Well-Being, Protein-Bound Toxins, and Dietary Fibre in Patients with Kidney Disease: Have We Been Missing the Obvious?
Abstract
1. Background
2. Results
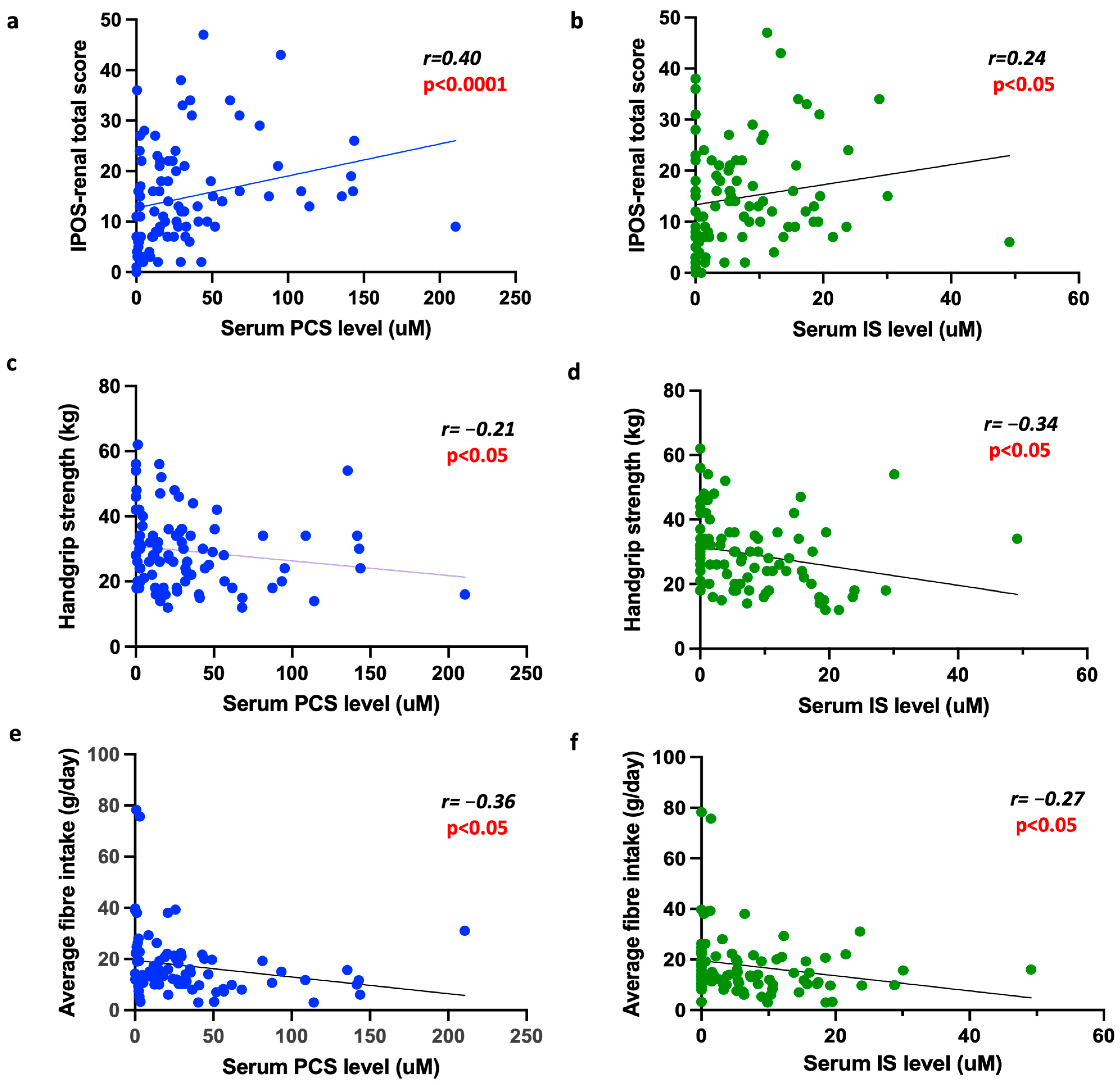
2.1. The Association Between PBTs and Patient-Reported Outcome Measures (PROMs)
2.2. The Association Between PBTs and Physical Ability
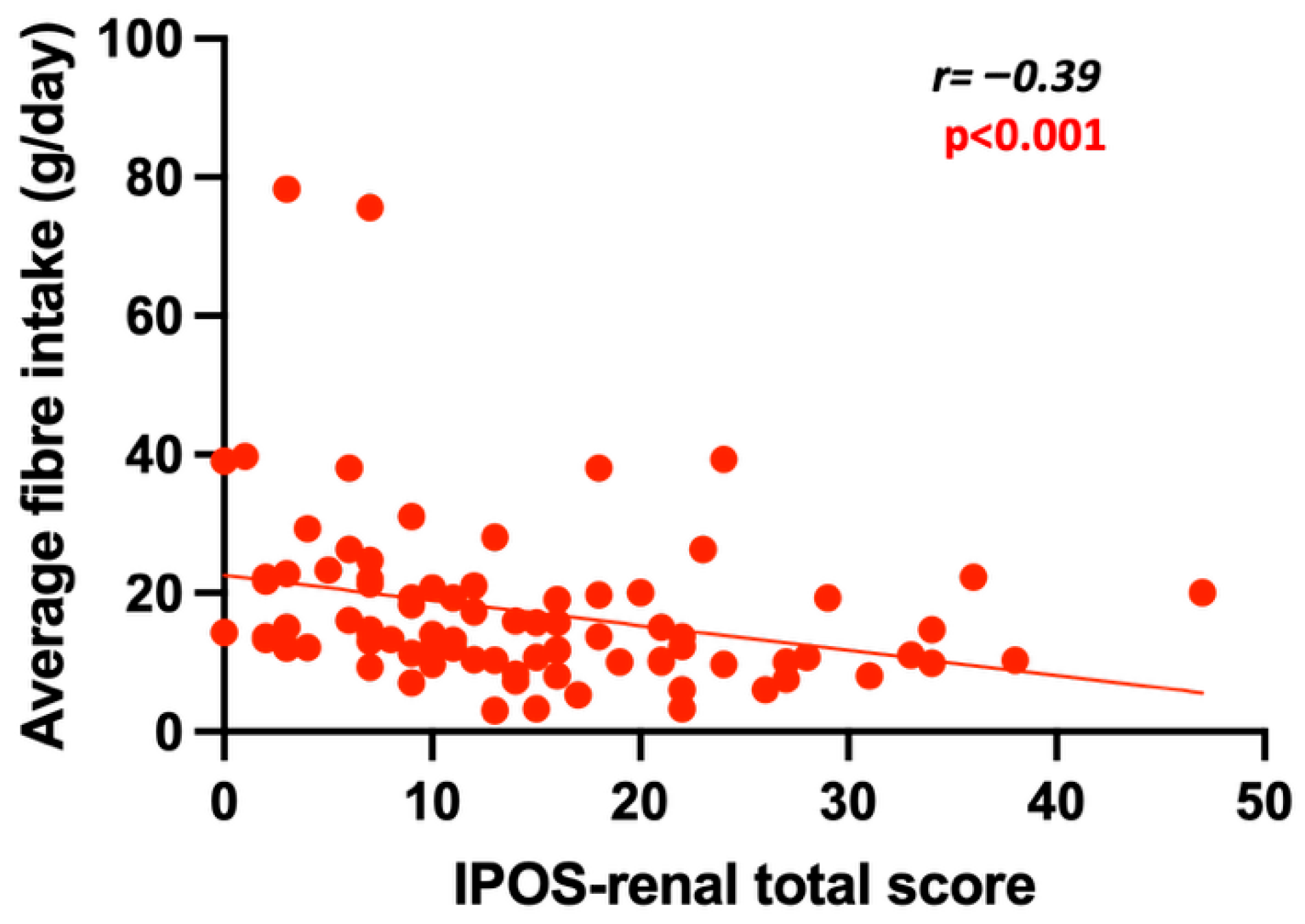
2.3. The Association Between Fibre Intake, PBTs and Patient-Focused Outcome Measures
2.4. The Association Between Albumin and Patient-Focused Outcomes
2.5. Comparison Between Transplant, HD and PD Patients
2.6. Comparison of Patient-Focused Outcome Measures Between Transplant, HD and PD Patients
2.7. Comparison of PBTs and Fibre Intake Between Transplant, HD and PD Patients
2.8. The Effect of Residual Kidney Function
3. Discussion
4. Conclusions
5. Methods
5.1. Diet History
5.2. Patient-Reported Outcome Measures (PROMs)
5.3. Physical Ability
5.4. Pathology
5.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanholder, R.; Glorieux, G.; De Smet, R.; Lameire, N.; European Uremic Toxin Work Group. New insights in uremic toxins. Kidney Int. Suppl. 2003, 63, S6–S10. [Google Scholar] [CrossRef]
- National Kidney Foundation. NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am. J. Kidney Dis. 1997, 30 (Suppl. S2), S67–S136. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Paniagua, R.; Amato, D.; Vonesh, E.; Correa-Rotter, R.; Ramos, A.; Moran, J.; Mujais, S.; Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J. Am. Soc. Nephrol. 2002, 13, 1307–1320. [Google Scholar] [CrossRef]
- Eknoyan, G.; Beck, G.J.; Cheung, A.K.; Daugirdas, J.T.; Greene, T.; Kusek, J.W.; Allon, M.; Bailey, J.; Delmez, J.A.; Depner, T.A.; et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 2002, 347, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Blake, P.G.; Boudville, N.; Davies, S.; de Arteaga, J.; Dong, J.; Finkelstein, F.; Foo, M.; Hurst, H.; Johnson, D.W.; et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit. Dial. Int. 2020, 40, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Malaweera, A.; Huang, L.; McMahon, L. Benefits and Pitfalls of Uraemic Toxin Measurement in Peritoneal Dialysis. J. Clin. Med. 2025, 14, 1395. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Verbeke, K.; Vanrenterghem, Y. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int. 2003, 64, 2238–2243. [Google Scholar] [CrossRef]
- Vanholder, R.; Meert, N.; Van Biesen, W.; Meyer, T.; Hostetter, T.; Dhondt, A.; Eloot, S. Why do patients on peritoneal dialysis have low blood levels of protein-bound solutes? Nat. Clin. Pract Nephrol. 2009, 5, 130–131. [Google Scholar] [CrossRef]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef]
- Chen, X.; Gao, S.; Ruan, M.; Chen, S.; Xu, J.; Xing, X.; Pan, X.; Mei, C.; Mao, Z. Shen-Shuai-Ning granule decreased serum concentrations of indoxyl sulphate in uremic patients undergoing peritoneal dialysis. Biosci. Rep. 2018, 38, BSR20171694. [Google Scholar] [CrossRef]
- Salmean, Y.A.; Segal, M.S.; Palii, S.P.; Dahl, W.J. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J. Ren. Nutr. 2015, 25, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, Q.; Zhao, J.; Lin, X.; He, S.; Wu, N.; Yao, Y.; Liang, W.; Zuo, X.; Ying, C. Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: A randomized crossover study. Am. J. Clin. Nutr. 2020, 111, 1087–1099. [Google Scholar] [CrossRef]
- Davison, S.N.; Klarenbach, S.; Manns, B.; Schnick-Makaroff, K.; Buzinski, R.; Corradetti, B.; Short, H.; Johnson, J.A. Patient-reported outcome measures in the care of in-centre hemodialysis patients. J. Patient-Rep. Outcomes 2021, 5 (Suppl. S2), 93. [Google Scholar] [CrossRef]
- Ozen, N.; Cepken, T.; Sousa, C.N. Does Adequate Hemodialysis Prevent Symptoms?: A National Cross-Sectional Survey. Clin. Nurs. Res. 2021, 30, 334–342. [Google Scholar] [CrossRef]
- Johnson, W.J.; Hagge, W.W.; Wagoner, R.D.; Dinapoli, R.P.; Rosevear, J.W. Effects of urea loading in patients with far-advanced renal failure. Mayo Clin. Proc. 1972, 47, 21–29. [Google Scholar] [PubMed]
- Blake, P.G.; Sombolos, K.; Abraham, G.; Weissgarten, J.; Pemberton, R.; Chu, G.L.; Oreopoulos, D.G. Lack of correlation between urea kinetic indices and clinical outcomes in CAPD patients. Kidney Int. 1991, 39, 700–706. [Google Scholar] [CrossRef]
- Moreno, F.; Lopez Gomez, J.M.; Sanz-Guajardo, D.; Jofre, R.; Valderrabano, F.; Spanish Cooperative Renal Patients Quality of Life Study Group. Quality of life in dialysis patients. A spanish multicentre study. Nephrol. Dial. Transplant. 1996, 11 (Suppl. S2), 125–129. [Google Scholar] [CrossRef]
- Hida, M.; Aiba, Y.; Sawamura, S.; Suzuki, N.; Satoh, T.; Koga, Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996, 74, 349–355. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Lesaffer, G. p-cresol: A toxin revealing many neglected but relevant aspects of uraemic toxicity. Nephrol. Dial. Transplant. 1999, 14, 2813–2815. [Google Scholar] [CrossRef]
- Hou, Y.C.; Liu, Y.M.; Liao, M.T.; Zheng, C.M.; Lu, C.L.; Liu, W.C.; Hung, K.-C.; Lin, S.-M.; Lu, K.-C. Indoxyl sulfate mediates low handgrip strength and is predictive of high hospitalization rates in patients with end-stage renal disease. Front. Med. 2023, 10, 1023383. [Google Scholar] [CrossRef]
- Lin, Y.L.; Liu, C.H.; Lai, Y.H.; Wang, C.H.; Kuo, C.H.; Liou, H.H.; Hsu, B.-G. Association of Serum Indoxyl Sulfate Levels with Skeletal Muscle Mass and Strength in Chronic Hemodialysis Patients: A 2-year Longitudinal Analysis. Calcif. Tissue Int. 2020, 107, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Caldiroli, L.; Armelloni, S.; Eskander, A.; Messa, P.; Rizzo, V.; Margiotta, E.; Cesari, M.; Vettoretti, S. Association between the uremic toxins indoxyl-sulfate and p-cresyl-sulfate with sarcopenia and malnutrition in elderly patients with advanced chronic kidney disease. Exp. Gerontol. 2021, 147, 111266. [Google Scholar] [CrossRef]
- Vanden Wyngaert, K.; Van Craenenbroeck, A.H.; Holvoet, E.; Calders, P.; Van Biesen, W.; Eloot, S. Composite Uremic Load and Physical Performance in Hemodialysis Patients: A Cross-Sectional Study. Toxins 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Viramontes-Horner, D.; Marquez-Sandoval, F.; Martin-del-Campo, F.; Vizmanos-Lamotte, B.; Sandoval-Rodriguez, A.; Armendariz-Borunda, J.; García-Bejarano, H.; Renoirte-López, K.; García-García, G. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J. Ren. Nutr. 2015, 25, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin. Kidney J. 2022, 15, 213–225. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.X.; Wu, Q.J.; Wang, Z.H.; Liu, H.; Dong, C.; Kuai, T.-T.; You, L.-L.; Xiao, J. Association of dietary fiber with subjective sleep quality in hemodialysis patients: A cross-sectional study in China. Ann. Med. 2023, 55, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Buxo, J.A.; Lowrie, E.G.; Lew, N.L.; Zhang, S.M.; Zhu, X.; Lazarus, J.M. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am. J. Kidney Dis. 1999, 33, 523–534. [Google Scholar] [CrossRef]
- Maiorca, R.; Brunori, G.; Zubani, R.; Cancarini, G.C.; Manili, L.; Camerini, C.; Movilli, E.; Pola, A.; D’Avolio, G.; Gelatti, U. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol. Dial. Transplant. 1995, 10, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Goodrose-Flores, C.; Bonn, S.; Klasson, C.; Helde Frankling, M.; Trolle Lagerros, Y.; Bjorkhem-Bergman, L. Appetite in Palliative Cancer Patients and Its Association with Albumin, CRP and Quality of Life in Men and Women-Cross-Sectional Data from the Palliative D-Study. Life 2022, 12, 671. [Google Scholar] [CrossRef]
- Karapinar, M.A.K.A.; Kirdi, N.; Yavuz, B.B. SAT0743-HPR Relation between serum albumin and physical performance and mobility in a community-based elderly people with osteoporosis. Ann. Rheum. Dis. 2018, 77, 1832. [Google Scholar] [CrossRef]
- Malaweera, A.; Huang, L.L.; McMahon, L.P. Clinical events and patient-reported outcome measures in peritoneal dialysis. BMC Nephrol. 2025, 26, 328. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, A.N.; Hoffman, A.T.; Morton, R.L.; Smyth, B.; Brown, M.A. Estimating a Minimal Important Difference for the EQ-5D-5L Utility Index in Dialysis Patients. Value Health 2024, 27, 469–477. [Google Scholar] [CrossRef]
- Fewings, A.; Vandelanotte, C.; Irwin, C.; Ting, C.; Williams, E.; Khalesi, S. The use and acceptability of diet-related apps and websites in Australia: Cross-sectional study. Digit. Health 2022, 8, 20552076221139091. [Google Scholar] [CrossRef]
- Chen, J.; Allman-Farinelli, M. Impact of Training and Integration of Apps into Dietetic Practice on Dietitians’ Self-Efficacy with Using Mobile Health Apps and Patient Satisfaction. JMIR Mhealth Uhealth 2019, 7, e12349. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Hurworth, M.; Giglia, R.; Trapp, G.; Strauss, P. Feasibility of a commercial smartphone application for dietary assessment in epidemiological research and comparison with 24-h dietary recalls. Nutr. J. 2018, 17, 5. [Google Scholar] [CrossRef]
- Elder, G.J.; Malik, A.; Lambert, K. Role of dietary phosphate restriction in chronic kidney disease. Nephrology 2018, 23, 1107–1115. [Google Scholar] [CrossRef]
- Raj, R.; Ahuja, K.; Frandsen, M.; Murtagh, F.E.M.; Jose, M. Validation of the IPOS-Renal Symptom Survey in Advanced Kidney Disease: A Cross-sectional Study. J. Pain. Symptom Manag. 2018, 56, 281–287. [Google Scholar] [CrossRef]
- Kremenova, Z.; Vlckova, K. Translation, cultural adaptation, and validation of the Integrated Palliative Outcome Scale-renal (IPOS-r) to Czech. BMC Palliat. Care 2022, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.; Mulhern, B.; Lancsar, E.; Lorgelly, P.; Ratcliffe, J.; Street, D.; Viney, R. The Use of a Discrete Choice Experiment Including Both Duration and Dead for the Development of an EQ-5D-5L Value Set for Australia. Pharmacoeconomics 2023, 41, 427–438. [Google Scholar] [CrossRef]
- Worboys, H.M.; Gray, L.; Burton, J.; Alava, M.H.; Greenwood, S.; Cooper, N. Mapping the Kidney Disease Quality-of-Life Questionnaire Onto the EQ-5D-5L Utility Index in Patients Undergoing Hemodialysis. Value Health 2025, 28, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Hardy, R.; Mortality Review, G.; Falcon Teams, H.A.S. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, c4467. [Google Scholar] [CrossRef]
- Strand, B.H.; Cooper, R.; Bergland, A.; Jorgensen, L.; Schirmer, H.; Skirbekk, V.; Emaus, N. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromso Study. J. Epidemiol. Community Health 2016, 70, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Gabrys, I.; Lightfoot, C.J.; Lambert, K.; Baker, L.A.; Billany, R.E.; Kanavaki, A.; Palmer, J.; Robinson, K.A.; Nixon, D.; et al. A Systematic Review of Handgrip Strength Measurement in Clinical and Epidemiological Studies of Kidney Disease: Toward a Standardized Approach. J. Ren. Nutr. 2022, 32, 371–381. [Google Scholar] [CrossRef]
- Stringa, N.; van Schoor, N.M.; Hoogendijk, E.O.; Milaneschi, Y.; Huisman, M. The phenotypic and genotypic association of grip strength with frailty, physical performance and functional limitations over time in older adults. Age Ageing 2023, 52, afad189. [Google Scholar] [CrossRef]
- Luque-Casado, A.; Novo-Ponte, S.; Sanchez-Molina, J.A.; Sevilla-Sanchez, M.; Santos-Garcia, D.; Fernandez-Del-Olmo, M. Test-Retest Reliability of the Timed up and Go Test in Subjects with Parkinson’s Disease: Implications for Longitudinal Assessments. J. Parkinsons Dis. 2021, 11, 2047–2055. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, Q.; Han, R.; De Bock, P.; Vassard-Yu, G.; Hallemans, A.; Van Laer, L. Reliability and Validity of Instrumented Timed up and Go Test in Typical Adults and Elderly: A Systematic Review. Arch. Phys. Med. Rehabil. 2025, 106, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Jamal, S.A.; Lok, C.E. Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Calaf, R.; Cerini, C.; Genovesio, C.; Verhaeghe, P.; Jourde-Chiche, N.; Berge-Lefranc, D.; Gondouin, B.; Dou, L.; Morange, S.; Argilés, A.; et al. Determination of uremic solutes in biological fluids of chronic kidney disease patients by HPLC assay. J. Chromatogr. 2011, 879, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
| All (n = 90) | PD (n = 30) | HD (n = 30) | Transplant (n = 30) | p Value | |
|---|---|---|---|---|---|
| Age (years) [Median (IQR)] | 63 (54–74) | 59 (45–74) | 72 (59–80) | 59 (54–67) | <0.05 |
| Male sex [N (%)] | 59 (66%) | 17 (57%) | 20 (67%) | 22 (73%) | NS |
| Aetiology of kidney disease [N (%)] | |||||
| 25 (28%) | 5 (17%) | 14(47%) | 6 (20%) | <0.05 |
| 27 (30%) | 11 (36%) | 4 (13%) | 12 (40%) | |
| 38 (42%) | 14 (47%) | 12 (40%) | 12 (40%) | |
| Prevalence of diabetes | 31 (34%) | 6 (20%) | 14 (47%) | 11 (37%) | NS |
| Charleson co-morbidity score (CC score) [Median (IQR)] | 5 (3–7) | 4 (3–6) a | 7 (5–8) | 4 (3–6) b | <0.01 |
| BMI (kg/m2) [Median (IQR)] | 27 (23–29) | 27 (24–29) | 25 (22–29) | 27 (23–31) | NS |
| Mobility inside [N (%)] | |||||
| 82 (91%) | 29 (97%) | 24 (80%) | 29 (97%) | NS |
| 4 (5%) | 1 (3%) | 3 (10%) | 0 (0%) | |
| 2 (2%) | 0 (0%) | 2 (7%) | 0 (0%) | |
| 2 (2%) | 0 (0%) | 1 (3%) | 1 (3%) | |
| Mobility outside [N (%)] | |||||
| 76 (84%) | 27 (90%) | 20 (67%) c | 29 (97%) | <0.001 |
| 4 (5%) | 1 (3%) | 3 (10%) c | 0 (0%) | |
| 8 (9%) | 2 (7%) | 6 (20%) c | 0 (0%) | |
| 2 (2%) | 0 (0%) | 1 (3%) | 1 (3%) | |
| PROM tool | |||||
| EQ-5D-5L index value Median (IQR) | 0.96 (0.89–1) | 0.96 (0.89–0.98) | 0.93 (0.79–0.98) | 1 (0.95–1) | NS |
| EQ-5D-5L visual analogue scale Median (IQR) | 80 (59–90) | 70 (63–88) | 65 (50–85) d | 85 (80–90) | <0.01 |
| IPOS-renal total score Median (IQR) | 13 (7–21) | 16(11–23) e | 13 (9–21) | 7 (3–16) | <0.05 |
| Physical ability | |||||
| HGS Kg) Median (IQR) | 28 (20–34) | 24 (18–31) f | 26 (18–34) g | 33 (28–45) | <0.001 |
| TUG (s) Median (IQR) | 10.4 (8.0–12.4) | 11.3 (9.1–14.1) | 11.6 (8.3–17.9) h | 9.4 (7.5–10.6) | <0.01 |
| PBTs | |||||
| PCS (µM) Median (IQR) | 20.7 (3.58–40.8) | 53.4 (12.8–98.6) | 25.1 (15.5–33.4) | 4.4 (1.2–15.1) | <0.0001 |
| IS (µM) Median (IQR) | 5.2 (0.42–11.1) | 9.0 (5.1–15.4) i | 8.4 (5.0–16.4) i | 0 (0–0.59) | <0.01 |
| Fibre intake | |||||
| g/day Median (IQR) | 13.8 (10.3–20.8) | 11.8 (8.3–15.7) | 13 (9.9–19.7) | 19.3 (13.5–26.3) j | <0.05 |
| PCS | ||||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis (Model 1) | Multivariable Analysis (Model 2) | Multivariable Analysis (Model 3) | |
| PROM tool | ||||
| EQ-5D-5L Index value (r, 95% CI) | r = −0.18 (−0.38–0.03) p = NS | N/A | N/A | N/A |
| EQ-5D-5L VAS (r, 95% CI) | r = −0.16 (−0.36–0.06) p = NS | N/A | N/A | N/A |
| IPOS-renal total score (r, 95% CI) | r = 0.40 (0.20–0.56) p < 0.0001 | p < 0.05 | p < 0.05 | No further significance |
| Physical ability | ||||
| HGS (Kg) (r, 95% CI) | r = −0.21 (−0.40–0.005) p < 0.05 | No further significance | No further significance | No further significance |
| TUG (seconds) (r, 95% CI) | r = 0.34 (0.13–0.51) p < 0.05 | No further significance | No further significance | No further significance |
| IS | ||||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis (Model 1) | Multivariable Analysis (Model 2) | Multivariable Analysis (Model 3) | |
| PROM tool | ||||
| EQ-5D-5L Index value (r, 95% CI) | r = −0.17 (−0.37–0.05) p = NS | N/A | N/A | N/A |
| EQ-5D-5L VAS (r, 95% CI) | r = −0.2 (−0.40–0.17) p = NS | N/A | N/A | N/A |
| IPOS-renal total score (r, 95% CI) | r = 0.24 (0.03–0.43) p < 0.05 | No further significance | No further significance | No further significance |
| Physical ability | ||||
| HGS (Kg) (r, 95% CI) | r = −0.34 (−0.52–0.14) p < 0.05 | p < 0.05 | p < 0.05 | No further significance |
| TUG (seconds) (r, 95% CI) | r = 0.30 (0.1–0.49) p < 0.01 | No further significance | No further significance | No further significance |
| Fibre Intake | ||
|---|---|---|
| Univariable Analysis | Multivariable Analysis * | |
| PROM Tool | ||
| EQ-5D-5L Index value (r, 95% CI) | r = 0.19 (−0.3–0.40) p = NS | N/A |
| EQ-5D-5L VAS (r, 95% CI) | r = 0.21 (−0.01–0.42) p = NS | N/A |
| IPOS total score (r, 95% CI) | r = −0.39 (−0.56–0.19) p < 0.001 | p < 0.05 |
| Physical ability | ||
| HGS (r, 95% CI) | r = 0.11 (−0.11–32) p = NS | N/A |
| TUG (r, 95% CI) | r = −0.28 (−0.47–0.06) p < 0.05 | No further significance |
| Serum Albumin | ||||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis (Model 1) | Multivariable Analysis (Model 2) | Multivariable Analysis (Model 3) | |
| PROM tool | ||||
| EQ-5D-5L Index value (r, 95% CI) | r = 0.23 (0.01–0.43) p < 0.05 | No further significance | No further significance | N/A |
| EQ-5D-5L VAS (r, 95% CI) | r = 0.17 (−0.05–0.38) p = NS | No further significance | No further significance | N/A |
| IPOS-renal total score (r, 95% CI) | r = −0.32 (−0.51–0.11) p < 0.01 | p < 0.05 | No further significance | p < 0.01 |
| Physical ability | ||||
| HGS (Kg) (r, 95% CI) | r = 0.32 (0.11–0.51) p < 0.01 | p < 0.01 | p < 0.05 | p < 0.01 |
| TUG (seconds) (r, 95% CI) | r = −0.39 (−0.56–0.19) p < 0.001 | No further significance | No further significance | No further significance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaweera, A.; Huang, L.L.; McMahon, L.P. Well-Being, Protein-Bound Toxins, and Dietary Fibre in Patients with Kidney Disease: Have We Been Missing the Obvious? Toxins 2025, 17, 548. https://doi.org/10.3390/toxins17110548
Malaweera A, Huang LL, McMahon LP. Well-Being, Protein-Bound Toxins, and Dietary Fibre in Patients with Kidney Disease: Have We Been Missing the Obvious? Toxins. 2025; 17(11):548. https://doi.org/10.3390/toxins17110548
Chicago/Turabian StyleMalaweera, Aruni, Louis L. Huang, and Lawrence P. McMahon. 2025. "Well-Being, Protein-Bound Toxins, and Dietary Fibre in Patients with Kidney Disease: Have We Been Missing the Obvious?" Toxins 17, no. 11: 548. https://doi.org/10.3390/toxins17110548
APA StyleMalaweera, A., Huang, L. L., & McMahon, L. P. (2025). Well-Being, Protein-Bound Toxins, and Dietary Fibre in Patients with Kidney Disease: Have We Been Missing the Obvious? Toxins, 17(11), 548. https://doi.org/10.3390/toxins17110548





