In Silico Binding Mode Analysis of Blarina Paralytic Peptides with the Human T-Type Ca Channel hCav3.2
Abstract
1. Introduction
2. Results and Discussion
2.1. Sequence Alignment and Structure Comparison Among BPPs and SYNs
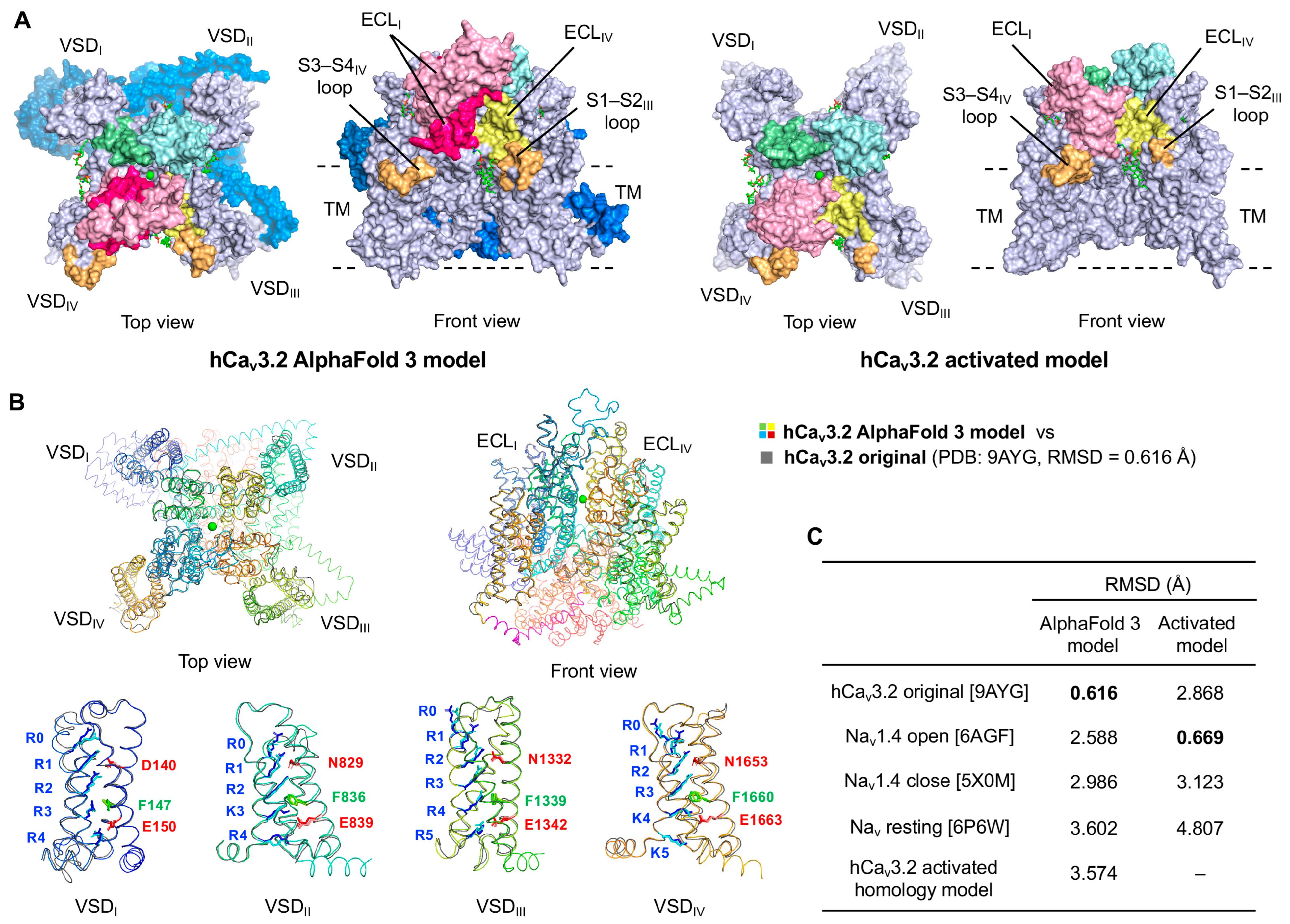
2.2. Construction of the Human T-Type Calcium Channel (hCav3.2) Models
2.3. PPI-Dock Models of the hCav3.2–BPP and SYN Complexes
3. Conclusions
4. Materials and Methods
4.1. Data Source
4.2. Molecular Modeling Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babcock, H.L. Some Observations on the Food Habits of the Short-tailed Shrew (Blarina brevicauda). Science 1914, 40, 526–530. [Google Scholar] [CrossRef]
- Hamilton, W.J., Jr. The Food of the Soricidae. J. Mammal. 1930, 11, 26–39. [Google Scholar] [CrossRef]
- Maier, T.J. Advances in the Biology of Shrews II. In Special Publication of the International Society of Shrew Biologists; Merritt, J.F., Churchfield, S., Hutterer, R., Sheftel, B.I., Eds.; International Society of Shrew Biologists: New York, NY, USA, 2005; pp. 361–366. [Google Scholar]
- Pearson, O.P. On the Cause and Nature of a Poisonous Action Produced by the Bite of a Shrew (Blarina brevicauda). J. Mammal. 1942, 23, 159–166. [Google Scholar] [CrossRef]
- Bowen, C.V.; DeBay, D.; Ewart, H.S.; Gallant, P.; Gormley, S.; Ilenchuk, T.T.; Iqbal, U.; Lutes, T.; Martina, M.; Mealing, G.; et al. In vivo Detection of Human TRPV6-rich Tumors with Anti-cancer Peptides Derived from Soricidin. PLoS ONE 2013, 8, e58866. [Google Scholar] [CrossRef]
- Kita, M.; Nakamura, Y.; Okumura, Y.; Ohdachi, S.D.; Oba, Y.; Yoshikuni, M.; Kido, H.; Uemura, D. Blarina Toxin, A Mammalian Lethal Venom from the Short-tailed Shrew Blarina brevicauda: Isolation and Characterization. Proc. Natl. Acad. Sci. USA 2004, 101, 7542–7547. [Google Scholar] [CrossRef]
- Kita, M.; Okumura, Y.; Ohdachi, S.D.; Oba, Y.; Yoshikuni, M.; Nakamura, Y.; Kido, H.; Uemura, D. Purification and Characterisation of Blarinasin, A New Tissue Kallikrein-like Protease from the Short-tailed Shrew Blarina brevicauda: Comparative Studies with Blarina Toxin. Biol. Chem. 2005, 386, 177–182. [Google Scholar] [CrossRef]
- Aminetzach, Y.T.; Srouji, J.R.; Kong, C.Y.; Hoekstra, H.E. Convergent Evolution of Novel Protein Function in Shrew and Lizard Venom. Curr. Biol. 2009, 19, 1925–1931. [Google Scholar] [CrossRef]
- Yano, Y.; Fukuoka, R.; Maturana, A.D.; Ohdachi, S.D.; Kita, M. Mammalian Neurotoxins, Blarina Paralytic Peptides, Cause Hyperpolarization of Human T-type Ca Channel hCav3.2 Activation. J. Biol. Chem. 2023, 299, 105066. [Google Scholar] [CrossRef]
- Hanf, Z.R.; Chavez, A.S. A Comprehensive Multi-omic Approach Reveals a Relatively Simple Venom in a Diet Generalist, the Northern Short-tailed Shrew, Blarina brevicauda. Genome Biol. Evol. 2020, 12, 1148–1166. [Google Scholar] [CrossRef]
- Gao, S.; Yao, X.; Yan, N. Structure of Human Cav2.2 Channel Blocked by the Painkiller Ziconotide. Nature 2021, 596, 143–147. [Google Scholar] [CrossRef]
- Weiss, N.; Zamponi, G.W. T-type Calcium Channels: From Molecule to Therapeutic Opportunities. Int. J. Biochem. Cell Biol. 2019, 108, 34–39. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Shin, H.S. Lack of Delta Waves and Sleep Disturbances during Non-rapid Eye Movement Sleep in Mice Lacking Alpha1G-subunit of T-type Calcium Channels. Proc. Natl. Acad. Sci. USA 2004, 101, 18195–18199. [Google Scholar] [CrossRef]
- Kim, D.; Park, D.; Choi, S.; Lee, S.; Sun, M.; Kim, C.; Shin, H.-S. Thalamic Control of Visceral Nociception Mediated by T-type Ca2+ Channels. Science 2003, 302, 117–119. [Google Scholar] [CrossRef]
- Sakkaki, S.; Gangarossa, G.; Lerat, B.; Francon, D.; Forichon, L.; Chemin, J.; Valjent, E.; Lerner-Natoli, M.; Lory, P. Blockade of T-type Calcium Channels Prevents Tonic-clonic Seizures in A Maximal Electroshock Seizure Model. Neuropharmacology 2016, 101, 320–329. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Jin, X.; Lyu, C.; Guo, Q.; Liu, T.; Chen, J.; Davakan, A.; Lory, P.; Yan, N. Structural Basis for Human Cav3.2 Inhibition by Selective Antagonists. Cell Res. 2024, 34, 440–450. [Google Scholar]
- Snutch, T.P.; Zamponi, G.W. Recent Advances in the Development of T-type Calcium Channel Blockers for Pain Intervention. Br. J. Pharmacol. 2018, 175, 2375–2383. [Google Scholar] [CrossRef]
- Fukunaga, K.; Izumi, H.; Yabuki, Y.; Shinoda, Y.; Shioda, N.; Han, F. Alzheimer’s Disease Therapeutic Candidate SAK3 Is an Enhancer of T-type Calcium Channels. J. Pharmacol. Sci. 2019, 139, 51–58. [Google Scholar] [CrossRef]
- Liston, D.R.; Vanderhaeghen, J.-J.; Rossier, J. Presence in Brain of Synenkephalin, a Proenkephalin-immunoreactive Protein Which Does Not Contain Enkephalin. Nature 1983, 302, 62–65. [Google Scholar] [CrossRef]
- Comb, M.; Seeburg, P.H.; Adelman, J.; Eiden, L.; Herbert, E. Primary Structure of the Human Met- and Leu-enkephalin Precursor and Its mRNA. Nature 1982, 295, 663–666. [Google Scholar] [CrossRef]
- Stern, A.S.; Jones, B.N.; Shively, J.E.; Stein, S.; Undenfriend, S. Two Adrenal Opioid Polypeptides: Proposed Intermediates in the Processing of Proenkephalin. Proc. Natl. Acad. Sci. USA 1981, 78, 1962–1966. [Google Scholar] [CrossRef]
- Hughes, J.; Smith, T.W.; Kosterlitz, H.W.; Fothergill, L.A.; Morgan, B.A.; Morris, H.R. Identification of Two Related Pentapeptides from the Brain with Potent Opiate Agonist Activity. Nature 1975, 258, 577–579. [Google Scholar] [CrossRef]
- Rodriguez-Vida, M.I.; Kleid, M.C.; Ase, A.; Finkielman, S.; Nahmod, V.E.; Vindrola, O. Synenkephalin Processing in Embryonic Rat Brain. Dev. Brain Res. 1994, 77, 151–156. [Google Scholar] [CrossRef]
- Noda, M.; Furutani, Y.; Takahashi, H.; Toyosato, M.; Hirose, T.; Inayama, S.; Nakanishi, S.; Numa, S. Cloning and Sequence Analysis of cDNA for Bovine Adrenal Preproenkephalin. Nature 1982, 295, 202–206. [Google Scholar] [CrossRef]
- Gubler, U.; Seeburg, P.; Hoffman, B.J.; Gage, L.P.; Udenfriend, S. Molecular Cloning Establishes Proenkephalin as Precursor of Enkephalin-containing Peptides. Nature 1982, 295, 206–208. [Google Scholar] [CrossRef]
- Goumon, Y.; Lugardon, K.; Gadroy, P.; Strub, J.M.; Welters, I.D.; Stefano, G.B.; Aunis, D.; Metz-Boutigue, M.H. Processing of Proenkephalin-A in Bovine Chromaffin Cells: Identification of Natural Derived Fragments by N-Terminal Sequencing and Matrix-assisted Laser Desorption Ionization-time of Flight Mass Spectrometry. J. Biol. Chem. 2000, 275, 38355–38362. [Google Scholar] [CrossRef]
- Saravia, F.; Ase, A.; Aloyz, R.; Kleid, M.C.; Ines, M.; Vida, R.; Nahmod, V.E.; Vindrola, O. Differential Posttranslational Processing of Proenkephalin in Rat Bone Marrow and Spleen Mononuclear Cells: Evidence for Synenkephalin Cleavage. Endocrinology 1993, 132, 1431–1437. [Google Scholar] [CrossRef]
- Vindrola, O.; Padros, M.R.; Sterin-Prync, A.; Ase, A.; Finkielman, S.; Nahmod, V.E. Proenkephalin System in Human Polymorphonuclear Cells. Production and Release of a Novel 1.0 kDa Peptide Derived from Synenkephalin. J. Clin. Investig. 1990, 86, 531–537. [Google Scholar] [CrossRef]
- Kowalski, K.; Marciniak, P.; Rosinski, G.; Rychlik, L. Evaluation of the Physiological Activity of Venom from the Eurasian Water Shrew Neomys fodiens. Front. Zool. 2017, 14, 46. [Google Scholar] [CrossRef]
- Kowalski, K.; Marciniak, P.; Rychlik, L. A New, Widespread Venomous Mammal Species: Hemolytic Activity of Sorex araneus Venom Is Similar to That of Neomys fodiens Venom. Zool. Lett. 2022, 8, 7. [Google Scholar] [CrossRef]
- Casewell, N.R.; Petras, D.; Card, D.C.; Suranse, V.; Mychajliw, A.M.; Richards, D.; Koludarov, I.; Albulescu, L.-O.; Slagboom, J.; Hempel, B.-F.; et al. Solenodon Genome Reveals Convergent Evolution of Venom in Eulipotyphlan Mammals. Proc. Natl. Acad. Sci. USA 2019, 116, 25745–25755. [Google Scholar] [CrossRef]
- Fukuoka, R.; Yano, Y.; Hara, N.; Sadamoto, C.; Maturana, A.D.; Kita, M. Hyperpolarization Modulation of the T-Type hCav3.2 Channel by Human Synenkephalin [1–53], a Shrew Neurotoxin Analogue without Paralytic Effects. Angew. Chem. Int. Ed. 2025, 64, e202503891. [Google Scholar] [CrossRef]
- Lecchi, P.; Loh, Y.P.; Snell, C.R.; Pannell, L.K. The Structure of Synenkephalin (Pro-Enkephalin1-73) Is Dictated by Three Disulfide Bridges. Biochem. Biophys. Res. Commun. 1997, 232, 800–805. [Google Scholar] [CrossRef]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular Diversity and Accelerated Evolution of C-type Lectin-like Proteins from Snake Venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, Function and Evolution of Three-finger Toxins: Mini Proteins with Multiple Targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Undheim, E.A.B.; Grimm, L.L.; Low, C.-F.; Morgenstern, D.; Herzig, V.; Zobel-Thropp, P.; Pineda, S.S.; Habib, R.; Dziemborowicz, S.; Fry, B.G.; et al. Weaponization of a Hormone: Convergent Recruitment of Hyperglycemic Hormone into the Venom of Arthropod Predators. Structure 2015, 23, 1283–1292. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and Snake Venom Evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Duda, T.F.; Palumbi, S.R. Molecular Genetics of Ecological Diversification: Duplication and Rapid Evolution of Toxin Genes of the Venomous Gastropod Conus. Proc. Natl. Acad. Sci. USA 1999, 96, 6820–6823. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, G.; Wu, Q.; Wu, K.; Li, R.; Lei, J.; Pan, X.; Yan, N. Cryo-EM Structures of Apo and Antagonist-bound Human Cav3.1. Nature 2019, 576, 492–497. [Google Scholar] [CrossRef]
- He, L.; Yu, Z.; Geng, Z.; Huang, Z.; Zhang, C.; Dong, Y.; Gao, Y.; Wang, Y.; Chen, Q.; Sun, L.; et al. Structure, Gating, and Pharmacology of Human CaV3.3 channel. Nat. Commun. 2022, 13, 2084. [Google Scholar] [CrossRef]
- Wu, J.; Yan, Z.; Li, Z.; Yan, C.; Lu, S.; Dong, M.; Yan, N. Structure of the Voltage-gated Calcium Channel Cav1.1 Complex. Science 2015, 350, aad2395. [Google Scholar] [CrossRef]
- Pan, X.; Li, Z.; Zhou, Q.; Shen, H.; Wu, K.; Huang, X.; Chen, J.; Zhang, J.; Zhu, X.; Lei, J.; et al. Structure of the Human Voltage-gated Sodium Channel Nav1.4 in Complex with β1. Science 2018, 362, eaau2486. [Google Scholar]
- Tsuda, T.; Arai, A.; Kita, M. Ligand-dissociation-type N,N-Dimethylaminopyrene Probes for in situ Site-specific Protein Labeling. Chem. Asian J. 2022, 17, e202200631. [Google Scholar] [CrossRef]
- Nakatani, M.; Ebihara, S.; Kita, M. Development of Diazirine–alkyne Tag for Protein–ligand Interaction Analysis with Photoaffinity Labeling. Chem. Lett. 2024, 53, upae142. [Google Scholar] [CrossRef]
- Escoda, L.; Castresana, J. The Genome of the Pyrenean Desman and the Effects of Bottlenecks and Inbreeding on the Genomic Landscape of an Endangered Species. Evol. Appl. 2021, 14, 1898–1913. [Google Scholar] [CrossRef]
- Noda, M.; Teranishi, Y.; Takahashi, H.; Toyosato, M.; Notake, M.; Nakanishi, A.; Numa, S. Isolation and Structural Organization of the Human Preproenkephalin Gene. Nature 1982, 297, 431–434. [Google Scholar] [CrossRef]
- Rosen, H.; Douglass, J.; Herbert, E. Isolation and Characterization of the Rat Proenkephalin Gene. J. Biol. Chem. 1984, 259, 14309–14313. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Williams, C.; Sabol, S.L. Rat Brain Preproenkephalin mRNA. cDNA Cloning, Primary Structure, and Distribution in the Central Nervous System. J. Biol. Chem. 1984, 259, 14301–14308. [Google Scholar] [CrossRef]
- Howells, R.D.; Kilpatrick, D.L.; Bhatt, R.; Monahan, J.J.; Poonian, M.; Udenfriend, S. Molecular Cloning and Sequence Determination of Rat Preproenkephalin cDNA: Sensitive Probe for Studying Transcriptional Changes in Rat Tissues. Proc. Natl. Acad. Sci. USA 1984, 81, 7651–7655. [Google Scholar] [CrossRef]
- Zurawski, G.; Benedik, M.; Kamb, B.J.; Abrams, J.S.; Zurawski, S.M.; Lee, F.D. Activation of Mouse T-Helper Cells Induces Abundant Preproenkephalin mRNA Synthesis. Science 1986, 232, 772–775. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Steinegger, M.; Söding, J. MMseqs2 Desktop and Local Web Server App for Fast, Interactive Sequence Searches. Bioinformatics 2019, 35, 2856–2858. [Google Scholar] [CrossRef]
- Itakura, M.; Utomo, D.H.; Kita, M. Development of Actin Dimerization Inducers Inspired by Actin-depolymerizing Macrolides. Chem. Commun. 2024, 60, 4910–4913. [Google Scholar] [CrossRef]
- Sun, Y.; Dakiiwa, A.; Zhang, M.; Shibata, T.; Kita, M. Inhibition of Lysosomal Cathepsin A and Neuraminidase 1 Interaction by Anti-Obesity Cyclic Peptide. Chem. Eur. J. 2024, 30, e202402049. [Google Scholar] [CrossRef] [PubMed]



| Name | Organism | Locus (Accession No.) | Reference |
|---|---|---|---|
| BPP1/2 | Blarina brevicauda | MT559766 | [10] |
| saSYN | Sorex araneus | NC_073303 | – 1 |
| scSYN | Sorex cinereus | NW_026606059 | – 1 |
| eSYN | Erinaceus europaeus | NW_006804460 | – 1 |
| gSYN | Galemys pyrenaicus | JAGFMF010011524 | [47] |
| hSYN | Homo sapiens | PENK_HUMAN (P01210) | [38,48] |
| rSYN | Rattus norvegicus | AH002996 | [49,50,51] |
| mSYN | Mus musculus | NP_001335138 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, N.; Sadamoto, C.; Fukuoka, R.; Yano, Y.; Maturana, A.D.; Kita, M. In Silico Binding Mode Analysis of Blarina Paralytic Peptides with the Human T-Type Ca Channel hCav3.2. Toxins 2025, 17, 549. https://doi.org/10.3390/toxins17110549
Hara N, Sadamoto C, Fukuoka R, Yano Y, Maturana AD, Kita M. In Silico Binding Mode Analysis of Blarina Paralytic Peptides with the Human T-Type Ca Channel hCav3.2. Toxins. 2025; 17(11):549. https://doi.org/10.3390/toxins17110549
Chicago/Turabian StyleHara, Nozomi, Chihiro Sadamoto, Ryo Fukuoka, Yusuke Yano, Andres D. Maturana, and Masaki Kita. 2025. "In Silico Binding Mode Analysis of Blarina Paralytic Peptides with the Human T-Type Ca Channel hCav3.2" Toxins 17, no. 11: 549. https://doi.org/10.3390/toxins17110549
APA StyleHara, N., Sadamoto, C., Fukuoka, R., Yano, Y., Maturana, A. D., & Kita, M. (2025). In Silico Binding Mode Analysis of Blarina Paralytic Peptides with the Human T-Type Ca Channel hCav3.2. Toxins, 17(11), 549. https://doi.org/10.3390/toxins17110549







