Pore-Forming Protein LIN-24 Enhances Starvation Resilience in Caenorhabditis elegans by Modulating Lipid Metabolism and Mitochondrial Dynamics
Abstract
1. Introductions
2. Results
2.1. LIN-24 in C. elegans Is Upregulated in Response to Starvation and May Play a Role in Fatty Acid Metabolism
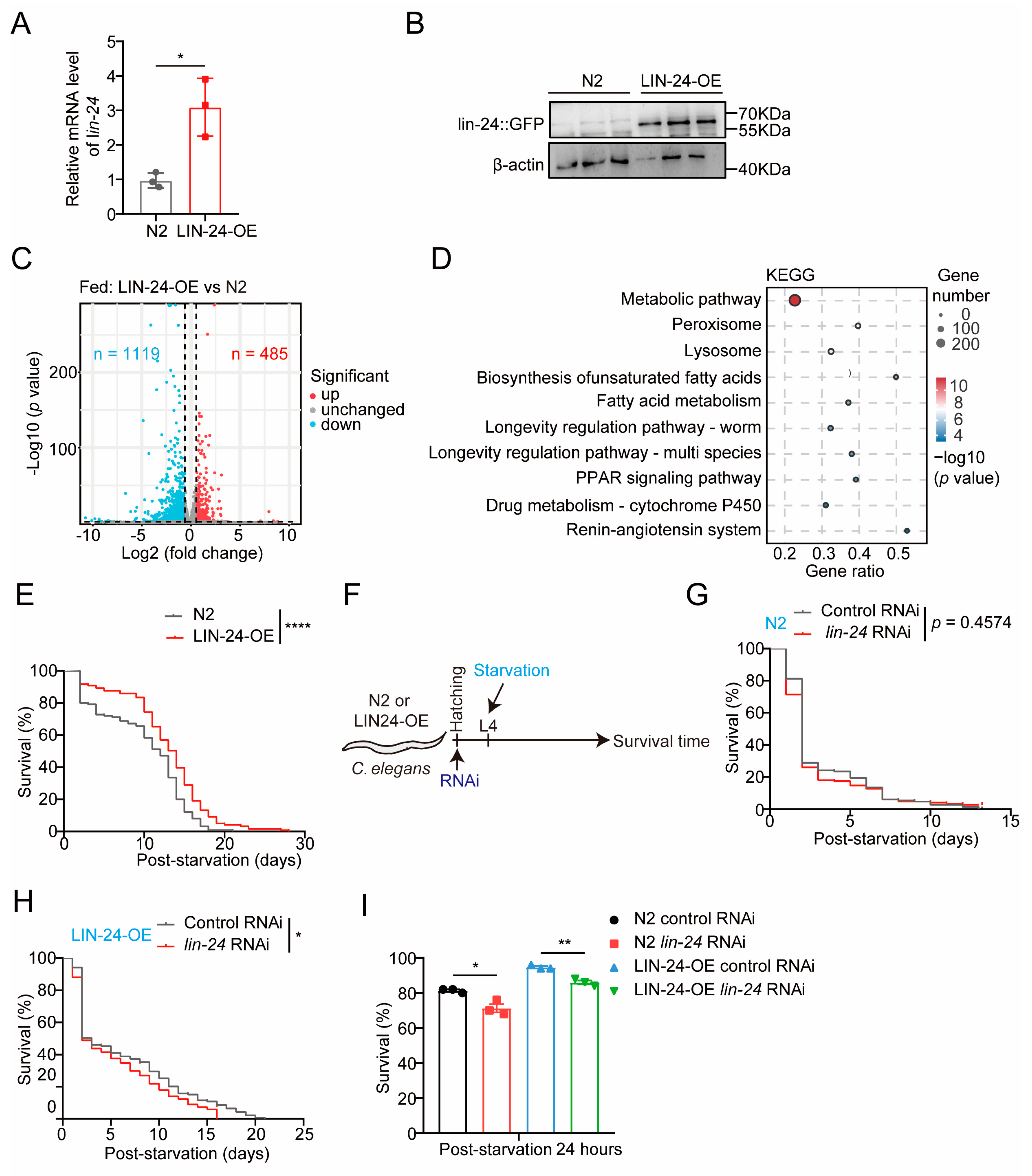
2.2. LIN-24 Overexpression Enhances Starvation Resistance in C. elegans
2.3. LIN-24 Promotes Fatty Acid Metabolism During Starvation
2.4. LIN-24 Modulates Amino Acid Metabolism and Preserves Skeletal Muscle Integrity During Starvation
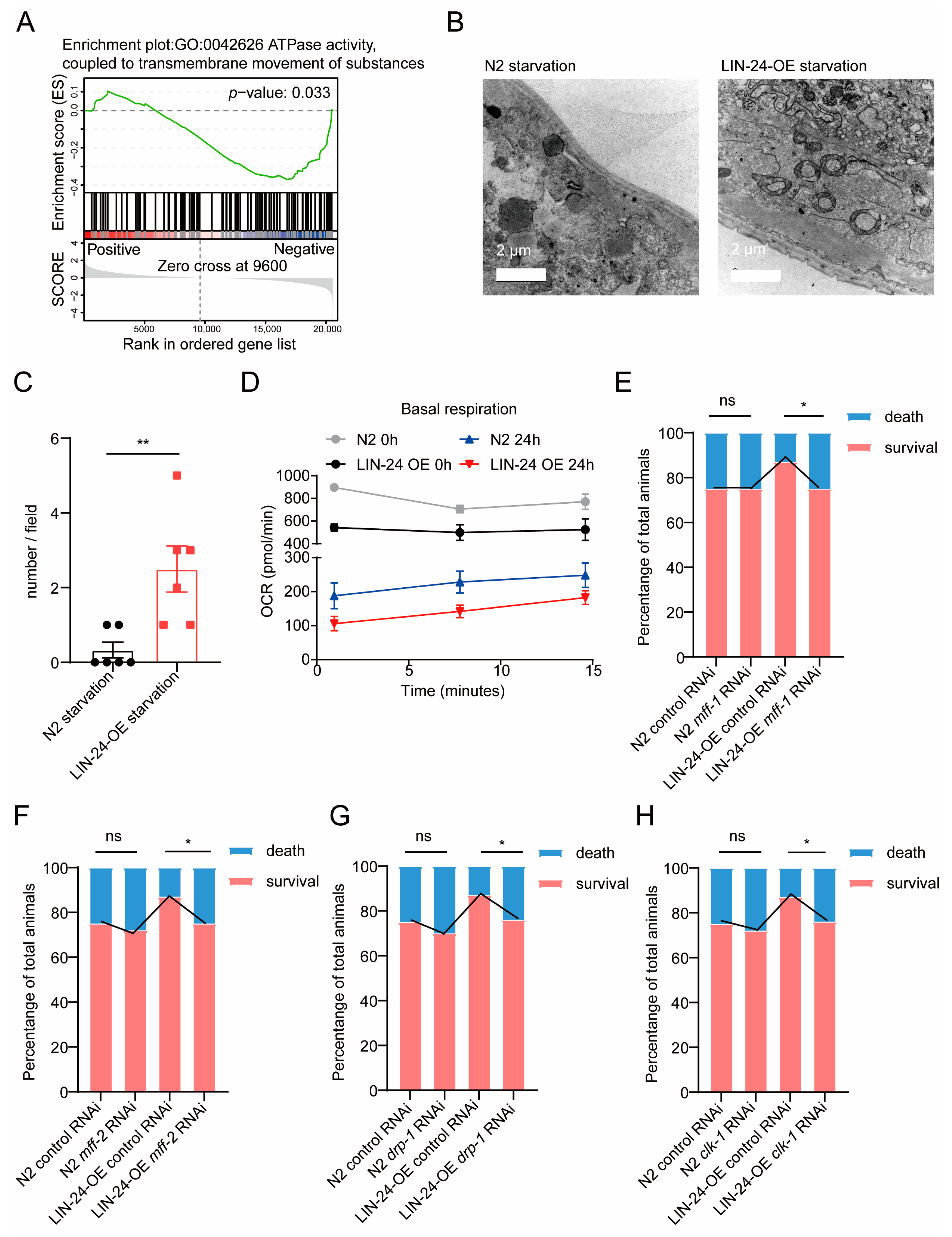
2.5. LIN-24 Promotes Donut-Shaped Mitochondria Formation to Enhance Starvation Resistance in C. elegans
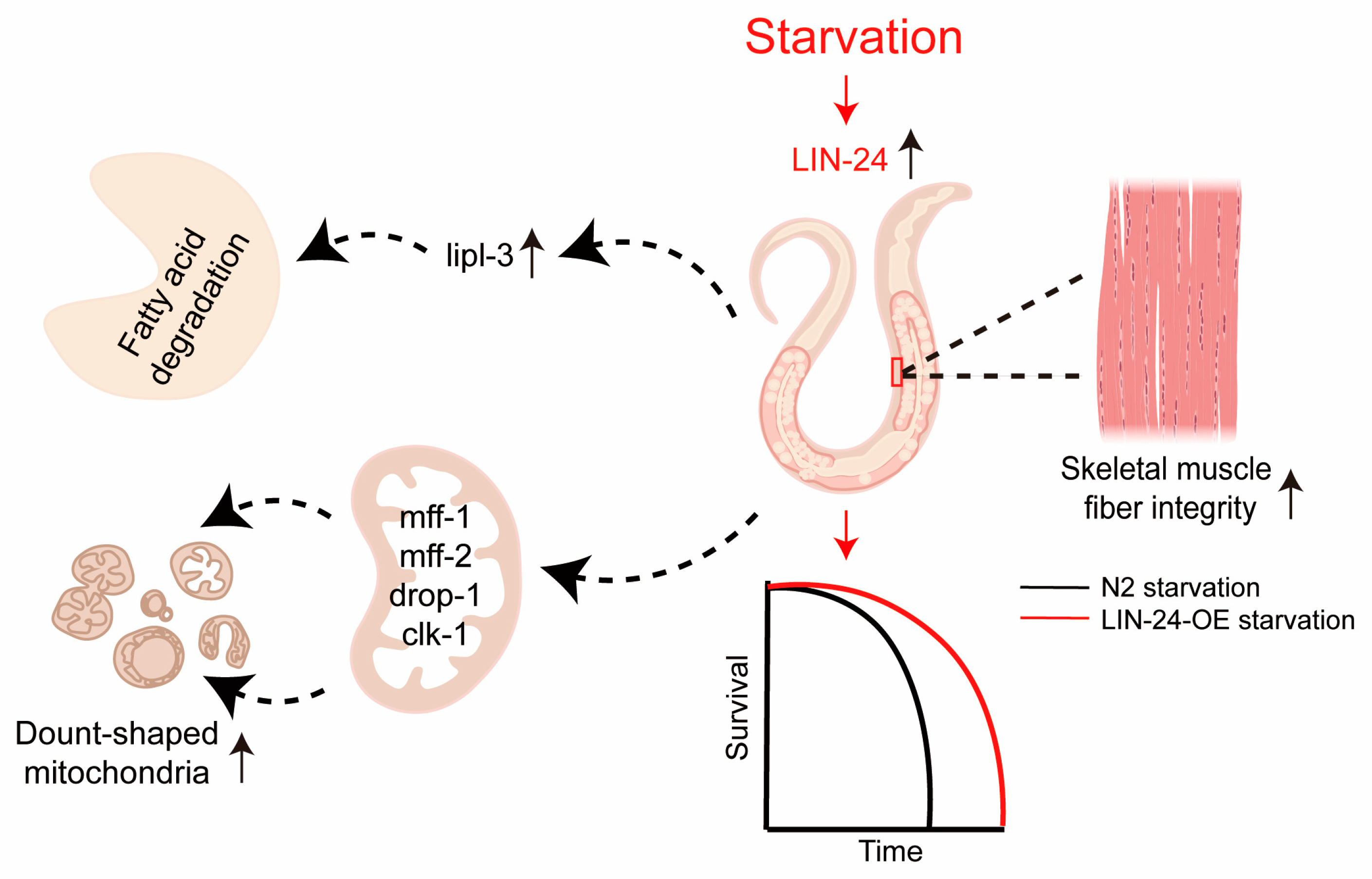
3. Discussion
4. Materials and Methods
4.1. C. elegans Strains and Culture
4.2. Starvation Resistance Assays
4.3. RNA Interference Starvation Resistance Assay
4.4. RNA Isolation and qPCR
4.5. RNA-Sequencing
4.6. RNA-Seq Analysis
4.7. Oil Red O Staining
4.8. Lipid Profiling
4.9. Live Nematode Respiration
4.10. Transmission Electron Microscopy
4.11. Western Blotting
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Jin, Z.; Summers, S.; Derous, D.; Li, M.; Li, B.; Li, L.; Speakman, J.R. Calorie restriction and calorie dilution have different impacts on body fat, metabolism, behavior, and hypothalamic gene expression. Cell Rep. 2022, 39, 110835. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F. Epigenetics: Tales of adversity. Nature 2010, 468, S20. [Google Scholar] [PubMed]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Füllgrabe, J.; Joseph, B. The hunger strikes back: An epigenetic memory for autophagy. Cell Death Differ. 2023, 30, 1404–1415. [Google Scholar] [CrossRef]
- Dworkin, J.; Harwood, C.S. Metabolic Reprogramming and Longevity in Quiescence. Annu. Rev. Microbiol. 2022, 76, 91–111. [Google Scholar] [CrossRef]
- van der Weijden, V.A.; Stötzel, M.; Iyer, D.P.; Fauler, B.; Gralinska, E.; Shahraz, M.; Meierhofer, D.; Vingron, M.; Rulands, S.; Alexandrov, T.; et al. FOXO1-mediated lipid metabolism maintains mammalian embryos in dormancy. Nat. Cell Biol. 2024, 26, 181–193. [Google Scholar] [CrossRef]
- Demoinet, E.; Li, S.; Roy, R. AMPK blocks starvation-inducible transgenerational defects in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2017, 114, E2689–E2698. [Google Scholar]
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Szczesny, P.; Iacovache, I.; Muszewska, A.; Ginalski, K.; van der Goot, F.G.; Grynberg, M. Extending the aerolysin family: From bacteria to vertebrates. PLoS ONE 2011, 6, e20349. [Google Scholar] [CrossRef]
- Ogawa, M.; Takabatake, T.; Takahashi, T.C.; Takeshima, K. Metamorphic change in EP37 expression: Members of the βγ-crystallin superfamily in newt. Dev. Genes Evol. 1997, 206, 417–424. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.Q.; Zhao, Z.; Deng, C.J. Animal secretory endolysosome channel discovery. Zool. Res. 2021, 42, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Gandhi, S.; Lata, K.; Chattopadhyay, K. Pore-forming toxins in infection and immunity. Biochem. Soc. Trans. 2021, 49, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; He, Y.Y.; Zhang, Y.; Lee, W.H.; Qian, J.Q.; Lai, R.; Jin, Y. A novel non-lens betagamma-crystallin and trefoil factor complex from amphibian skin and its functional implications. PLoS ONE 2008, 3, e1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Bian, X.L.; Zeng, L.; Pan, F.; Liu, L.Z.; Liang, J.Y.; Wang, L.Y.; Zhou, K.F.; Lee, W.H.; Xiang, Y.; et al. A cellular endolysosome-modulating pore-forming protein from a toad is negatively regulated by its paralog under oxidizing conditions. J. Biol. Chem. 2020, 295, 10293–10306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Lan, X.Q.; Wei, X.S.; Xu, S.M.; Liu, L.Z.; Bian, X.L.; Zeng, L.; Guo, X.L.; Guo, Y.Q.; Lee, W.H.; et al. Amphibian pore-forming protein βγ-CAT drives metabolite release from small extracellular vesicles through channel formation. Zool. Res. 2023, 44, 739–742. [Google Scholar] [CrossRef]
- Xiang, Y.; Yan, C.; Guo, X.L.; Zhou, K.F.; Li, S.A.; Gao, Q.; Wang, X.; Zhao, F.; Liu, J.; Lee, W.H.; et al. Host-derived, pore-forming toxin-like protein and trefoil factor complex protects the host against microbial infection. Proc. Natl. Acad. Sci. USA 2014, 111, 6702–6707. [Google Scholar] [CrossRef]
- Liu, L.Z.; Liu, L.; Shi, Z.H.; Bian, X.L.; Si, Z.R.; Wang, Q.Q.; Xiang, Y.; Zhang, Y. Amphibian pore-forming protein βγ-CAT drives extracellular nutrient scavenging under cell nutrient deficiency. iScience 2023, 26, 106598. [Google Scholar] [CrossRef]
- Bauer, M.; Hamm, A.C.; Bonaus, M.; Jacob, A.; Jaekel, J.; Schorle, H.; Pankratz, M.J.; Katzenberger, J.D. Starvation response in mouse liver shows strong correlation with life-span-prolonging processes. Physiol. Genom. 2004, 17, 230–244. [Google Scholar] [CrossRef]
- Hakvoort, T.B.; Moerland, P.D.; Frijters, R.; Sokolović, A.; Labruyère, W.T.; Vermeulen, J.L.; Ver Loren van Themaat, E.; Breit, T.M.; Wittink, F.R.; van Kampen, A.H.; et al. Interorgan coordination of the murine adaptive response to fasting. J. Biol. Chem. 2011, 286, 16332–16343. [Google Scholar] [CrossRef]
- Finn, P.F.; Dice, J.F. Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22, 830–844. [Google Scholar] [CrossRef]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.J.; Jarrell, T.A.; Brittin, C.A.; Wang, Y.; Bloniarz, A.E.; Yakovlev, M.A.; Nguyen, K.C.Q.; Tang, L.T.; Bayer, E.A.; Duerr, J.S.; et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 2019, 571, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.; Abreu-Goodger, C.; Hernandez-Mendoza, A.; Dimitrova Dinkova, T.D.; Padilla-Noriega, L.; Perez-Andrade, M.E.; Miranda-Rios, J. High-Throughput Profiling of Caenorhabditis elegans Starvation-Responsive microRNAs. PLoS ONE 2015, 10, e0142262. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tao, J.; Zhao, P.J.; Tang, W.; Xu, J.P.; Zhang, K.Q.; Zou, C.G. Adiponectin receptor PAQR-2 signaling senses low temperature to promote C. elegans longevity by regulating autophagy. Nat. Commun. 2019, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zeng, W.; Zhao, M.M.; Wang, J.; Wang, Q.; Chen, T.; Zhang, Y.; Lee, W.; Chen, S.; Zhang, Y.; et al. Caenorhabditis elegans LIN-24, a homolog of bacterial pore-forming toxin, protects the host from microbial infection. FASEB J. 2023, 37, e23162. [Google Scholar] [CrossRef]
- Watts, J.L.; Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 5854–5859. [Google Scholar] [CrossRef]
- An, L.; Fu, X.; Chen, J.; Ma, J. Application of Caenorhabditis elegans in Lipid Metabolism Research. Int. J. Mol. Sci. 2023, 24, 1173. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, Q.; Wu, H.; Li, W.; Qi, J.; Wu, Y.; Xiang, G.; Tang, H.; Yang, L.; Chen, K.; et al. Topology-dependent, bifurcated mitochondrial quality control under starvation. Autophagy 2020, 16, 562–574. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, L.; You, X.; Li, Z.; Li, C.; Chen, Y. PAQR9 regulates hepatic ketogenesis and fatty acid oxidation during fasting by modulating protein stability of PPARα. Mol. Metab. 2021, 53, 101331. [Google Scholar] [CrossRef]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Levy, T.; Voeltzke, K.; Hruby, L.; Alasad, K.; Bas, Z.; Snaebjörnsson, M.; Marciano, R.; Scharov, K.; Planque, M.; Vriens, K.; et al. mTORC1 regulates cell survival under glucose starvation through 2024, 4EBP1/2-mediated translational reprogramming of fatty acid metabolism. Nat. Commun. 2024, 15, 4083. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Ruvkun, G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol. 2013, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Mony, V.K.; Drangowska-Way, A.; Albert, R.; Harrison, E.; Ghaddar, A.; Horak, M.K.; Ke, W.; O’Rourke, E.J. Context-specific regulation of lysosomal lipolysis through network-level diverting of transcription factor interactions. Proc. Natl. Acad. Sci. USA 2021, 118, e2104832118. [Google Scholar] [CrossRef]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a "mediator" of systemic metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Rai, M.; Demontis, F. Systemic Nutrient and Stress Signaling via Myokines and Myometabolites. Annu. Rev. Physiol. 2016, 78, 85–107. [Google Scholar] [CrossRef]
- Hitachi, K.; Tsuchida, K. Role of microRNAs in skeletal muscle hypertrophy. Front. Physiol. 2013, 4, 408. [Google Scholar] [CrossRef]
- Pradhan, R.; Dieterich, W.; Natarajan, A.; Schwappacher, R.; Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia. Cancers 2024, 16, 1921. [Google Scholar] [CrossRef] [PubMed]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2023, 37, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, R.N.; Taylor, R.W.; Turnbull, D.M. Mutations causing mitochondrial disease: What is new and what challenges remain? Science 2015, 349, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef]
- Eisner, V.; Picard, M.; Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018, 20, 755–765. [Google Scholar] [CrossRef]
- Long, Q.; Zhao, D.; Fan, W.; Yang, L.; Zhou, Y.; Qi, J.; Wang, X.; Liu, X. Modeling of Mitochondrial Donut Formation. Biophys. J. 2015, 109, 892–899. [Google Scholar] [CrossRef]
- Ahmad, T.; Aggarwal, K.; Pattnaik, B.; Mukherjee, S.; Sethi, T.; Tiwari, B.K.; Kumar, M.; Micheal, A.; Mabalirajan, U.; Ghosh, B.; et al. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 2013, 4, e461. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, J.; Qi, T.; Zhu, S.; Yao, L.; Fang, G.; Li, Y.; Zang, X.; Xu, W.; Hao, W.; et al. Maternal aging increases offspring adult body size via transmission of donut-shaped mitochondria. Cell Res. 2023, 33, 821–834. [Google Scholar] [CrossRef]
- Monaghan, R.M.; Barnes, R.G.; Fisher, K.; Andreou, T.; Rooney, N.; Poulin, G.B.; Whitmarsh, A.J. A nuclear role for the respiratory enzyme CLK-1 in regulating mitochondrial stress responses and longevity. Nat. Cell Biol. 2015, 17, 782–792. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Jin, S.; Wang, X.; Qu, M.; Uhlén, P.; Tomilin, N.; Shupliakov, O.; Lendahl, U.; Nistér, M. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. Embo J. 2011, 30, 2762–2778. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, W.; van der Krift, F.; Rabouille, C. Modulation of the secretory pathway by amino-acid starvation. J. Cell Biol. 2018, 217, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Keenan, S.; Cooke, M.B.; Belski, R. The Effects of Intermittent Fasting Combined with Resistance Training on Lean Body Mass: A Systematic Review of Human Studies. Nutrients 2020, 12, 2349. [Google Scholar] [CrossRef]
- Tak, U.; Dokland, T.; Niederweis, M. Pore-forming Esx proteins mediate toxin secretion by Mycobacterium tuberculosis. Nat. Commun. 2021, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, L.; Xie, Y.; Liu, Y.; Li, S.; Yang, W.; Xu, B.; Ji, H.; Ding, L.; Wang, K.; et al. Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy. Nat. Commun. 2018, 9, 1752. [Google Scholar] [CrossRef]
- Wei, X.S.; Liu, L.Z.; Bian, X.L.; Zeng, L.; Lee, W.H.; Lin, L.; Zhang, Y.; Wang, Q.Q. Protein composition of extracellular vesicles from skin secretions of the amphibian Bombina maxima. Zool. Res. 2022, 43, 687–690. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone β-hydroxybutyrylation is critical in reversal of sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Yang, M.; Wang, J.; Huang, C.; Wu, A.; Cui, L.; Guo, Y.; Zeng, L.; Guo, X.; Zhang, Y.; et al. Pore-Forming Protein LIN-24 Enhances Starvation Resilience in Caenorhabditis elegans by Modulating Lipid Metabolism and Mitochondrial Dynamics. Toxins 2025, 17, 72. https://doi.org/10.3390/toxins17020072
Lan X, Yang M, Wang J, Huang C, Wu A, Cui L, Guo Y, Zeng L, Guo X, Zhang Y, et al. Pore-Forming Protein LIN-24 Enhances Starvation Resilience in Caenorhabditis elegans by Modulating Lipid Metabolism and Mitochondrial Dynamics. Toxins. 2025; 17(2):72. https://doi.org/10.3390/toxins17020072
Chicago/Turabian StyleLan, Xinqiang, Mengqi Yang, Jiali Wang, Chunping Huang, Andong Wu, Leilei Cui, Yingqi Guo, Lin Zeng, Xiaolong Guo, Yun Zhang, and et al. 2025. "Pore-Forming Protein LIN-24 Enhances Starvation Resilience in Caenorhabditis elegans by Modulating Lipid Metabolism and Mitochondrial Dynamics" Toxins 17, no. 2: 72. https://doi.org/10.3390/toxins17020072
APA StyleLan, X., Yang, M., Wang, J., Huang, C., Wu, A., Cui, L., Guo, Y., Zeng, L., Guo, X., Zhang, Y., Xiang, Y., & Wang, Q. (2025). Pore-Forming Protein LIN-24 Enhances Starvation Resilience in Caenorhabditis elegans by Modulating Lipid Metabolism and Mitochondrial Dynamics. Toxins, 17(2), 72. https://doi.org/10.3390/toxins17020072





