Annual and Seasonal Variations in Aflatoxin M1 in Milk: Updated Health Risk Assessment in Serbia
Abstract
1. Introduction
2. Results and Discussion
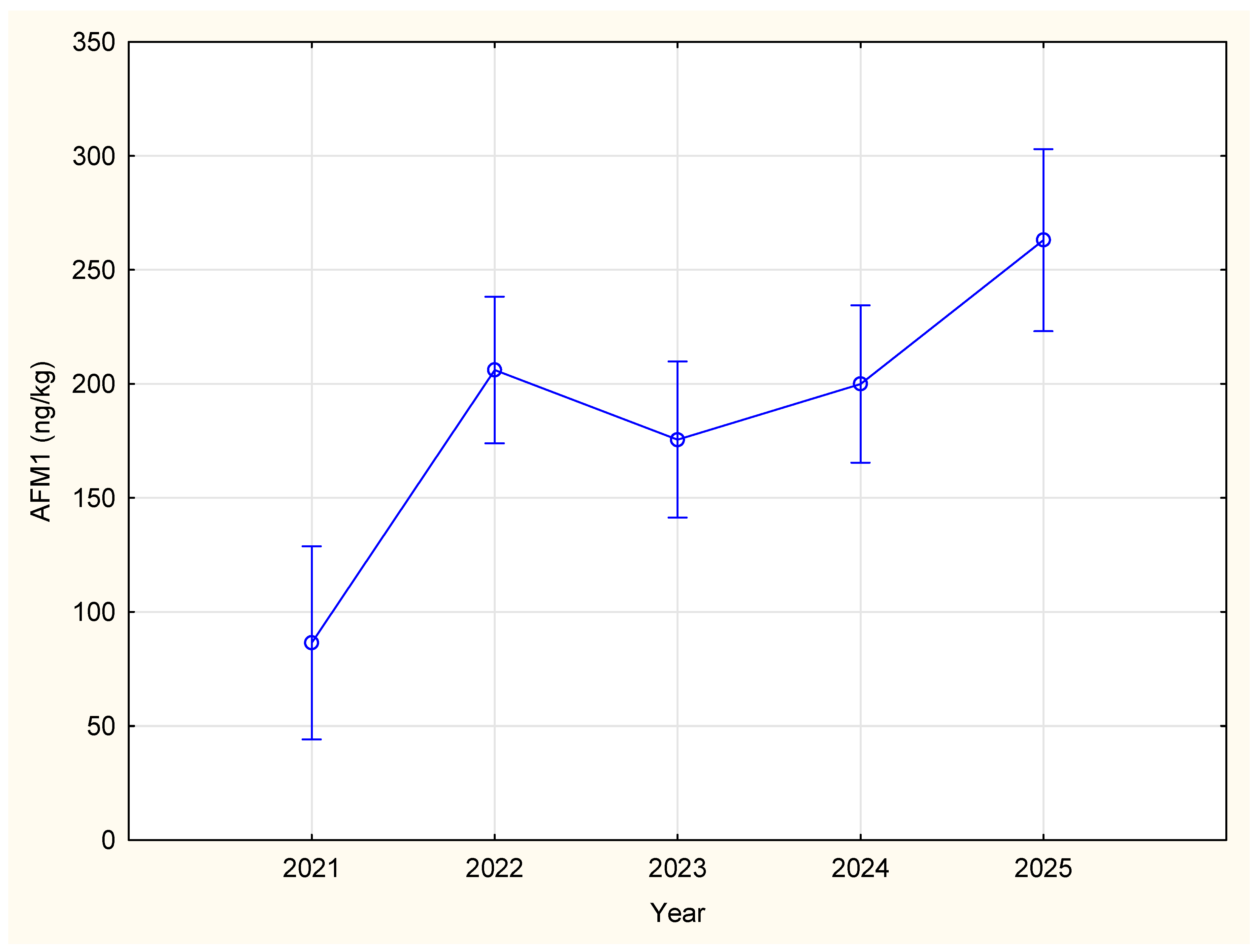
2.1. Annual Variations
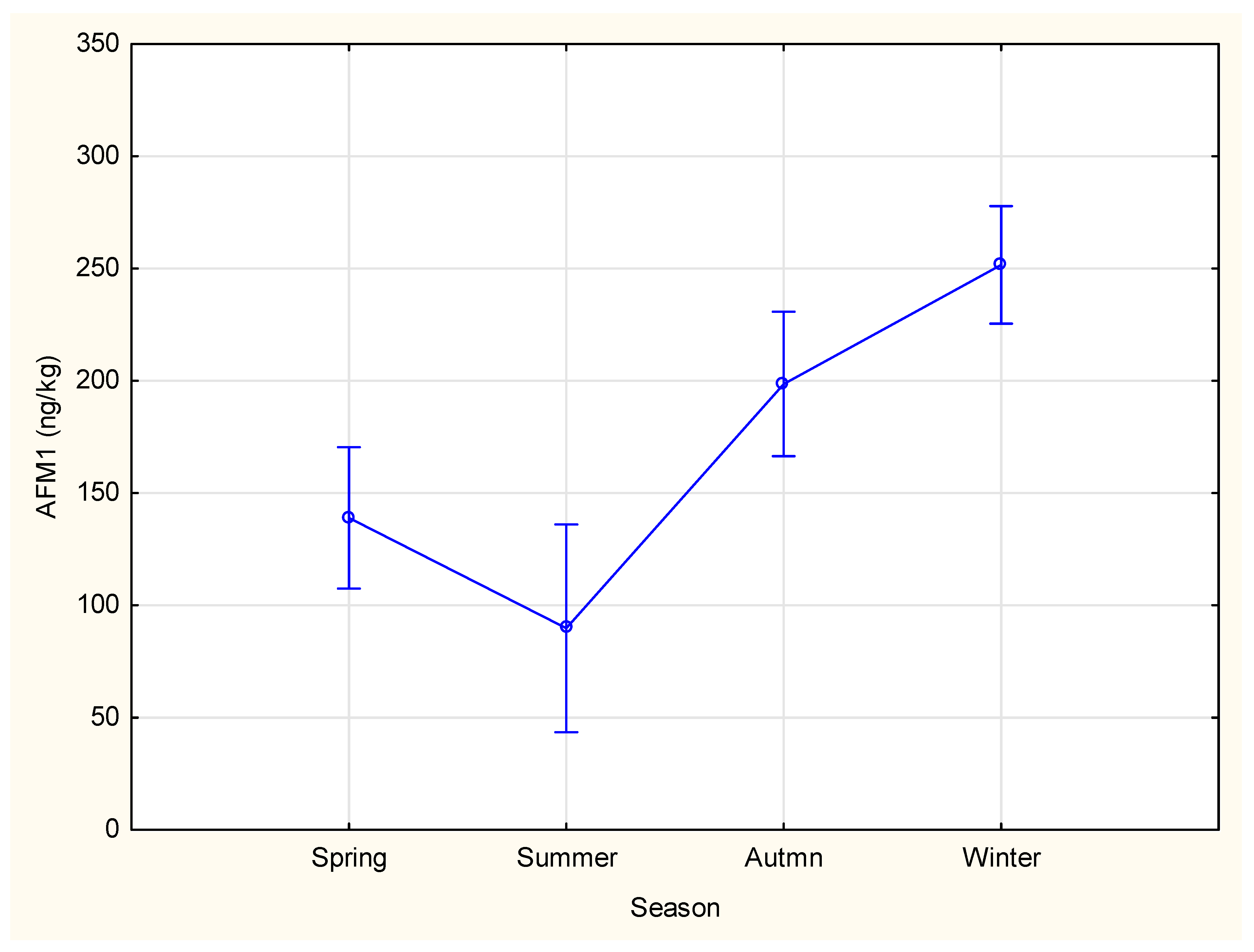
2.2. Seasonal Variations
2.3. Health Risk Assessment
2.4. Limitations
3. Conclusions
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Analysis
4.3. Health Risk Assessment
4.4. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Aflatoxin |
| AFB1 | Aflatoxin B1 |
| AFM1 | Aflatoxin M1 |
| b.w. | Body weight (kg) |
| BMDL10 | Benchmark dose lower confidence interval (400 ng/kg b.w. per day for AFB1; potency factor for AFM1 is 0.1 relative to AFB1) |
| Ci | AFM1 concentration level in milk (mean or selected percentile, ng/kg) |
| DL | Detection Limit |
| EDI | Estimated Daily Intake |
| EFSA | European Food Safety Authority |
| EU | European Union |
| FAO | Food and Agriculture Organization of the United Nations |
| IARC | International Agency for Research on Cancer |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| LB | Lower Bound |
| LOD | Limit of Detection |
| LSM | Least Squares Mean |
| MAX | Maximum |
| MIN | Minimum |
| ML | Maximum Level |
| MoE | Margin of Exposure |
| RHSS | Republic Hydrometeorological Service of Serbia |
| SE | Standard Error |
| SElm | Standard Error of the Least Squares Mean |
| TDI | Tolerable Daily Intake |
| UB | Upper Bound |
| UHT | Ultra-High Temperature |
| Vw | Consumed amount of milk (kg/day) |
| WHO | World Health Organization |
References
- Kolarić, L.; Minarovičová, L.; Lauková, M.; Kohajdová, Z.; Šimko, P. Elimination of aflatoxin M1 from milk: Current status and potential outline of applicable mitigation procedures. Trends Food Sci. Technol. 2024, 150, 104603. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). IARC Monograph on the Evaluation of Carcinogenic Risk to Humans; IARC: Lyon, France, 2002; Volume 82, p. 171. [Google Scholar]
- Britzi, M.; Friedman, S.; Miron, J.; Solomon, R.; Cuneah, O.; Shimshoni, J.A.; Soback, S.; Ashkenazi, R.; Armer, S.; Shlosberg, A. Carry-over of aflatoxin B1 to aflatoxin M1 in high-yielding Israeli cows in mid- and late-lactation. Toxins 2013, 5, 173–183. [Google Scholar] [CrossRef]
- Glamočić, D.; Horvatović, M.P.; Jajić, I.; Krstović, S.; Ivković, M.; Čuzdi, N. Corn silage as a source of aflatoxin B1 in feed for dairy cattle. Biotechnol. Lett. 2021, 26, 2759–2764. [Google Scholar] [CrossRef]
- Creppy, E.E. Update of survey, regulation, and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Ayar, A.; Sert, D.; Çon, A.H. A study on the occurrence of aflatoxin in raw milk due to feeds. J. Food Saf. 2007, 27, 199–207. [Google Scholar] [CrossRef]
- Van Egmond, H.P. Introduction. In Mycotoxins in Dairy Products; Elsevier Applied Science: London, UK; New York, NY, USA, 1989; pp. 1–9. [Google Scholar][Green Version]
- Gimeno, A. Aflatoxin M1 in milk: Risks for public health, prevention and control. Aliment. Anim. 2004, 49, 32–44. [Google Scholar][Green Version]
- European Commission. Commission Directive 100/2003 of 31 October 2003 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed. Off. J. Eur. Union 2003, L285, 33–37. [Google Scholar][Green Version]
- Serbian Regulation. Regulations on the quality of animal feed. Off. Gaz. Rep. Serbia 2014, 27. [Google Scholar][Green Version]
- European Commission. Commission Regulation 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar][Green Version]
- FDA (United States Food and Drug Administration). Compliance Policy Guide Sec. 527.400: Aflatoxin M1 in Milk. FDA Regulatory Guidance for Mycotoxins. Available online: https://www.aflatoxinpartnership.org/wp-content/uploads/2021/05/NGFAComplianceGuide-FDARegulatoryGuidanceforMycotoxins8-2011.pdf (accessed on 12 November 2024).[Green Version]
- Serbian Regulation. Regulations on the quantities of pesticides, metals and metalloids, and other toxic substances, chemotherapeutics, anabolics, and other substances that may be present in foodstuffs. Off. Gaz. Rep. Serbia 2011, 28. [Google Scholar][Green Version]
- EFSA (European Food Safety Authority). Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to aflatoxin B1 as an undesirable substance in animal feed. EFSA J. 2004, 2, 39. [Google Scholar] [CrossRef]
- Pecorelli, I.; Branciari, R.; Roila, R.; Ranucci, D.; Bibi, R.; van Asselt, M.; Valiani, A. Evaluation of Aflatoxin M1 enrichment factor in different cow milk cheese hardness category. Ital. J. Food Saf. 2020, 9, 8419. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Verdini, E.; Ranucci, D.; Valiani, A.; Pelliccia, A.; Fioroni, L.; Pecorelli, I. A study of the occurrence of Aflatoxin M1 in Milk supply chain over a seven-year period (2014–2020): Human exposure assessment and risk characterization in the population of Central Italy. Foods 2021, 10, 1529. [Google Scholar] [CrossRef]
- Jajić, I.; Glamočić, D.; Krstović, S.; Polovinski Horvatović, M. Aflatoxin M1 occurrence in Serbian milk and its impact on legislative. J. Hell. Vet. Med. Soc. 2019, 69, 1283–1290. [Google Scholar] [CrossRef]
- Udovički, B.; Audenaert, K.; De Saeger, S.; Rajković, A. Overview on the mycotoxins incidence in Serbia in the period 2004–2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef]
- Rama, A.; Latifi, F.; Bajraktari, D.; Ramadani, N. Assessment of aflatoxin M1 levels in pasteurized and UHT milk consumed in Prishtina, Kosovo. Food Control 2015, 57, 351–354. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Ganev, K.C.; Khaneghah, A.M.; Portela, J.B.; Cruz, A.G.; Granato, D.; Corassin, C.H.; Oliveira, C.A.F.; Sant’Ana, A.S. The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: A review. Food Control 2016, 68, 310–329. [Google Scholar] [CrossRef]
- Jakšić, S.; Živkov Baloš, M.; Prodanov Radulović, J.; Jajić, I.; Krstović, S.; Stojanov, I.; Mašić, Z. Aflatoxin M1 in milk and assessing the possibility of its occurrence in milk products. Arch. Vet. Med. 2017, 10, 37–49. [Google Scholar] [CrossRef]
- Zakaria, L. An overview of Aspergillus species associated with plant diseases. Pathogens 2024, 13, 813. [Google Scholar] [CrossRef]
- RHSS (Republic Hydrometeorological Service of Serbia). Annual Agrometeorological Analysis Archives; RHSS (Republic Hydrometeorological Service of Serbia): Belgrade, Serbia, 2020.
- RHSS (Republic Hydrometeorological Service of Serbia). Annual Agrometeorological Analysis Archives; RHSS (Republic Hydrometeorological Service of Serbia): Belgrade, Serbia, 2021.
- RHSS (Republic Hydrometeorological Service of Serbia). Annual Agrometeorological Analysis Archives; RHSS (Republic Hydrometeorological Service of Serbia): Belgrade, Serbia, 2022.
- RHSS (Republic Hydrometeorological Service of Serbia). Annual Agrometeorological Analysis Archives; RHSS (Republic Hydrometeorological Service of Serbia): Belgrade, Serbia, 2023.
- RHSS (Republic Hydrometeorological Service of Serbia). Annual Agrometeorological Analysis Archives; RHSS (Republic Hydrometeorological Service of Serbia): Belgrade, Serbia, 2024.
- RHSS (Republic Hydrometeorological Service of Serbia). Monthly Agrometeorological Analysis Archives; RHSS (Republic Hydrometeorological Service of Serbia): Belgrade, Serbia, 2025.
- Matić, J.; Mandić, A.; Mastilović, J.; Mišan, A.; Beljkas, B.; Milovanović, I. Contaminations of raw materials and food products with mycotoxins. Food Process. Qual. Saf. 2008, 35, 65–70. [Google Scholar]
- Janković, V.V.; Vukojević, J.B.; Lakićević, B.M.; Mitrović, R.R.; Vuković, D. Presence of moulds and aflatoxin M1 in milk. Zb. Matice Srp. Za Prir. Nauk. 2009, 117, 63–68. [Google Scholar] [CrossRef]
- Polovinski, M.; Glamočić, D. Two-year study of the incidence of aflatoxin M1 in milk in the region of Serbia. Biotechnol. Anim. Husb. 2009, 25, 713–718. [Google Scholar]
- Kos, J.; Mastilović, J.; Janic-Hajnal, E.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Control 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Živančev, J.; Antić, I.; Buljovčić, M.; Bulut, S.; Kocić-Tanackov, S. Review of the occurrence of mycotoxins in Serbian food items in the period from 2005 to 2022. Food Feed Res. 2022, 49, 155–172. [Google Scholar] [CrossRef]
- Udovički, B. Dietary Exposure Assessment of Aflatoxin B1 in the Republic of Serbia and Decontamination Efficiency by Ultraviolet Irradiation. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2020. [Google Scholar]
- Udovički, B.; Keskić, T.; Aleksić, B.; Smigić, N.; Rajković, A. Second-order probabilistic assessment of chronic dietary exposure to aflatoxin M1 in Serbia. Food Chem. Toxicol. 2023, 178, 113906. [Google Scholar] [CrossRef]
- Milićević, D.; Petronijević, R.; Petrović, Z.; Đinović-Stojanović, J.; Jovanović, J.; Baltić, T.; Janković, S. Impact of climate change on aflatoxin M1 contamination of raw milk with special focus on climate conditions in Serbia. J. Sci. Food Agric. 2019, 99, 5202–5210. [Google Scholar] [CrossRef]
- Pleadin, J.; Kos, J.; Radić, B.; Vulić, A.; Kudumija, N.; Radović, R.; Janić Hajnal, E.; Mandić, A.; Anić, M. Aflatoxins in maize from Serbia and Croatia: Implications of climate change. Foods 2023, 12, 548. [Google Scholar] [CrossRef]
- Bilandžić, N.; Božić, Đ.; Đokić, M.; Sedak, M.; Kolanović, B.S.; Varenina, I.; Tanković, S.; Cvetnić, Ž. Seasonal effect on aflatoxin M1 contamination in raw and UHT milk from Croatia. Food Control 2014, 40, 260–264. [Google Scholar] [CrossRef]
- Tomašević, I.; Petrović, J.; Jovetić, M.; Raičević, S.; Milojević, M.; Miočinović, J. Two-year survey on the occurrence and seasonal variation of aflatoxin M1 in milk and milk products in Serbia. Food Control 2015, 56, 64–70. [Google Scholar] [CrossRef]
- Miočinović, J.; Keškić, T.; Miloradović, Z.; Kos, A.; Tomašević, I.; Puđa, P. The aflatoxin M1 crisis in the Serbian dairy sector: The year after. Food Addit. Contam. Part B 2017, 10, 1–4. [Google Scholar] [CrossRef]
- Jauković, M.M.; Rokvić, N.I.; Vuksan, A.D. Recent aflatoxin levels in maize, feed mixtures, milk and cheese in Serbia. Zb. Matice Srp. Za Prir. Nauk. 2024, 146, 81–89. [Google Scholar] [CrossRef]
- Đekić, I.; Petrović, J.; Jovetić, M.; Redžepović-Đorđević, A.; Štulić, M.; Lorenzo, J.M.; Iammarino, M.; Tomašević, I. Aflatoxins in milk and dairy products: Occurrence and exposure assessment for the Serbian population. Appl. Sci. 2020, 10, 7420. [Google Scholar] [CrossRef]
- Đurđević, V.; Vuković, A.; Mandić, V.M. Climate Changes Observed in Serbia and Future Climate Projections Based on Different Scenarios of Future Emissions; United Nations Development Programme (UNDP): Belgrade, Serbia, 2018; ISBN 978-86-7728-301-8. [Google Scholar]
- Ferrari, L.; Rizzi, N.; Grandi, E.; Clerici, E.; Tirloni, E.; Stella, S.; Bernardi, C.E.M.; Pinotti, L. Compliance between food and feed safety: Eight-year survey (2013–2021) of aflatoxin M1 in raw milk and aflatoxin B1 in feed in northern Italy. Toxins 2023, 15, 168. [Google Scholar] [CrossRef]
- Statistical Office of the Republic of Serbia, Statistical Yearbook 2014; Statistical Office of the Republic of Serbia: Belgrade, Serbia. Available online: https://pod2.stat.gov.rs/objavljenepublikacije/god/sgs2014.pdf (accessed on 15 June 2025).
- Institute of Public Health of Serbia. Results of the Investigation of the Health Status of the Serbian Population—2013; Official Bulletin of the Republic of Serbia: Belgrade, Serbia, 2014. [Google Scholar]
- EFSA (European Food Safety Authority). Opinion of the Scientific Panel on Contaminants in the Food Chain on the risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T. Uncertainties in the risk assessment of three mycotoxins: Aflatoxin, ochratoxin, and zearalenone. Can. J. Physiol. Pharmacol. 1990, 68, 1017–1024. [Google Scholar] [CrossRef]
- Fallah, A.A. Seasonal variation of aflatoxin M1 contamination in industrial and traditional Iranian dairy products. Food Control 2011, 22, 1653–1656. [Google Scholar] [CrossRef]
- Xiong, J.L.; Wang, Y.M.; Ma, M.R.; Liu, J.X. Seasonal variation of aflatoxin M1 in raw milk from the Yangtze River Delta region of China. Food Control 2013, 34, 703–706. [Google Scholar] [CrossRef]
- Bilandžić, N.; Božić, Đ.; Đokić, M.; Sedak, M.; Kolanović, B.S.; Varenina, I.; Tanković, S.; Cvetnić, Z. Seasonal occurrence of aflatoxin M1 in raw milk during a six-year period in Croatia. Food Control 2022, 11, 1959. [Google Scholar] [CrossRef]
- Özbey, G.; Kabak, B. Seasonal variation of aflatoxin M1 level in cow milk from Turkey. J. Food Saf. Food Qual. 2023, 74, 144–148. [Google Scholar] [CrossRef]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety). Principles and Methods for the Risk Assessment of Chemicals in Food; International Programme on Chemical Safety, Environmental Health Criteria: Geneva, Switzerland, 2009; p. 240. [Google Scholar]
- EFSA (European Food Safety Authority). Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Human Health Risk Assessment Toolkit: Chemical Hazards; International Programme on Chemical Safety. WHO: Geneva, Switzerland. Available online: https://www.who.int/publications/i/item/9789240035720 (accessed on 15 June 2025).
| Year | LSM | SELSM | MIN | MAX | n |
|---|---|---|---|---|---|
| 2021 | 86.40 a | 21.55 | 44.11 | 128.69 | 132 |
| 2022 | 206.051 b | 16.39 | 173.87 | 238.23 | 228 |
| 2023 | 175.54 b | 17.46 | 141.27 | 209.81 | 201 |
| 2024 | 199.95 b | 17.59 | 165.42 | 234.48 | 198 |
| 2025 | 263.05 c | 20.35 | 223.12 | 302.99 | 148 |
| Season | LSM | SELSM | MIN | MAX | n |
|---|---|---|---|---|---|
| Spring | 138.88 a | 16.04 | 107.40 | 170.36 | 235 |
| Summer | 89.74 a | 23.55 | 43.52 | 135.97 | 109 |
| Autumn | 198.56 b | 16.43 | 166.32 | 230.81 | 224 |
| Winter | 251.60 c | 13.35 | 225.39 | 277.81 | 339 |
| AFM1 Level | Raw Milk (ng/kg) | EDI (ng/kg b.w.) | MoE |
|---|---|---|---|
| n | 907 | ||
| n (%) above ML | 636 (70.1) | ||
| LB mean | 189.8 | 0.336 | 11,904 |
| UB mean | 190.4 | 0.337 | 11,871 |
| P50 | 98 | 0.173 | 23,060 |
| P75 | 228 | 0.404 | 9912 |
| P90 | 491 | 0.869 | 4603 |
| P95 | 684 | 1.211 | 3304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstović, S.; Jakšić, S.; Miljanić, J.; Iličić, B.; Živkov Baloš, M.; Guljaš, D.; Damjanović, M.; Jajić, I. Annual and Seasonal Variations in Aflatoxin M1 in Milk: Updated Health Risk Assessment in Serbia. Toxins 2025, 17, 544. https://doi.org/10.3390/toxins17110544
Krstović S, Jakšić S, Miljanić J, Iličić B, Živkov Baloš M, Guljaš D, Damjanović M, Jajić I. Annual and Seasonal Variations in Aflatoxin M1 in Milk: Updated Health Risk Assessment in Serbia. Toxins. 2025; 17(11):544. https://doi.org/10.3390/toxins17110544
Chicago/Turabian StyleKrstović, Saša, Sandra Jakšić, Jelena Miljanić, Borislav Iličić, Milica Živkov Baloš, Darko Guljaš, Marko Damjanović, and Igor Jajić. 2025. "Annual and Seasonal Variations in Aflatoxin M1 in Milk: Updated Health Risk Assessment in Serbia" Toxins 17, no. 11: 544. https://doi.org/10.3390/toxins17110544
APA StyleKrstović, S., Jakšić, S., Miljanić, J., Iličić, B., Živkov Baloš, M., Guljaš, D., Damjanović, M., & Jajić, I. (2025). Annual and Seasonal Variations in Aflatoxin M1 in Milk: Updated Health Risk Assessment in Serbia. Toxins, 17(11), 544. https://doi.org/10.3390/toxins17110544






