Characterization of Alpha-Bungarotoxin Antibodies Prepared by Different Strategies
Abstract
1. Introduction
2. Results
2.1. Acquisition of MBP-α-BGT
2.2. Specificity of Antisera Prepared by Three Methods
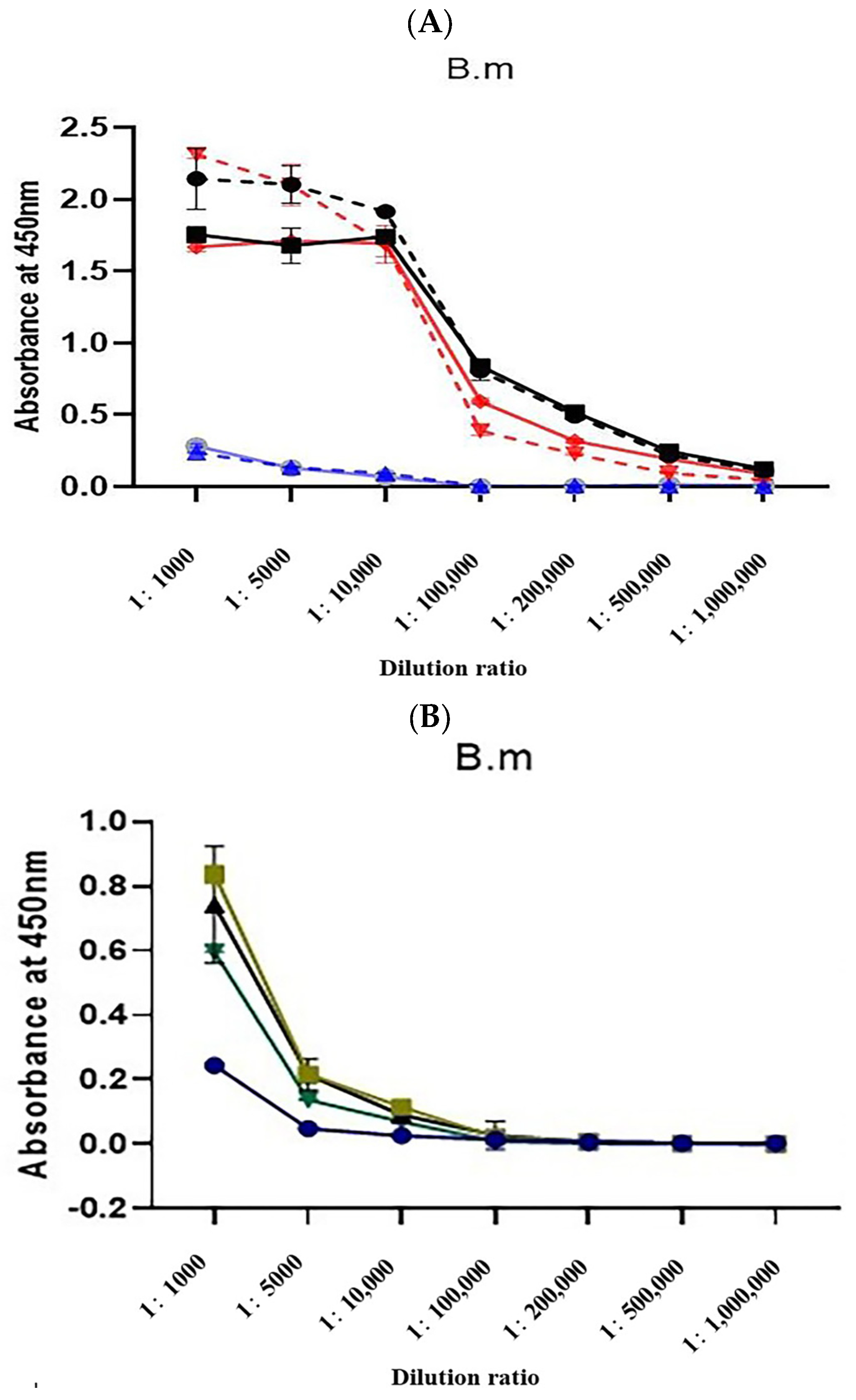
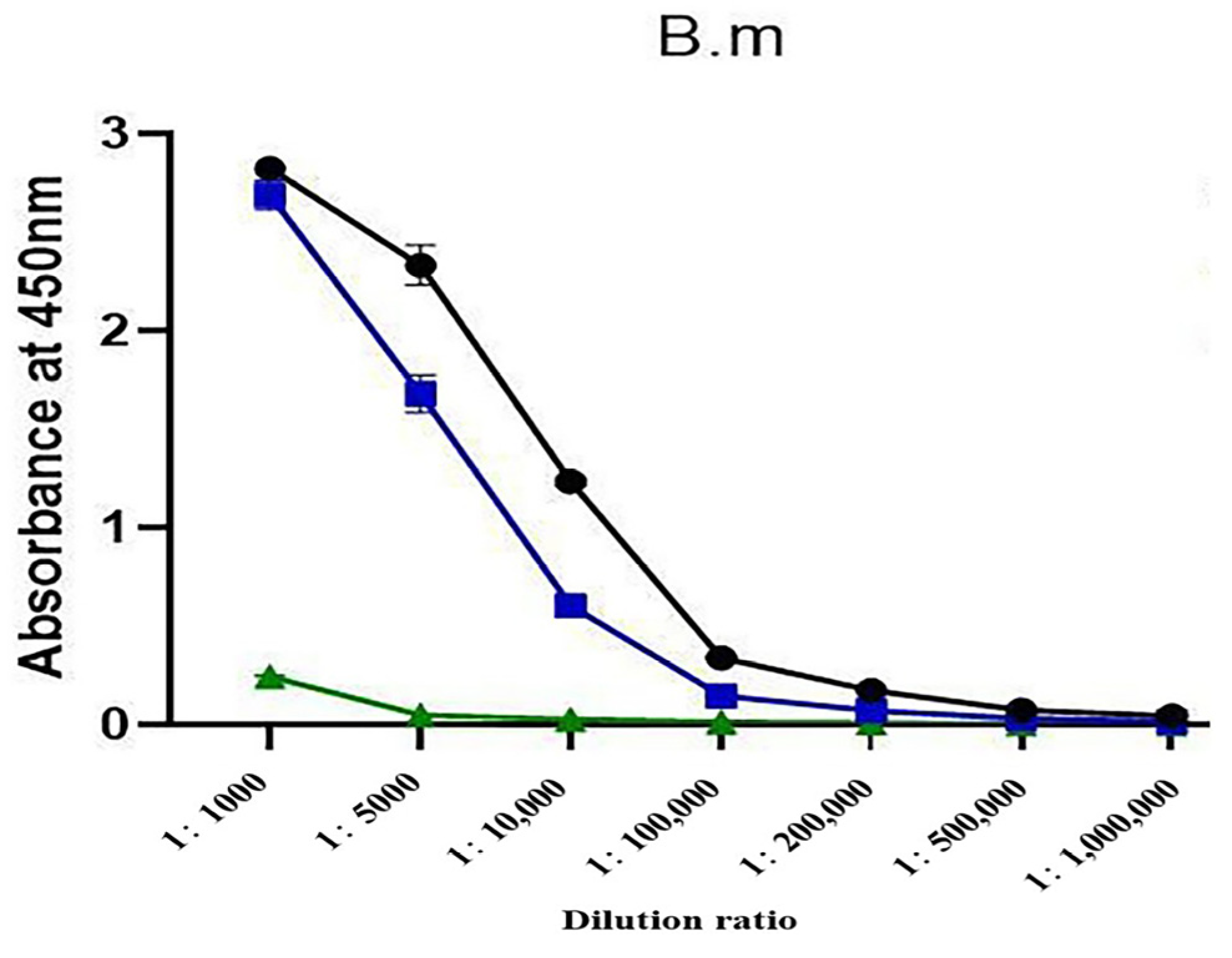
2.3. Changes in the Three Antisera Titers
2.4. Antibody Titer Determination
2.5. Total Antigen Dose for Each of the Three Immunization Methods
2.6. Comparison of Neutralization Activity of the Three Antibodies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Animals and Ethics Statement
4.2.2. Antigen Detoxification
4.2.3. Protein Determination
4.2.4. Expression of Maltose Binding Protein Fusion α-BGT
4.2.5. Lethality and Dose Conversion
4.2.6. Methods of Immunizing Rabbits with Three Antigens
4.2.7. Western Blotting Assessment of Antisera Specificity
4.2.8. Indirect ELISA Determination of Antisera Titers
4.2.9. Purification of Antibodies
4.2.10. Animal Protection Experiment
4.2.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhang, J.R.; Lu, H.J.; Zhao, L.; Chen, J.; Zhang, H.F.; Wei, X.S.; Zhang, L.Y.; Wu, X.B.; Lee, W.H. Immunoreactivity and neutralization study of Chinese Bungarus multicinctus antivenin and lab-prepared anti-bungarotoxin antisera towards purified bungarotoxins and snake venoms. PLoS Negl. Trop. Dis. 2020, 14, e0008873. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.T.; Höjer, J.; Du, N.T. Clinical features of 60 consecutive ICU-treated patients envenomed by Bungarus multicinctus. Southeast Asian J. Trop. Med. Public Health 2009, 40, 518–524. [Google Scholar] [PubMed]
- Aird, S.D.; Wombleb, G.C.; Yates, J.R., III; Grinc, P.R. Primary structure of g-bungarotoxin, a new postsynaptic neurotoxin from venom of Bungarus multicinctus. Toxicon 1999, 37, 609–625. [Google Scholar] [CrossRef]
- Tu, A.T. Neurotoxins of animal venoms: Snakes. Annu. Rev. Biochem. 1973, 42, 235–258. [Google Scholar] [CrossRef]
- Dufton, M.J.; Hider, R.C. Conformational properties of the neurotoxins and cytotoxins isolated from Elapid snake venoms. CRC Crit. Rev. Biochem. 1983, 14, 113–171. [Google Scholar] [CrossRef]
- Walkinshaw, M.D.; Saenger, W.; Maelicke, A. Three-dimensional structure of the “long” neurotoxin from cobra venom. Proc. Natl. Acad. Sci. USA 1980, 77, 2400–2404. [Google Scholar] [CrossRef]
- Nirthanan, S.; Gwee, M.C.E. Three-Finger α-Neurotoxins and the Nicotinic Acetylcholine Receptor, Forty Years On. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, J.; Peng, L.; Yang, Y.; Sun, Z. Compositional and toxicological investigation of pooled venom from farm-raised Naja atra. J. Venom Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210040. [Google Scholar] [CrossRef]
- Tan, K.Y.; Ng, T.S.; Bourges, A.; Ismail, A.K.; Maharani, T.; Khomvilai, S.; Sitprija, V.; Tan, N.H.; Tan, C.H. Geographical variations in king cobra (Ophiophagus hannah) venom from Thailand, Malaysia, Indonesia and China: On venom lethality, antivenom immunoreactivity and in vivo neutralization. Acta Trop. 2020, 203, 105311. [Google Scholar] [CrossRef]
- Ha, T.H.; Höjer, J.; Trinh, X.K.; Nguyen, T.D. A controlled clinical trial of a novel antivenom in patients envenomed by Bungarus multicinctus. J. Med. Toxicol. 2010, 6, 393–397. [Google Scholar] [PubMed]
- Huang, Q.; Wang, F.; Yang, H.; Valitutto, M.; Songer, M. Will the COVID-19 outbreak be a turning point for China’s wildlife protection: New developments and challenges of wildlife conservation in China. Biol. Conserv. 2021, 254, 108937. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mei, F.; Lu, C. COVID-19, a critical juncture in China’s wildlife protection? Hist. Philos. Life Sci. 2021, 43, 46. [Google Scholar] [CrossRef] [PubMed]
- Ratanabanangkoon, K.; Tan, K.Y.; Eursakun, S.; Tan, C.H.; Simsiriwong, P.; Pamornsakda, T.; Wiriyarat, W.; Klinpayom, C.; Tan, N.H. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLoS Negl. Trop. Dis. 2016, 10, e0004565. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Bon, C.; Burnouf, T.; Gutierrez, J.M.; Padilla, A.; Warrell, D.A. WHO Guidelines for the Production, Control and Regulations of Snake Antivenoms Immunoglobulins. 2010. Available online: https://www.who.int/publications/m/item/snake-antivenom-immunoglobulins-annex-5-trs-no-1004 (accessed on 30 October 2013).
- Brown, N.; Landon, J. Antivenom: The most cost-effective treatment in the world? Toxicon 2010, 55, 1405–1407. [Google Scholar] [CrossRef]
- Gutierrez, J.M. Global Availability of Antivenoms: The Relevance of Public Manufacturing Laboratories. Toxins 2018, 11, 5. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Burnouf, T.; Harrison, R.A.; Calvete, J.J.; Kuch, U.; Warrell, D.A.; Williams, D.J. A multicomponent strategy to improve the availability of antivenom for treating snakebite envenoming. Bull World Health Organ. 2014, 92, 526–532. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Higashi, H.G.; Wen, F.H.; Burnouf, T. Strengthening antivenom production in Central and South American public laboratories: Report of a workshop. Toxicon 2007, 49, 30–35. [Google Scholar] [CrossRef]
- Chippaux, J.P. Emergency immunotherapy: Snake and scorpion antivenoms. Biol. Aujourdhui. 2010, 204, 61–70. [Google Scholar] [CrossRef]
- Zolfagharina, H.; Dounighi, N.M. Progress and improvement of the manufacturing process of snake antivenom. Arch. Razi Inst. 2013, 68, 1–10. [Google Scholar]
- Shan, L.L.; Gao, J.F.; Zhang, Y.X.; Shen, S.S.; He, Y.; Wang, J.; Ma, X.M.; Ji, X. Proteomic characterization and comparison of venoms from two elapid snakes (Bungarus multicinctus and Naja atra) from China. J. Proteomics 2016, 138, 83–94. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Pawade, B.S.; Salvi, N.C.; Shaikh, I.K.; Waghmare, A.B.; Jadhav, N.D.; Wagh, V.B.; Pawade, A.S.; Waykar, I.G.; Potnis-Lele, M. Rapid and selective detection of experimental snake envenomation—Use of gold nanoparticle based lateral flow assay. Toxicon 2016, 119, 299–306. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the Inhibitory Potential of Varespladib for Snakebite Envenomation. Molecules 2018, 23, 391. [Google Scholar] [CrossRef]


| Antibodies | Amount of Antigen | Antibody Titers |
|---|---|---|
| Detoxifying α-BGT antibody | 4.8 mg | 250,000 |
| Natural α-BGT antibody | 0.6 mg | 250,000 |
| MBP-α-BGT antibody | 4.8 mg | 5000 |
| Antiserum | Toxins | LD50 (mg/kg) i.p | Antiserum Dose (µg/Mouse) | Number of Mice (n = 6) | ED50 (mg/kg) | Reference | |
|---|---|---|---|---|---|---|---|
| Died | Lived | ||||||
| Detoxifying α-BGT antibody | α-BGT | 0.2 µg/g | 62.5 | 6 | 0 | 11.14 | Lin Bo (2020) [2] |
| 125 | 4 | 2 | |||||
| 250 | 4 | 2 | |||||
| 500 | 0 | 6 | |||||
| Natural α-BGT antibody | 62.5 | 6 | 0 | 11.136 | Present work | ||
| 125 | 4 | 2 | |||||
| 250 | 3 | 3 | |||||
| 500 | 1 | 5 | |||||
| 1000 | 0 | 6 | |||||
| MBP α-BGT antibody | 4000 | 6 | 0 | >200 | Present work | ||
| Not added | 0 | 6 | 0 | Not available | Present work | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zhang, G.; Zhao, L.; Yuan, Y.; Gong, B.; Han, B.; Lee, W.-H. Characterization of Alpha-Bungarotoxin Antibodies Prepared by Different Strategies. Toxins 2025, 17, 601. https://doi.org/10.3390/toxins17120601
Lu H, Zhang G, Zhao L, Yuan Y, Gong B, Han B, Lee W-H. Characterization of Alpha-Bungarotoxin Antibodies Prepared by Different Strategies. Toxins. 2025; 17(12):601. https://doi.org/10.3390/toxins17120601
Chicago/Turabian StyleLu, Huijuan, Guowen Zhang, Lin Zhao, Ying Yuan, Bing Gong, Bin Han, and Wen-Hui Lee. 2025. "Characterization of Alpha-Bungarotoxin Antibodies Prepared by Different Strategies" Toxins 17, no. 12: 601. https://doi.org/10.3390/toxins17120601
APA StyleLu, H., Zhang, G., Zhao, L., Yuan, Y., Gong, B., Han, B., & Lee, W.-H. (2025). Characterization of Alpha-Bungarotoxin Antibodies Prepared by Different Strategies. Toxins, 17(12), 601. https://doi.org/10.3390/toxins17120601





