Biological Control of Microcystis aeruginosa Through Sequestration in Pseudofaeces Produced by the Freshwater Gastropod, Sinotaia aeruginosa
Abstract
1. Introduction
2. Results
2.1. The Process of Suspension Filtration in Sinotaia Aeruginosa
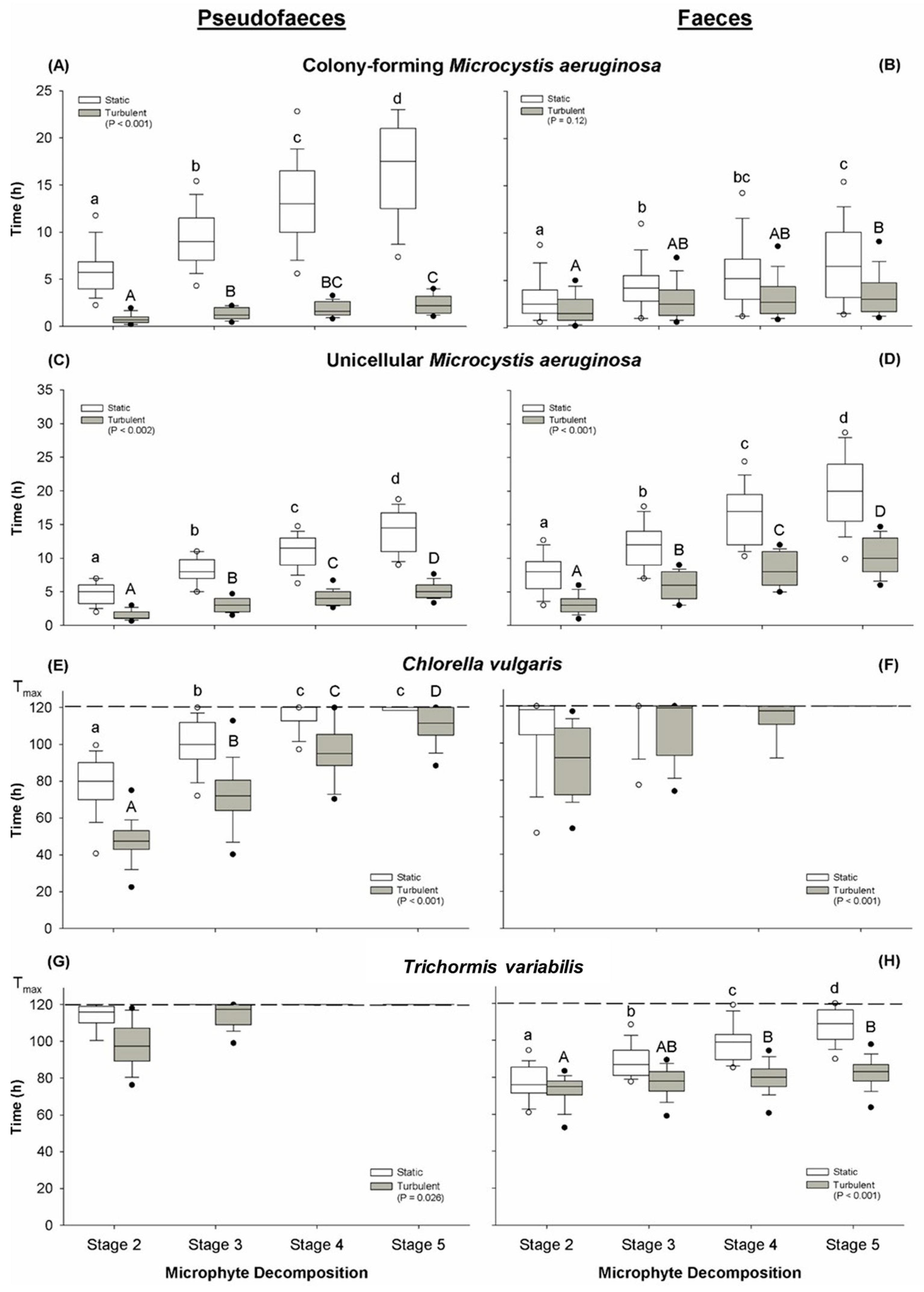
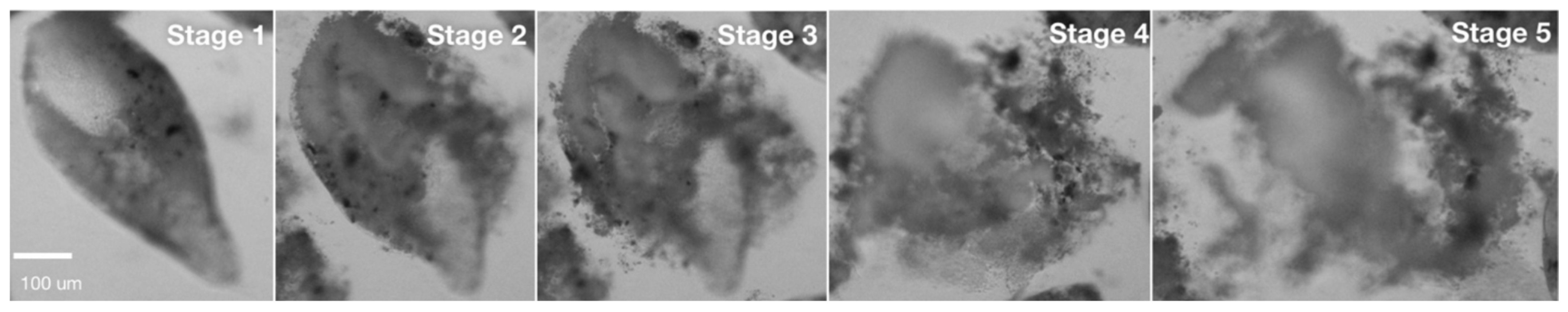
2.2. Structure and Decomposition of Pseudofaeces Produced
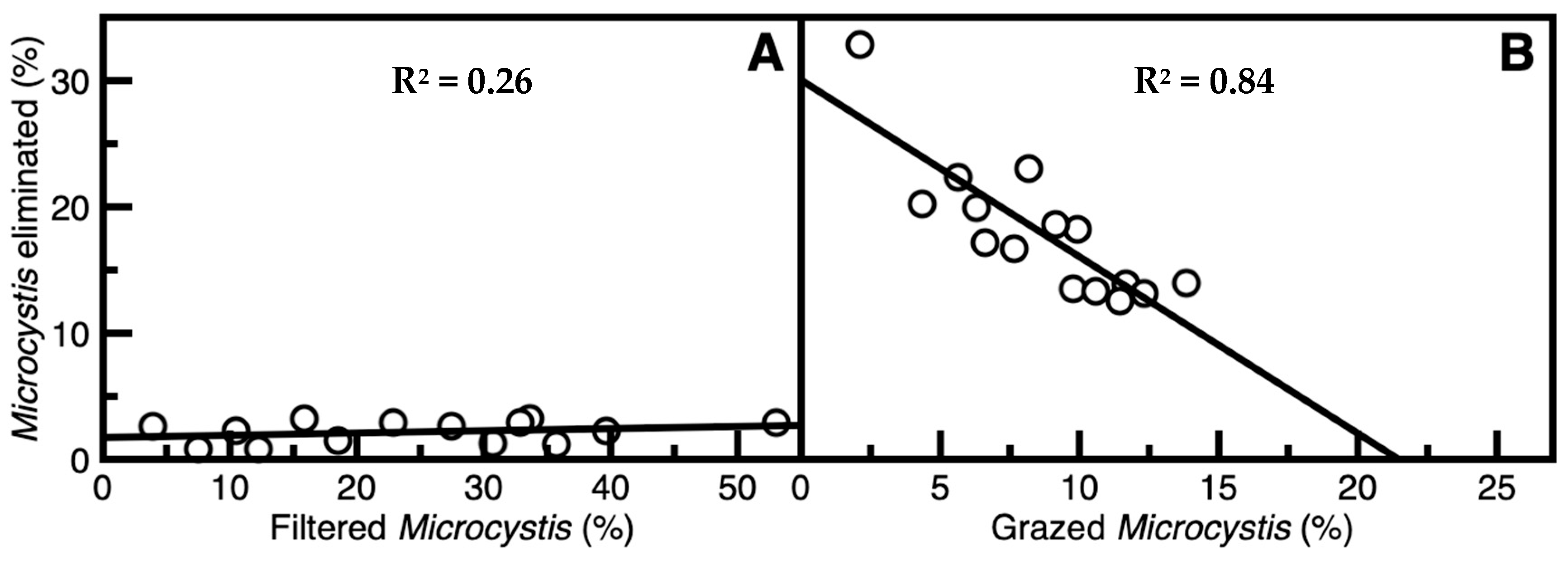
2.3. Quantifying Removal of M. aeruginosa via Suspension Filtration and Grazing
2.4. Structure and Decomposition of Faeces Produced
2.5. Structure and Decomposition of Secondary Faeces Produced
3. Discussion
3.1. Disassembly of Pseudofaeces and Faeces
3.2. Removal and Elimination of Microcystis Through Ingestion by S. aeruginosa
3.3. Filter-Feeding Snails in the Biological Control of M. aeruginosa
4. Conclusions
5. Materials and Methods
5.1. Collection and Maintenance of Sinotaia aeruginosa
5.2. Cyanobacterial and Algal Species Collection and Culture
5.3. Microscopic Characterisation of Suspension Filtering Process
5.4. Filtration Rate and Removal of M. aeruginosa Through Suspension Filtration and Grazing of Mobile Snails
5.5. Decomposition Time of Pseudofaeces and Faeces
5.6. Sample Processing and Chlorophyll-A Measurement
5.7. Optics and Photography
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Cai, H.; Yu, G.; Jiang, H. Insights into Extracellular Polymeric Substances of Cyanobacterium Microcystis aeruginosa Using Fractionation Procedure and Parallel Factor Analysis. Water Res. 2013, 47, 2005–2014. [Google Scholar] [CrossRef]
- Mishra, S.; Stumpf, R.P.; Schaeffer, B.A.; Werdell, P.J. Recent Changes in Cyanobacteria Algal Bloom Magnitude in Large Lakes across the Contiguous United States. Sci. Total Environ. 2023, 897, 165253. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the Global Expansion of Harmful Cyanobacterial Blooms: Moving Targets in a Human- and Climatically-Altered World. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Feng, L.; Dai, Y.; Hu, C.; Gibson, L.; Tang, J.; Lee, Z.; Wang, Y.; Cai, X.; Liu, J.; et al. Global Mapping Reveals Increase in Lacustrine Algal Blooms over the Past Decade. Nat. Geosci. 2022, 15, 130–134. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Wilhelm, S.W.; Bullerjahn, G.S.; Paerl, H.W. Climate Change and the Aquatic Continuum: A Cyanobacterial Comeback Story. Environ. Microbiol. Rep. 2023, 15, 3–12. [Google Scholar] [CrossRef]
- Erratt, K.J.; Creed, I.F.; Lobb, D.A.; Smol, J.P.; Trick, C.G. Climate Change Amplifies the Risk of Potentially Toxigenic Cyanobacteria. Glob. Change Biol. 2023, 29, 5240–5249. [Google Scholar] [CrossRef]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived Intensification in Harmful Algal Blooms Is a Wave of Cumulative Threat to the Aquatic Ecosystems. Biology 2022, 11, 852. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Briland, R.D.; Stone, J.P.; Manubolu, M.; Lee, J.; Ludsin, S.A. Cyanobacterial Blooms Modify Food Web Structure and Interactions in Western Lake Erie. Harmful Algae 2020, 92, 101586. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Safe Recreational Water Environments: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003; Volume 1. [Google Scholar]
- Dyble, J.; Fahnenstiel, G.L.; Litaker, R.W.; Millie, D.F.; Tester, P.A. Microcystin Concentrations and Genetic Diversity of Microcystis in the Lower Great Lakes. Environ. Toxicol. 2008, 23, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sabart, M.; Pobel, D.; Briand, E.; Combourieu, B.; Salençon, M.J.; Humbert, J.F.; Latour, D. Spatiotemporal Variations in Microcystin Concentrations and in the Proportions of Microcystin-Producing Cells in Several Microcystis aeruginosa Populations. Appl. Environ. Microbiol. 2010, 76, 4750–4759. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Recommendations for Cyanobacteria and Cyanotoxin Monitoring in Recreational Waters; United States Environmental Protection Agency: Washington, DC, USA, 2019.
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The Role of Benthic Invertebrate Species in Freshwater Ecosystems. BioScience 1999, 49, 119. [Google Scholar] [CrossRef]
- Arango, C.P.; Riley, L.A.; Tank, J.L.; Hall, R.O. Herbivory by an Invasive Snail Increases Nitrogen Fixation in a Nitrogen-Limited Stream. Can. J. Fish. Aquat. Sci. 2009, 66, 1309–1317. [Google Scholar] [CrossRef]
- Zheng, Z.; Lv, J.; Lu, K.; Jin, C.; Zhu, J.; Liu, X. The Impact of Snail (Bellamya aeruginosa) Bioturbation on Sediment Characteristics and Organic Carbon Fluxes in an Eutrophic Pond. Clean Soil Air Water 2011, 39, 566–571. [Google Scholar] [CrossRef]
- Declerck, C.H. The Evolution of Susupension Feeding in Gastropods. Biol. Rev. 1995, 70, 549–569. [Google Scholar] [CrossRef]
- Kamimura, S.; Tsuchiya, M. The Effect of Feeding Behavior of the Gastropods Batillaria zonalis and Cerithideopsilla cingulata on Their Ambient Environment. Mar. Biol. 2004, 144, 705–712. [Google Scholar] [CrossRef]
- Han, S.; Yan, S.; Chen, K.; Zhang, Z.; Zed, R.; Zhang, J.; Song, W.; Liu, H. 15N Isotope Fractionation in an Aquatic Food Chain: Bellamya aeruginosa (Reeve) as an Algal Control Agent. J. Environ. Sci. 2010, 22, 242–247. [Google Scholar] [CrossRef]
- Olden, J.D.; Ray, L.; Mims, M.C.; Horner-Devine, M.C. Filtration Rates of the Non-Native Chinese Mystery Snail (Bellamya chinensis) and Potential Impacts on Microbial. Limnetica 2013, 32, 107–120. [Google Scholar] [CrossRef]
- Fang, L.; Wong, P.K.; Lin, L.; Lan, C.; Qiu, J. Impact of Invasive Apple Snails in Hong Kong on Wetland Macrophytes, Nutrients, Phytoplankton and Filamentous Algae. Freshw. Biol. 2010, 55, 1191–1204. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Liu, X. Can the Freshwater Snail Bellamya aeruginosa (Mollusca) Affect Phytoplankton Community and Water Quality? Hydrobiologia 2013, 707, 147–157. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Song, Z.; Xie, Z.; Li, L.; Song, L. Bioaccumulation of Microcystins in Two Freshwater Gastropods from a Cyanobacteria-Bloom Plateau Lake, Lake Dianchi. Environ. Pollut. 2012, 164, 227–234. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Yang, W.; Zheng, Z.; Zhu, J. Gut Microbiota of Freshwater Gastropod (Bellamya aeruginosa) Assist the Adaptation of Host to Toxic Cyanobacterial Stress. Toxins 2023, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Yokoyama, A.; Ishikawa, K.; Kumagai, M.; Watanabe, M.F.; Park, H.-D. Accumulation and Depuration of Microcystin Produced by the Cyanobacterium Microcystis in a Freshwater Snail. Limnology 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The Cyanotoxin-Microcystins: Current Overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Bullerjahn, G.S.; McKay, R.M.L. The Complicated and Confusing Ecology of Microcystis Blooms. mBio 2020, 11, e00529-20. [Google Scholar] [CrossRef]
- Qu, M.; Lefebvre, D.D.; Wang, Y.; Qu, Y.; Zhu, D.; Ren, W. Algal Blooms: Proactive Strategy. Science 2014, 346, 175–176. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Igwaran, A.; Kayode, A.J.; Moloantoa, K.M.; Khetsha, Z.P.; Unuofin, J.O. Cyanobacteria Harmful Algae Blooms: Causes, Impacts, and Risk Management. Water Air Soil Pollut. 2024, 235, 71. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Han, S.; Su, H.; Xia, W.; Wang, H.; Liu, Y.; Zhang, L.; Ke, Z.; Zhang, X.; et al. Nontraditional Biomanipulation: A Powerful Ecotechnology to Combat Cyanobacterial Blooms in Eutrophic Freshwaters. Innov. Life 2023, 1, 100038. [Google Scholar] [CrossRef]
- Anabtawi, H.M.; Lee, W.H.; Al-Anazi, A.; Mohamed, M.M.; Aly Hassan, A. Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water 2024, 16, 224. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony Formation in the Cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques, 1st ed.; Elsevier Science & Technology: Chantilly, France, 2005; ISBN 978-0-08-045650-8. [Google Scholar]
- Iannino, A.; Vosshage, A.T.L.; Weitere, M.; Fink, P. High Nutrient Availability Leads to Weaker Top-down Control of Stream Periphyton: Compensatory Feeding in Ancylus fluviatilis. Freshw. Biol. 2019, 64, 37–45. [Google Scholar] [CrossRef]
- Groendahl, S.; Fink, P. High Dietary Quality of Non-Toxic Cyanobacteria for a Benthic Grazer and Its Implications for the Control of Cyanobacterial Biofilms. BMC Ecol. 2017, 17, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Zeng, Q.; Gu, X.; Mao, Z. Growth and Photosynthetic Activity of Microcystis Colonies after Gut Passage through Silver Carp and Bighead Carp. Acta Ecol. Sin. 2014, 34, 1707–1715. [Google Scholar] [CrossRef]
- Reynolds, C.S. Variability in the Provision and Function of Mucilage in Phytoplankton: Facultative Responses to the Environment. Hydrobiologia 2007, 578, 37–45. [Google Scholar] [CrossRef]
- Bitterlich, G. Digestive Enzyme Pattern of Two Stomachless Filter Feeders, Silver Carp, Hypophthalmichthys molitrix val., and Bighead Carp, Aristichthys nobilis rich. J. Fish. Biol. 1985, 27, 103–112. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, Y.; Zhang, Z.; Chen, Y.; Qin, W.; Guan, W.; Li, G.; Yu, H.; Dai, C.; Li, R.; et al. Dual Characteristics of Bellamya aeruginosa Encountering Microcystis aeruginosa: Algal Control and Toxin Depuration. Ecotoxicol. Environ. Saf. 2023, 252, 114596. [Google Scholar] [CrossRef]
- Ma, T.; Gong, S.; Zhou, K.; Zhu, C.; Deng, K.; Luo, Q.; Wang, Z. Laboratory Culture of the Freshwater Benthic Gastropod Bellamya aeruginosa (Reeve) and Its Utility as a Test Species for Sediment Toxicity. J. Environ. Sci. 2010, 22, 304–313. [Google Scholar] [CrossRef]
- Yongjiu, C.; Zhijun, G.; Boqiang, Q. Community Structure and Diversity of Macrozoobenthos in Lake Taihu, a Large Shallow Eutrophic Lake in China. Biodivers. Sci. 2010, 18, 50. [Google Scholar] [CrossRef]
- Gao, F.; Deng, J.; Xu, Z.; Ning, Y.; Yin, H.; Gao, J. Ecological Characteristics of Macrobenthic Communities in the Chaohu Lake Basin and Their Relationship with Environmental Factors. J. Anim. Vet. Adv. 2011, 10, 627–634. [Google Scholar] [CrossRef]
- Cook, P.M. A Ciliary Feeding Mechanism in Viviparus viviparus (L.). J. Molluscan Stud. 1949, 27, 265–271. [Google Scholar] [CrossRef]
- Höckelmann, C.; Pusch, M. The Respiration and Filter-Feeding Rates of the Snail Viviparus viviparus (Gastropoda) under Simulated Stream Conditions. Arch. Für Hydrobiol. 2000, 149, 553–568. [Google Scholar] [CrossRef]
- Tashiro, J.S.; Colman, S.D. Filter-Feeding in the Freshwater Prosobranch Snail Bithynia tentaculata: Bioenergetic Partitioning of Ingested Carbon and Nitrogen. Am. Midl. Nat. 1982, 107, 114. [Google Scholar] [CrossRef]
- Qu, M.; Anderson, S.; Lyu, P.; Malang, Y.; Lai, J.; Liu, J.; Jiang, B.; Xie, F.; Liu, H.H.T.; Lefebvre, D.D.; et al. Effective Aerial Monitoring of Cyanobacterial Harmful Algal Blooms Is Dependent on Understanding Cellular Migration. Harmful Algae 2019, 87, 101620. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Matthijs, H.C.P.; Visser, P.M. Harmful Cyanobacteria; Aquatic Ecology Series; Springer: Dordrecht Norwell, MA, USA, 2005; ISBN 978-1-4020-3022-2. [Google Scholar]
- Velasco, L.; Navarro, J. Feeding Physiology of Infaunal (Mulinia edulis) and Epifaunal (Mytilus chilensis) Bivalves under a Wide Range of Concentrations and Qualities of Seston. Mar. Ecol. Prog. Ser. 2002, 240, 143–155. [Google Scholar] [CrossRef]
- Velasco, L.; Navarro, J. Feeding Physiology of Two Bivalves under Laboratory and Field Conditions in Response to Variable Food Concentrations. Mar. Ecol. Prog. Ser. 2005, 291, 115–124. [Google Scholar] [CrossRef]
- Coughlan, J. The Estimation of Filtering Rate from the Clearance of Suspensions. Mar. Biol. 1969, 2, 356–358. [Google Scholar] [CrossRef]
- International Organization for Standardization Water Quality. Measurement of Biochemical Parameters—Spectrometric Determination of the Chlorophyll-A Concentration. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10260:ed-1:v1:en:biblref:1 (accessed on 21 January 2019).

| Cyanobacteria | Chlorophyta | ||||
|---|---|---|---|---|---|
| Process | Time to Release | Microcystis aeruginosa (Unicellular) | Microcystis aeruginosa (Colony-Forming) | Trichormus variabilis (Colony-Forming) | Chlorella vulgaris (Unicellular) |
| Suspension filtering | pseudofaeces (s) | 131 ± 9.2 | 112 ± 13.6 | 141 ± 10 | 125 ± 5.8 |
| Grazing | faeces (h) | 8.3 ± 0.7 | 9.1 ± 2.3 | 8.9 ± 0.8 | 9.4 ± 0.6 |
| Stage | Shape | Surface | Content Release | Notes |
|---|---|---|---|---|
| 1 | Tight rope-shaped | Smooth and constricting content | None | Original status |
| 2 | Rope- shaped, but inflated | Smooth with loose content | Few cells | |
| 3 | Inflated, slight loss of original shape | Loose with coarse edges | Numerous cells | |
| 4 | Deformed, major loss of integrity | Unrestricting with coarse edges | Majority of cells | |
| 5 | Deformed, complete loss of integrity | No clear edges | All cells | Completely decomposed |
| Taxon | Cyanobacteria | Chlorophyta | ||
|---|---|---|---|---|
| Species: | Microcystis aeruginosa | Microcystis aeruginosa | Trichormus variabilis | Chlorella vulgaris |
| Strain ID: | † FACHB-905 | Collected from Dianshan Lake, China | Collected from Dianshan Lake, China | † FACHB-8 |
| Cellular structure: | Unicellular | Irregularly shaped colony-forming | Filamentous colony- forming | Unicellular |
| Cell size *: (µm) | 3–5 | 4–7 | 5–8 | 3–5 |
| Colony size *: (µm) | N/A | 100–500 | 60–250 | N/A |
| External mucilage: | No | Yes | No | No |
| Distribution in water column: | Suspended uniformly throughout | Buoyant, floats to surface | Floats to surface, may attach to substrate | Sinks to benthic zone, suspended by turbulence |
| Stage | Shape | Surface | Content Release | Notes |
|---|---|---|---|---|
| 1 | Fusiform shape, connected through mucilage | Smooth and constricting content | None | Original status |
| 2 | No change | Smooth; small rupture points | Few cells | |
| 3 | Slight loss of original shape | Edge discernible; Multiple rupture points | Numerous cells | |
| 4 | Deformed, major loss of integrity | Most barrier missing | Majority of cells | |
| 5 | Deformed, complete loss of integrity | No clear edges | All cells | Completely decomposed |
| Characteristics | Pseudofaeces | Faeces | Secondary Ingestion Faeces |
|---|---|---|---|
| Shape | Rope-shaped | Fusiform | Fusiform |
| Configuration | Trapped suspended particles with mucilage | Thick mucilage outside | Thick mucilage outside & mucilage inside |
| Hardness | Soft | Firm | Firm |
| Colour | Colour of contents | Colour of contents | Colour of contents |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madison, B.N.; Qu, M.; Gavrin, E.; Ren, W.; Wang, Y.; Lefebvre, D.D. Biological Control of Microcystis aeruginosa Through Sequestration in Pseudofaeces Produced by the Freshwater Gastropod, Sinotaia aeruginosa. Toxins 2025, 17, 536. https://doi.org/10.3390/toxins17110536
Madison BN, Qu M, Gavrin E, Ren W, Wang Y, Lefebvre DD. Biological Control of Microcystis aeruginosa Through Sequestration in Pseudofaeces Produced by the Freshwater Gastropod, Sinotaia aeruginosa. Toxins. 2025; 17(11):536. https://doi.org/10.3390/toxins17110536
Chicago/Turabian StyleMadison, Barry N., Mingzhi Qu, Elliot Gavrin, Wenwei Ren, Yuxiang Wang, and Daniel D. Lefebvre. 2025. "Biological Control of Microcystis aeruginosa Through Sequestration in Pseudofaeces Produced by the Freshwater Gastropod, Sinotaia aeruginosa" Toxins 17, no. 11: 536. https://doi.org/10.3390/toxins17110536
APA StyleMadison, B. N., Qu, M., Gavrin, E., Ren, W., Wang, Y., & Lefebvre, D. D. (2025). Biological Control of Microcystis aeruginosa Through Sequestration in Pseudofaeces Produced by the Freshwater Gastropod, Sinotaia aeruginosa. Toxins, 17(11), 536. https://doi.org/10.3390/toxins17110536





