Detection of Ochratoxin A in Tissues of Wild Boars (Sus scrofa) from Southern Italy
Abstract
1. Introduction
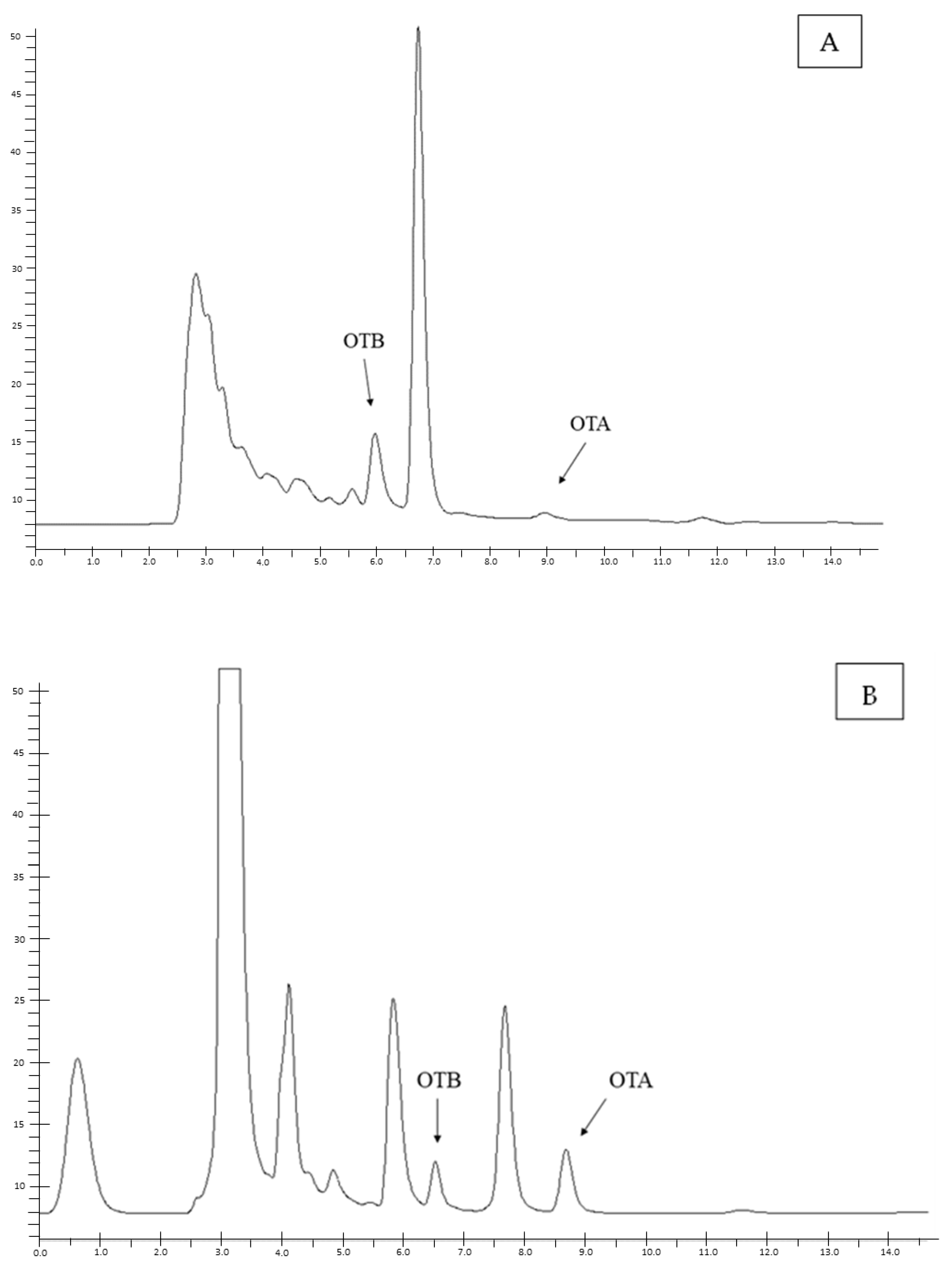
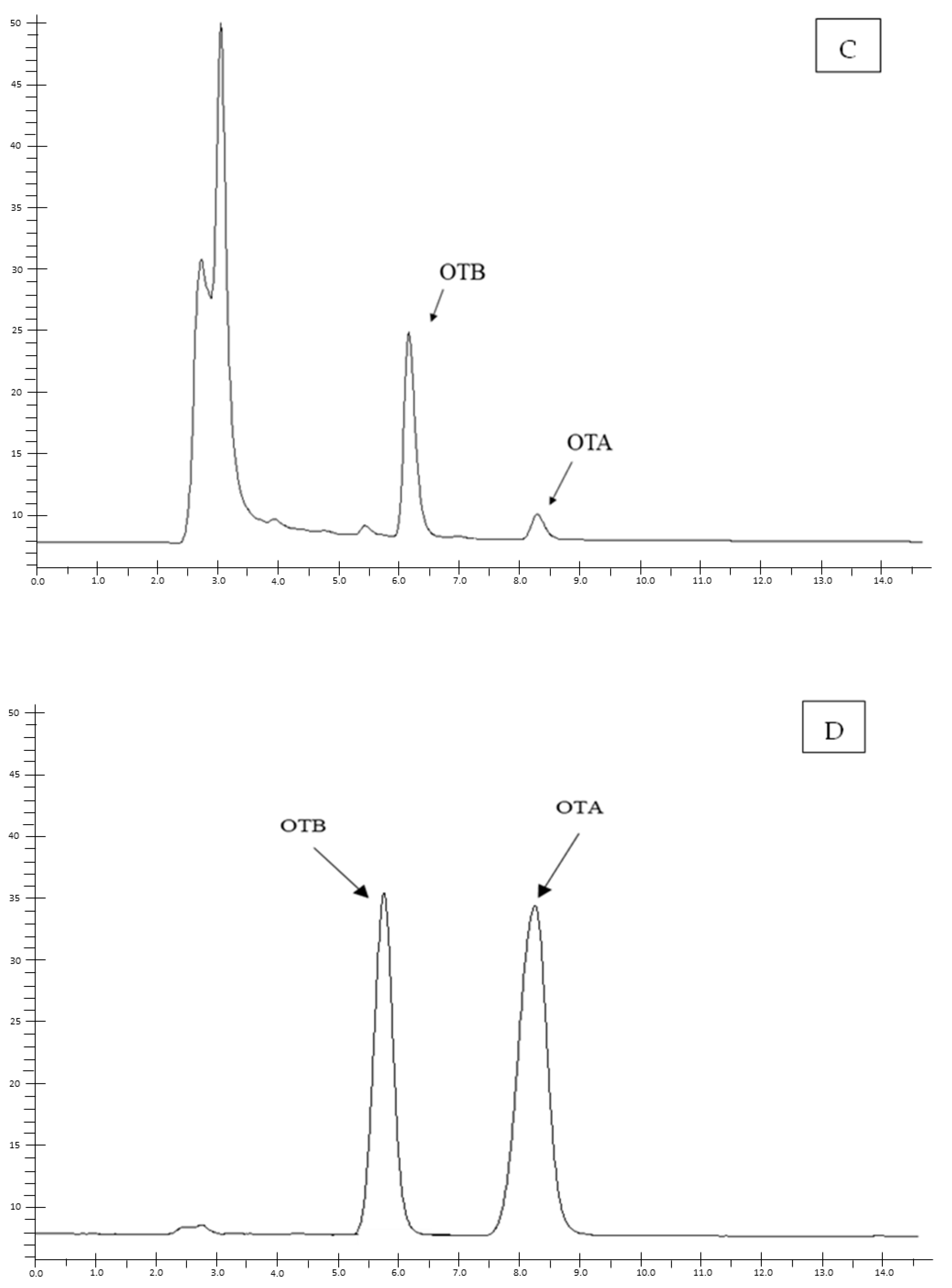
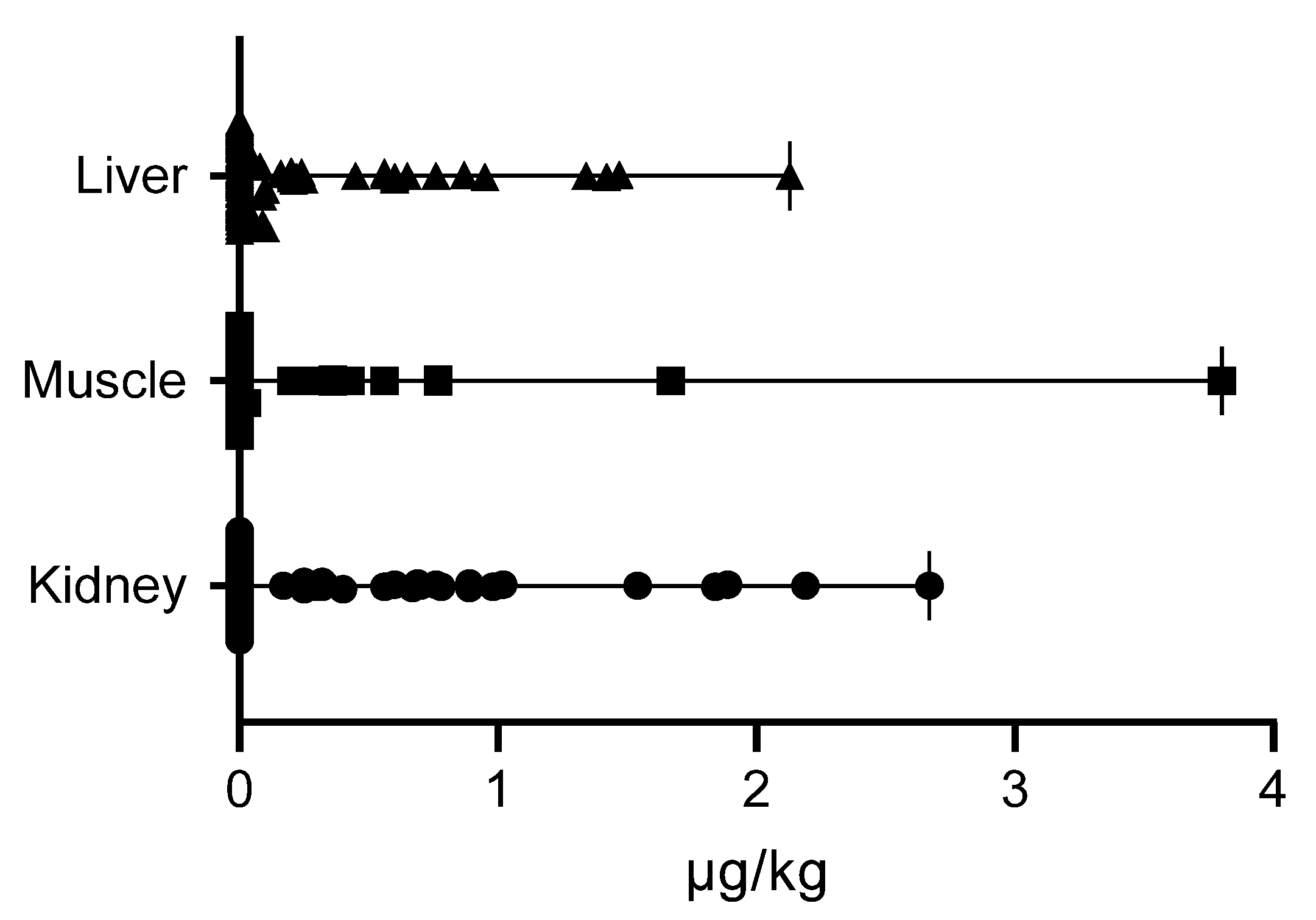
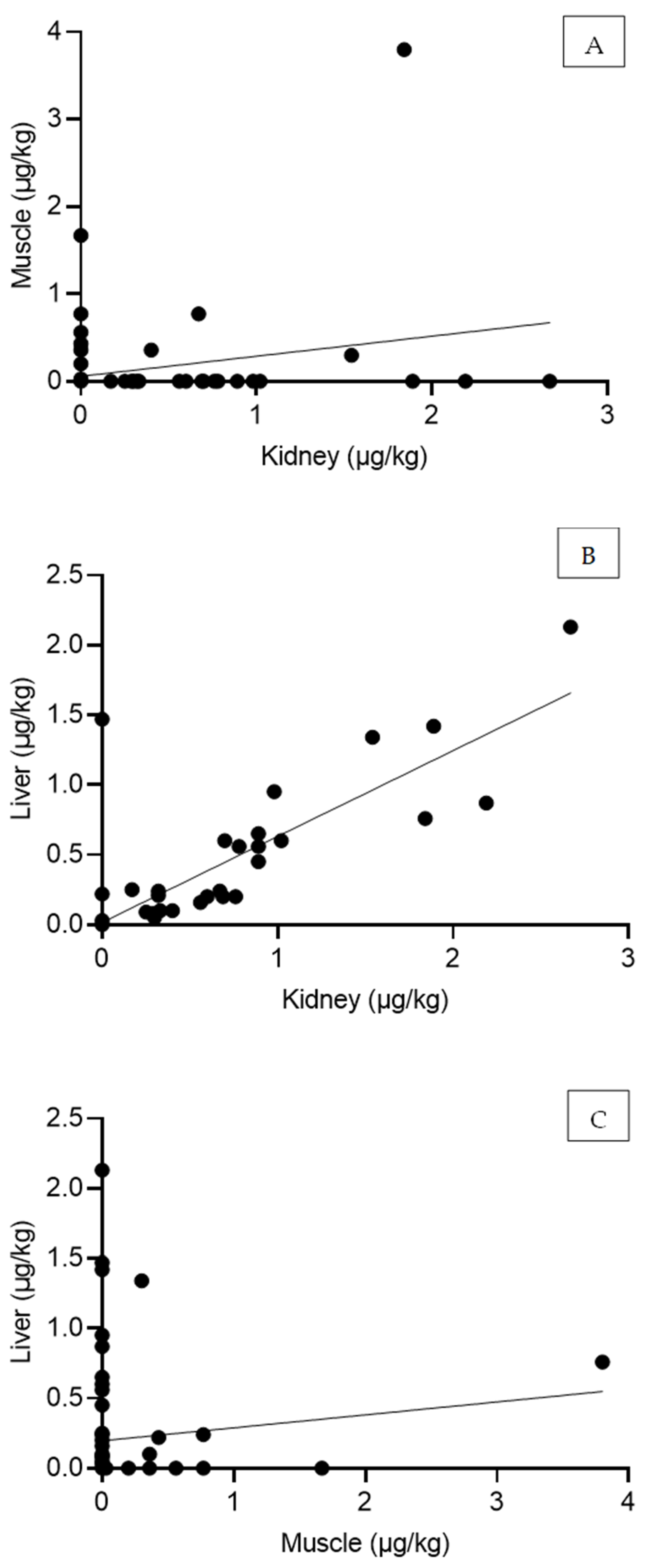
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Samples Collection and Ethical Approval
5.2. Reagents
5.3. Chromatographic Method
5.3.1. Sample Preparation and OTA Quantification
5.3.2. Method Validation
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EFSA | European Food Safety Authority |
| HPLC | High-performance Liquid Chromatography |
| IARC | International Agency for Research on Cancer |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| OTA | Ochratoxin A |
| RSD | Relative standard deviation |
| SD | Standard deviation |
References
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Pexara, A.; Solomakos, N.; Govaris, A. Ochratoxin A in Slaughtered Pigs and Pork Products. Toxins 2022, 14, 67. [Google Scholar] [CrossRef]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef]
- Khoi, C.S.; Chen, J.H.; Lin, T.Y.; Chiang, C.K.; Hung, K.Y. Ochratoxin A-Induced Nephrotoxicity: Up-to-Date Evidence. Int. J. Mol. Sci. 2021, 22, 11237. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Vaccaro, E.; Ballistreri, G.; Restucci, B.; Paciello, O.; Florio, S.; Ciarcia, R. Protective Effects of a Red Orange and Lemon Extract (RLE) on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2024, 13, 289. [Google Scholar] [CrossRef]
- Hou, L.; Gan, F.; Zhou, X.; Zhou, Y.; Qian, G.; Liu, Z.; Huang, K. Immunotoxicity of ochratoxin A and aflatoxin B1 in combination is associated with the nuclear factor kappa B signaling pathway in 3D4/21 cells. Chemosphere 2018, 199, 718–727. [Google Scholar] [CrossRef]
- Dönmez-Altuntaş, H.; Hamurcu, Z.; Imamoglu, N.; Liman, B.C. Effects of ochratoxin A on micronucleus frequency in human lymphocytes. Die Nahr. 2003, 47, 33–35. [Google Scholar] [CrossRef]
- Więckowska, M.; Cichon, N.; Szelenberger, R.; Gorniak, L.; Bijak, M. Ochratoxin A and Its Role in Cancer Development: A Comprehensive Review. Cancers 2024, 16, 3473. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Bozzo, G.; Ceci, E.; Bonerba, E.; Di Pinto, A.; Tantillo, G.; De Giglio, E. Occurrence of Ochratoxin A in the wild boar (Sus scrofa): Chemical and histological analysis. Toxins 2012, 4, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Colomer, J.; Massei, G.; Roos, D.; Rosell, C.; Rodríguez-Teijeiro, J.D. What drives wild boar density and population growth in Mediterranean environments? Sci. Total Environ. 2024, 931, 172739. [Google Scholar] [CrossRef] [PubMed]
- Luci, G.; Intorre, L.; Ferruzzi, G.; Mani, D.; Giuliotti, L.; Pretti, C.; Tognetti, R.; Bertini, S.; Meucci, V. Determination of ochratoxin A in tissues of wild boar (Sus scrofa L.) by enzymatic digestion (ED) coupled to high-performance liquid chromatography with a fluorescence detector (HPLC-FLD). Mycotoxin Res. 2018, 34, 1–8. [Google Scholar] [CrossRef]
- Iemmi, T.; Menozzi, A.; Meucci, V.; Magnini, I.; Battaglia, F.; Severino, L.; Ariano, A.; Bertini, S. Ochratoxin A Levels in Tissues of Wild Boars (Sus scrofa) from Northern Italy. Toxins 2020, 12, 706. [Google Scholar] [CrossRef]
- Colombino, E.; Capella, S.; Casalinuovo, F.; Racco, R.; Pruiti, F.; Volante, M.; Di Marco Lo Presti, V.; Belluso, E.; Capucchio, M.T. Malignant peritoneal mesothelioma in a boar who lived in Calabria (Italy): Wild animal as sentinel system of human health. Sci. Total Environ. 2019, 683, 267–274. [Google Scholar] [CrossRef]
- EFSA. Outcome of a public consultation on the risk assessment of ochratoxin A in food. EFSA Support. Publ. 2020, 17, 1845E. [Google Scholar] [CrossRef]
- Parussolo, G.; Oliveira, M.S.; Garcia, M.V.; Bernardi, A.O.; Lemos, J.G.; Stefanello, A.; Mallmann, C.A.; Copetti, M.V. Ochratoxin A production by Aspergillus westerdijkiae in Italian-type salami. Food Microbiol. 2019, 83, 134–140. [Google Scholar] [CrossRef]
- Zjalic, S.; Markov, K.; Loncar, J.; Jakopovic, Z.; Beccaccioli, M.; Reverberi, M. Biocontrol of Occurrence Ochratoxin A in Wine: A Review. Toxins 2024, 16, 277. [Google Scholar] [CrossRef]
- Capei, R.; Pettini, L.; Mandò Tacconi, F. Occurrence of Ochratoxin A in breakfast cereals and sweet snacks in Italy: Dietary exposure assessment. Ann. Ig. Med. Prev. E Di Comunita 2019, 31, 130–139. [Google Scholar] [CrossRef]
- Jota Baptista, C.; Seixas, F.; Gonzalo-Orden, J.M.; Patinha, C.; Pato, P.; Ferreira da Silva, E.; Merino-Goyenechea, L.J.; Oliveira, P.A. Heavy metals and metalloids in wild boars (Sus scrofa)—A silent but serious public health hazard. Vet. Res. Commun. 2024, 48, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, A.; Dalin, A.-M.; Pettersson, J.; Persson, S. Concentrations of cadmium, lead, arsenic, and some essential metals in wild boar from Sweden. Eur. J. Wildl. Res. 2021, 67, 18. [Google Scholar] [CrossRef]
- Gajęcka, M.; Sławuta, P.; Nicpoń, J.; Kołacz, R.; Kiełbowicz, Z.; Zielonka, Ł.; Dąbrowski, M.; Szweda, W.; Gajęcki, M.; Nicpoń, J. Zearalenone and its metabolites in the tissues of female wild boars exposed per os to mycotoxins. Toxicon 2016, 114, 1–12. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Ferrara, G.; Montagnaro, S.; Meucci, V.; Intorre, L.; Bacci, S.; Esposito, L.; Piscopo, N.; Rubino, A.; et al. Zearalenone (ZEN) and Its Metabolite Levels in Tissues of Wild Boar (Sus scrofa) from Southern Italy: A Pilot Study. Toxins 2023, 15, 56. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Stoev, S.D.; Denev, S.A. Porcine/chicken or human nephropathy as the result of joint mycotoxins interaction. Toxins 2013, 5, 1503–1530. [Google Scholar] [CrossRef]
- Marin, D.E.; Braicu, C.; Gras, M.A.; Pistol, G.C.; Petric, R.C.; Berindan Neagoe, I.; Palade, M.; Taranu, I. Low level of ochratoxin A affects genome-wide expression in kidney of pig. Toxicon 2017, 136, 67–77. [Google Scholar] [CrossRef]
- Chen, D.; Cao, X.; Tao, Y.; Wu, Q.; Pan, Y.; Huang, L.; Wang, X.; Wang, Y.; Peng, D.; Liu, Z.; et al. Development of a sensitive and robust liquid chromatography coupled with tandem mass spectrometry and a pressurized liquid extraction for the determination of aflatoxins and ochratoxin A in animal derived foods. J. Chromatogr. A 2012, 1253, 110–119. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; Lizarraga Pérez, E.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. [Google Scholar] [CrossRef]
- Hanif, N.Q. Ochratoxicosis in monograstric animals-a review. J. Bioresour. Manag. 2016, 3, 3. [Google Scholar]
- Markov, K.; Pleadin, J.; Bevardi, M.; Vahčić, N.; Sokolić-Mihalak, D.; Frece, J. Natural occurrence of aflatoxin B1, ochratoxin A and citrinin in Croatian fermented meat products. Food Control 2013, 34, 312–317. [Google Scholar] [CrossRef]
- Ali, S.; Battaglini Franco, B.; Theodoro Rezende, V.; Gabriel Dionisio Freire, L.; Lima de Paiva, E.; Clara Fogacio Haikal, M.; Leme Guerra, E.; Eliana Rosim, R.; Gustavo Tonin, F.; Savioli Ferraz, I.; et al. Exposure assessment of children to dietary mycotoxins: A pilot study conducted in Ribeirão Preto, São Paulo, Brazil. Food Res. Int. (Ott. Ont.) 2024, 180, 114087. [Google Scholar] [CrossRef] [PubMed]
- Pietri, A.; Bertuzzi, T.; Gualla, A.; Piva, G. Occurrence of ochratoxin A in raw ham muscles and in pork products from northern Italy. Ital. J. Food Sci./Riv. Ital. Di Sci. Degli Aliment. 2006, 18, 99–106. [Google Scholar]
- Armorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Ochratoxin A in artisan salami produced in Veneto (Italy). Food Addit. Contam. Part B 2016, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety, A. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to ochratoxin A in food. EFSA J. 2006, 4, 365. [Google Scholar] [CrossRef]
- Longobardi, C.; Ferrara, G.; Andretta, E.; Montagnaro, S.; Damiano, S.; Ciarcia, R. Ochratoxin A and Kidney Oxidative Stress: The Role of Nutraceuticals in Veterinary Medicine—A Review. Toxins 2022, 14, 398. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Food safety implications of ochratoxin A in animal-derived food products. Vet. J. (Lond. Engl. 1997) 2012, 192, 286–292. [Google Scholar] [CrossRef]
- Damiano, S.; Longobardi, C.; Ferrara, G.; Piscopo, N.; Riccio, L.; Russo, V.; Meucci, V.; De Marchi, L.; Esposito, L.; Florio, S.; et al. Oxidative Status and Histological Evaluation of Wild Boars‘ Tissues Positive for Zearalenone Contamination in the Campania Region, Southern Italy. Antioxidants 2023, 12, 1748. [Google Scholar] [CrossRef]
- Ferrara, G.; Nocera, F.P.; Longobardi, C.; Ciarcia, R.; Fioretti, A.; Damiano, S.; Iovane, G.; Pagnini, U.; Montagnaro, S. Retrospective Serosurvey of Three Porcine Coronaviruses among the Wild Boar (Sus scrofa) Population in the Campania Region of Italy. J. Wildl. Dis. 2022, 58, 887–891. [Google Scholar] [CrossRef]
- Monaci, L.; Tantillo, G.; Palmisano, F. Determination of ochratoxin A in pig tissues by liquid-liquid extraction and clean-up and high-performance liquid chromatography. Anal. Bioanal. Chem. 2004, 378, 1777–1782. [Google Scholar] [CrossRef]
- European Community. Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. O. J. Eur. Communities 2002, 50, 8–36. [Google Scholar]
| Parameters | Muscle | Liver | Kidney | |
|---|---|---|---|---|
| OTA | ||||
| LOD (μg/kg) | 0.01 | 0.01 | 0.01 | |
| LOQ (μg/kg) | 0.02 | 0.02 | 0.02 | |
| r2 | 0.998 | 0.996 | 0.995 | |
| Repeatability (µg/kg) | ||||
| 0.25 | Mean concentration ± SD RSD (%) | 0.23 ± 0.01 6.20 | 0.22 ± 0.01 3.64 | 0.22 ± 0.016.15 |
| 2.50 | Mean concentration ± SD RSD (%) | 2.50 ± 0.25 10.00 | 2.32 ± 0.27 11.89 | 2.21 ± 0.209.08 |
| 10.0 | Mean concentration ± SD RSD (%) | 10.07 ± 0.30 3.03 | 9.73 ± 0.70 7.22 | 9.45 ± 0.222.30 |
| Reproducibility (µg/kg) | ||||
| 0.25 | Mean concentration ± SD RSD (%) | 0.23 ± 0.02 8.02 | 0.22 ± 0.02 8.65 | 0.21 ± 0.016.17 |
| 2.50 | Mean concentration ± SD RSD (%) | 2.29 ± 0.30 13.37 | 2.41 ± 0.31 4.03 | 2.11 ± 0.209.48 |
| 10.0 | Mean concentration ± SD RSD (%) | 9.74 ± 0.33 3.45 | 9.52 ± 0.43 4.48 | 9.50 ± 0.232.42 |
| Recovery (%) | ||||
| 0.25 | 91.57 ± 8.01 | 90.29 ± 7.67 | 87.86 ± 4.10 | |
| 2.5 | 94.14 ± 12.64 | 97.86 ± 15.04 | 83.86 ± 7.71 | |
| 10.0 | 98.17 ± 3.37 | 95.80 ± 4.38 | 94.43 ± 2.22 | |
| Sex | N | Kidney | Muscle | Liver |
|---|---|---|---|---|
| Male | 10 | 0.65 (0.32–2.67) | 0.43 (0.08–3.80) | 0.21 (0.10–2.13) |
| Female | 16 | 0.68 (0.17–2.19) | 0.56 (0.03–3.80) | 0.25 (0.03–1.47) |
| Age Class | N | Kidney | Muscle | Liver |
|---|---|---|---|---|
| Young | 17 | 0.65 (0.25–2.67) | 0.77 (0.20–3.80) | 0.22 (0.03–2.13) |
| Adult | 9 | 0.67 (0.17–1.89) | 0.25 (0.03–0.56) | 0.24 (0.08–1.42) |
| r | p | |
|---|---|---|
| Kidney vs. Muscle | 0.26 (0.025–0.46) | 0.03 |
| Kidney vs. Liver | 0.84 (0.75–0.89) | 2.90 × 10⁻²⁰ |
| Muscle vs. Liver | 0.11 (-0.12–0.33) | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiano, S.; Longobardi, C.; De Marchi, L.; Piscopo, N.; Meucci, V.; Lenzi, A.; Ciarcia, R. Detection of Ochratoxin A in Tissues of Wild Boars (Sus scrofa) from Southern Italy. Toxins 2025, 17, 74. https://doi.org/10.3390/toxins17020074
Damiano S, Longobardi C, De Marchi L, Piscopo N, Meucci V, Lenzi A, Ciarcia R. Detection of Ochratoxin A in Tissues of Wild Boars (Sus scrofa) from Southern Italy. Toxins. 2025; 17(2):74. https://doi.org/10.3390/toxins17020074
Chicago/Turabian StyleDamiano, Sara, Consiglia Longobardi, Lucia De Marchi, Nadia Piscopo, Valentina Meucci, Alessio Lenzi, and Roberto Ciarcia. 2025. "Detection of Ochratoxin A in Tissues of Wild Boars (Sus scrofa) from Southern Italy" Toxins 17, no. 2: 74. https://doi.org/10.3390/toxins17020074
APA StyleDamiano, S., Longobardi, C., De Marchi, L., Piscopo, N., Meucci, V., Lenzi, A., & Ciarcia, R. (2025). Detection of Ochratoxin A in Tissues of Wild Boars (Sus scrofa) from Southern Italy. Toxins, 17(2), 74. https://doi.org/10.3390/toxins17020074







