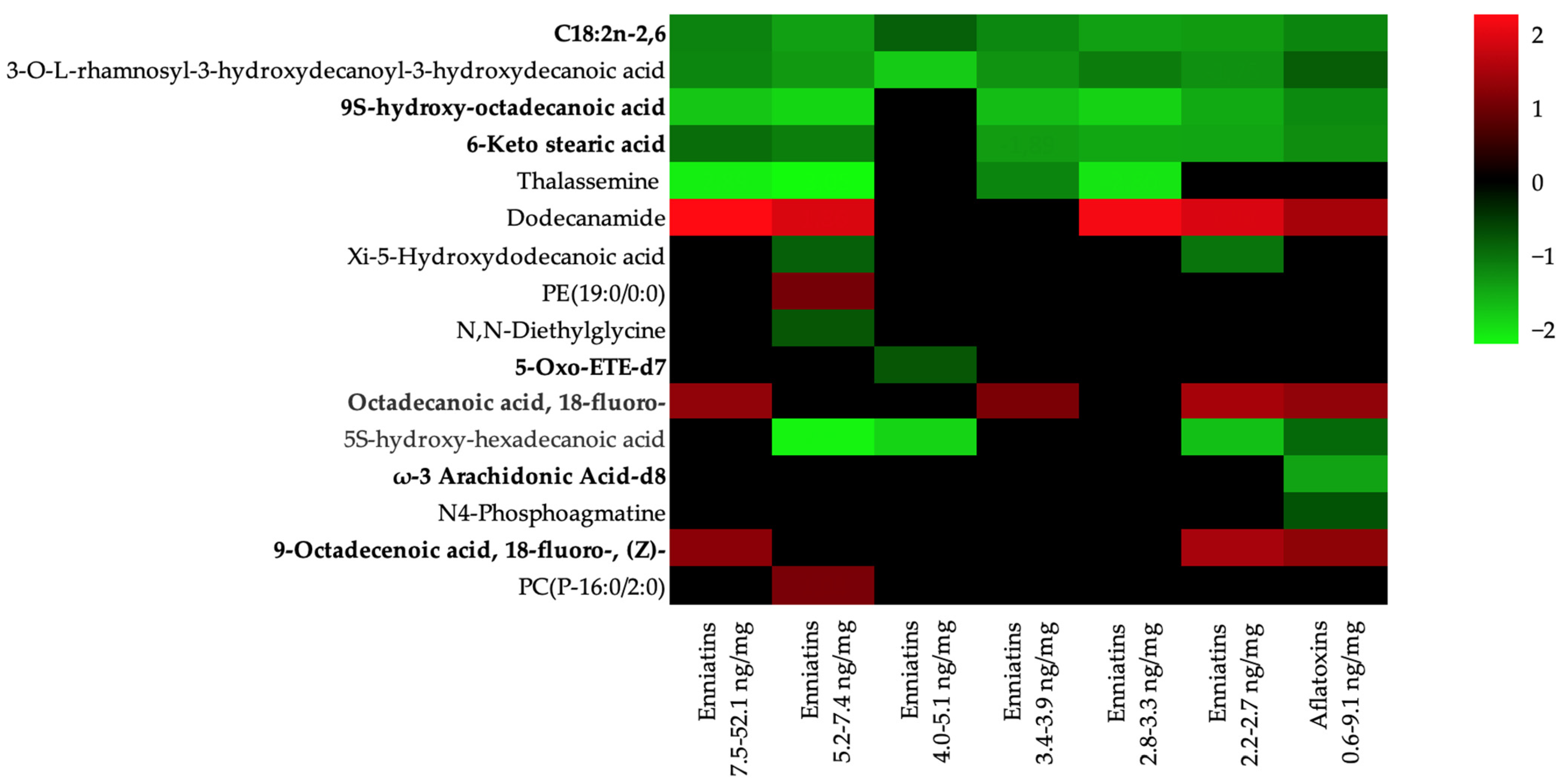

| Pathway | Hit | Metabolite | Enniatins 2.2–52.1 ng/mg (Log FC) | Aflatoxins 0.6–9.1 ng/mg (Log FC) | Regulation | Pathway Unadjusted p-Value | Pathway FDR |
|---|---|---|---|---|---|---|---|
| Biosynthesis of unsaturated fatty acids | Linoleic acid | C18:2n-2,6 | −1.7 ± 0.3 | −1.6 ± 0.0 | Down | <0.001 | 0.007 |
| Octadecanoic acid/stearic acid | 9S-hydroxy-octadecanoic acid | −2.4 ± 0.2 | −1.7 ± 0.0 | Down | <0.001 | 0.007 | |
| Octadecanoic acid/stearic acid | Octadecanoic acid, 18-fluoro- | 1.2 ± 0.2 | 1.2 ± 0.0 | Up | <0.001 | 0.007 | |
| Octadecanoic acid/stearic acid | 6-Keto stearic acid | −1.8 ± 0.3 | −1.7 ± 0.0 | Down | <0.001 | 0.007 | |
| Arachidonic acid metabolite | 5-Oxo-ETE-d7 | −1.0 ± 0.0 | − | Down | <0.001 | 0.007 | |
| Arachidonic acid metabolite | ω-3 Arachidonic Acid-d8 | − | −2.0 ± 0.0 | Down | <0.001 | 0.007 | |
| (9Z)-Octadecenoic acid/Oleic acid | 9-Octadecenoic acid, 18-fluoro-, (Z)- | 1.2 ± 0.2 | 1.2 ± 0.0 | Up | <0.001 | ||
| Fatty acid biosynthesis | Decanoic acid | 3-O-L-rhamnosyl-3-hydroxydecanoyl-3-hydroxydecanoic acid | −1.8 ± 0.3 | −1.1 ± 0.0 | Down | 0.025 | 0.608 |
| Dodecanoic acid | Dodecanamide | 2.0 ± 0.2 | 1.4 ± 0.0 | Up | 0.025 | 0.608 | |
| Dodecanoic acid | Xi-5-Hydroxydodecanoic acid | −1.3 ± 0.2 | − | Down | 0.025 | 0.608 | |
| Hexadecanoic acid | 5S-hydroxy-hexadecanoic acid | −2.6 ± 0.3 | −1.3 ± 0.0 | Down | 0.025 | 0.608 | |
| Glycerophospholipid metabolism | Glycerophosphoethanolamine | PE(19:0/0:0) | 1.0 ± 0.0 | − | Up | 0.012 | 0.501 |
| Ether lipid metabolism | Glycerophosphoethanolamine | PE(19:0/0:0) | 1.0 ± 0.0 | − | Up | 0.030 | 0.608 |
| Glycerophosphocholine | PC(P-16:0/2:0) | 1.0 ± 0.0 | − | Up | 0.030 | 0.608 | |
| Linoleic acid metabolism | Linoleic acid | C18:2n-2,6 | −1.7 ± 0.3 | −1.6 ± 0.0 | Down | 0.068 | 0.991 |
| Glycine, serine, and threonine metabolism | Phosphagen/creatine | Thalassemine | −2.6 ± 0.7 | Down | 0.076 | 0.991 | |
| Glycine | N,N-Diethylglycine | −1.0 ± 0.0 | − | Down | 0.076 | 0.991 | |
| Arginine and proline metabolism | Phosphagen/creatine | Thalassemine | −2.6 ± 0.7 | Down | 0.089 | 0.991 | |
| Agmatine | N4-Phosphoagmatine | − | −1.0 ± 0.0 | Down | 0.089 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasí-Navarro, N.; Lombardi, S.; Vila-Donat, P.; Llop, S.; Vioque, J.; Soler-Blasco, R.; Esplugues, A.; Manyes, L.; Lozano, M. Metabolomic Profiling of Human Urine Related to Mycotoxin Exposure. Toxins 2025, 17, 75. https://doi.org/10.3390/toxins17020075
Dasí-Navarro N, Lombardi S, Vila-Donat P, Llop S, Vioque J, Soler-Blasco R, Esplugues A, Manyes L, Lozano M. Metabolomic Profiling of Human Urine Related to Mycotoxin Exposure. Toxins. 2025; 17(2):75. https://doi.org/10.3390/toxins17020075
Chicago/Turabian StyleDasí-Navarro, Nuria, Sonia Lombardi, Pilar Vila-Donat, Sabrina Llop, Jesus Vioque, Raquel Soler-Blasco, Ana Esplugues, Lara Manyes, and Manuel Lozano. 2025. "Metabolomic Profiling of Human Urine Related to Mycotoxin Exposure" Toxins 17, no. 2: 75. https://doi.org/10.3390/toxins17020075
APA StyleDasí-Navarro, N., Lombardi, S., Vila-Donat, P., Llop, S., Vioque, J., Soler-Blasco, R., Esplugues, A., Manyes, L., & Lozano, M. (2025). Metabolomic Profiling of Human Urine Related to Mycotoxin Exposure. Toxins, 17(2), 75. https://doi.org/10.3390/toxins17020075







