Building Sub-Saharan African PBPK Populations Reveals Critical Data Gaps: A Case Study on Aflatoxin B1
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
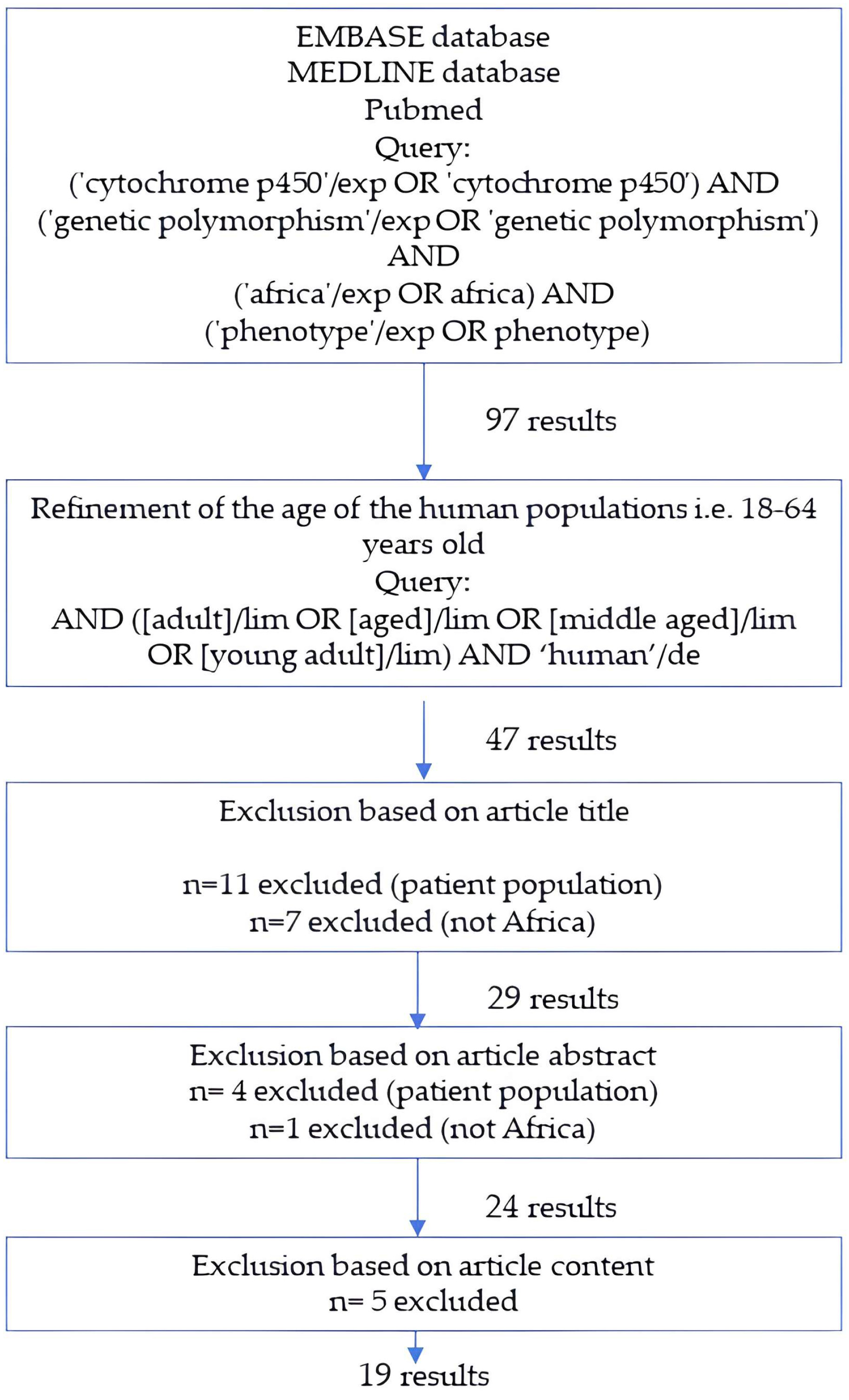
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bains, R.K. African variation at Cytochrome P450 genes Evolutionary aspects and the implications for the treatment of infectious diseases. Evolution 2013, 2012, 118–134. [Google Scholar] [CrossRef]
- Rajman, I.; Knapp, L.; Morgan, T.; Masimirembwa, C. African Genetic Diversity: Implications for Cytochrome P450-mediated Drug Metabolism and Drug Development. eBioMedicine 2017, 17, 67–74. [Google Scholar] [CrossRef]
- Bilinsky, L.M.; Thomas, D.J.; Fisher, J.W. Using mathematical modeling to infer the valence state of arsenicals in tissues: A PBPK model for dimethylarsinic acid (DMAV) and dimethylarsinous acid (DMAIII) in mice. J. Theor. Biol. 2019, 461, 215–229. [Google Scholar] [CrossRef]
- Kim, C.S.; Ross, I.A.; Sandberg, J.A.; Preston, E. Quantitative low-dose assessment of seafood toxin, domoic acid, in the rat brain: Application of physiologically-based pharmacokinetic (PBPK) modeling. Environ. Toxicol. Pharmacol. 1998, 6, 49–58. [Google Scholar] [CrossRef]
- Buur, J.L.; Baynes, R.E.; Riviere, J.E. Estimating meat withdrawal times in pigs exposed to melamine contaminated feed using a physiologically based pharmacokinetic model. Regul. Toxicol. Pharmacol. 2008, 51, 324–331. [Google Scholar] [CrossRef]
- Moreau, M.; Nong, A. Evaluating hexabromocyclododecane (HBCD) toxicokinetics in humans and rodents by physiologically based pharmacokinetic modeling. Food Chem. Toxicol. 2019, 133, 110785. [Google Scholar] [CrossRef]
- Komprda, J.; Komprdová, K.; Domínguez-Romero, E.; Mikeš, O.; Řiháčková, K.; Čupr, P.; Černá, M.; Scheringer, M. Dynamics of PCB exposure in the past 50 years and recent high concentrations in human breast milk: Analysis of influencing factors using a physiologically based pharmacokinetic model. Sci. Total Environ. 2019, 690, 388–399. [Google Scholar] [CrossRef]
- Lootens, O.; De Boevre, M.; Ning, J.; Gasthuys, E.; Van Bocxlaer, J.; De Saeger, S.; Vermeulen, A. Building a Human Physiologically Based Pharmacokinetic Model for Aflatoxin B1 to Simulate Interactions with Drugs. Pharmaceutics 2023, 15, 894. [Google Scholar] [CrossRef] [PubMed]
- Bois, F.Y.; Jamei, M.; Clewell, H.J.; PBPK, H.J.C. PBPK modelling of inter-individual variability in the pharmacokinetics of environmental chemicals modelling of inter-individual variability in the pharmacokinetics of environmental chemicals. Toxicology 2010, 278, 256–267. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Wan, L.; Fu, J.; Huo, Y.; Zhao, Y.; Guo, C. Gene Polymorphisms Affecting the Pharmacokinetics and Pharmacodynamics of Donepezil Efficacy. Front. Pharmacol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhang, X.-H.; Liu, J.; Sun, L.-N.; Shen, Y.-W.; Zhou, C.; Zhang, H.-W.; Xie, L.-J.; Chen, J.; Liu, Y.; et al. Effects of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of proton pump inhibitors. Pharmacol. Res. 2019, 152, 104606. [Google Scholar] [CrossRef] [PubMed]
- Galal, S. Demographics of Africa-Statistics & Facts Statista. Available online: https://www.statista.com/topics/7928/demographics-of-africa/#topicOverview (accessed on 15 May 2024).
- Pfennig, A.; Petersen, L.N.; Kachambwa, P.; Lachance, J. Evolutionary Genetics and Admixture in African Populations. Genome Biol. Evol. 2023, 15, evad054. [Google Scholar] [CrossRef]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; McCarthy, S.A.; Hui, R.; Almarri, M.A.; Ayub, Q.; Danecek, P.; Chen, Y.; Felkel, S.; Hallast, P.; Kamm, J.; et al. Insights into human genetic variation and population history from 929 diverse genomes. Science 2020, 367, eaay5012. [Google Scholar] [CrossRef]
- Mallick, S.; Li, H.; Lipson, M.; Mathieson, I.; Gymrek, M.; Racimo, F.; Zhao, M.; Chennagiri, N.; Nordenfelt, S.; Tandon, A.; et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature 2016, 538, 201–206. [Google Scholar] [CrossRef]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef]
- Tishkoff, S.A.; Reed, F.A.; Friedlaender, F.R.; Ehret, C.; Ranciaro, A.; Froment, A.; Hirbo, J.B.; Awomoyi, A.A.; Bodo, J.-M.; Doumbo, O.; et al. The Genetic Structure and History of Africans and African Americans. Science 2009, 324, 1035. [Google Scholar] [CrossRef]
- Sahel and West Africa Club Secretariat. Maps Sahel and West Africa Club Secretariat. Available online: https://www.oecd.org/swac/maps/ (accessed on 19 February 2024).
- African Union. Member States. Available online: https://au.int/en/member_states/countryprofiles2 (accessed on 5 August 2024).
- EMA Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-reflection-paper-use-extrapolation-development-medicines-paediatrics-revision-1_en.pdf (accessed on 3 June 2025).
- Food and Drug Administration. Physiologically Based Pharmacokinetic Analyses—Format and Content Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2018. [Google Scholar]
- Shebley, M.; Sandhu, P.; Riedmaier, A.E.; Jamei, M.; Narayanan, R.; Patel, A.; Peters, S.A.; Reddy, V.P.; Zheng, M.; de Zwart, L.; et al. Physiologically Based Pharmacokinetic Model Qualification and Reporting Procedures for Regulatory Submissions: A Consortium Perspective. Clin. Pharmacol. Ther. 2018, 104, 88–110. [Google Scholar] [CrossRef]
- World Bank Group. Population, Total. Available online: https://data360.worldbank.org/en/indicator/WB_HNP_SP_POP_TOTL?country=TGO (accessed on 16 September 2025).
- Ngaimisi, E.; Habtewold, A.; Minzi, O.; Makonnen, E.; Mugusi, S.; Amogne, W.; Yimer, G.; Riedel, K.-D.; Janabi, M.; Aderaye, G.; et al. Importance of Ethnicity, CYP2B6 and ABCB1 Genotype for Efavirenz Pharmacokinetics and Treatment Outcomes: A Parallel-Group Prospective Cohort Study in Two Sub-Saharan Africa Populations. PLoS ONE 2013, 8, e67946. [Google Scholar] [CrossRef]
- Bunu, S.J.; Owaba, A.D.; Vaikosen, E.N.; Ebeshi, B. The CYP2B6 Gene Polymorphism and Phenotypic Correlation of Efavirenz-Based Combination Therapy Among the Niger Delta Ethnic Population: Implications in Modern Pharmacogenomics. Pharmacogenom. Pers. Med. 2022, 15, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Skelton, M.; Ren, Y.; Smith, P.; Takuva, S.; Dandara, C. High predictive value of CYP2B6 SNPs for steady-state plasma efavirenz levels in South African HIV/AIDS patients. Pharmacogenet. Genom. 2013, 23, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Matimba, A.; Oluka, M.N.; Ebeshi, B.U.; Sayi, J.; O Bolaji, O.; Guantai, A.N.; Masimirembwa, C.M. Establishment of a biobank and pharmacogenetics database of African populations. Eur. J. Hum. Genet. 2008, 16, 780–783. [Google Scholar] [CrossRef]
- Gounden, V.; van Niekerk, C.; Snyman, T.; George, J.A. Presence of the CYP2B6 516G> T polymorphism, increased plasma Efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res. Ther. 2010, 7, 32. [Google Scholar] [CrossRef]
- Viljoen, M.; Karlsson, M.O.; Meyers, T.M.; Gous, H.; Dandara, C.; Rheeders, M. Influence of CYP2B6 516G>T polymorphism and interoccasion variability (IOV) on the population pharmacokinetics of efavirenz in HIV-infected South African children. Eur. J. Clin. Pharmacol. 2012, 68, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Twesigomwe, D.; Drögemöller, B.I.; Wright, G.E.B.; Adebamowo, C.; Agongo, G.; Boua, P.R.; Matshaba, M.; Paximadis, M.; Ramsay, M.; Simo, G.; et al. Characterization of CYP2B6 and CYP2A6 Pharmacogenetic Variation in Sub-Saharan African Populations. Clin. Pharmacol. Ther. 2023, 115, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Mbavha, B.T.; Kanji, C.R.; Stadler, N.; Stingl, J.; Stanglmair, A.; Scholl, C.; Wekwete, W.; Masimirembwa, C. Population genetic polymorphisms of pharmacogenes in Zimbabwe, a potential guide for the safe and efficacious use of medicines in people of African ancestry. Pharmacogenet. Genom. 2022, 32, 173–182. [Google Scholar] [CrossRef]
- Aklillu, E.; Herrlin, K.; Gustafsson, L.L.; Bertilsson, L.; Ingelman-Sundberg, M. Evidence for environmental influence on CYP2D6-catalysed debrisoquine hydroxylation as demonstrated by phenotyping and genotyping of Ethiopians living in Ethiopia or in Sweden. Pharmacogenetics 2002, 12, 375–383. [Google Scholar] [CrossRef]
- Mitchell, C.; Gregersen, N.; Krause, A. Novel CYP2C9 and VKORC1 gene variants associated with warfarin dosage variability in the South African black population. Pharmacogenomics 2011, 12, 953–963. [Google Scholar] [CrossRef]
- Bolaji, O.O.; Sadare, I.O.; Babalola, C.P.; Ogunbona, F.A. Polymorphic oxidative metabolism of proguanil in a Nigerian population. Eur. J. Clin. Pharmacol. 2002, 57, 543–545. [Google Scholar] [CrossRef]
- Dandara, C.; Masimirembwa, C.M.; Magimba, A.; Sayi, J.; Kaaya, S.; Sommers, D.K.; Snyman, J.; Hasler, J.A. Genetic polymorphism of CYP2D6 and CYP2C19 in East- and Southern African populations including psychiatric patients. Eur. J. Clin. Pharmacol. 2001, 57, 11–17. [Google Scholar] [CrossRef]
- Drögemöller, B.I.; Wright, G.E.; Niehaus, D.J.; Koen, L.; Malan, S.; Da Silva, D.M.; Hillermann–Rebello, R.; La Grange, A.M.; Venter, M.; Warnich, L. Characterization of the Genetic Profile of CYP2C19 in Two South African Populations. Pharmacogenomics 2010, 11, 1095–1103. [Google Scholar] [CrossRef]
- Dandara, C.; Lombard, Z.; Plooy, I.D.; McLellan, T.; Norris, S.A.; Ramsay, M. Genetic variants in CYP (-1A2, -2C9, -2C19, -3A4 and -3A5), VKORC1 and ABCB1 genes in a black South African population: A window into diversity. Pharmacogenomics 2011, 12, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Miura, J.; Obua, C.; Abbo, C.; Kaneko, S.; Tateishi, T. Cytochrome P450 2C19 genetic polymorphisms in Ugandans. Eur. J. Clin. Pharmacol. 2009, 65, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Masimirembwa, C.; Bertilsson, L.; Johansson, I.; Hasler, J.A.; Ingelman-Sundberg, M. Phenotyping and genotyping of S-mephenytoin hydroxylase (cytochrome P450 2C19) in a Shona population of Zimbabwe. Clin. Pharmacol. Ther. 1995, 57, 656–661. [Google Scholar] [CrossRef]
- Nsabiyumva, F.; Furet, Y.; Autret, E.; Jonville, A.P.; Breteau, M. Oxidative polymorphism of dextromethorphan in a Burundi population. Eur. J. Clin. Pharmacol. 1991, 41, 75–77. [Google Scholar] [CrossRef]
- Sommers, D.; Moncrieff, J.; Avenant, J. Non-correlation between debrisoquine and metoprolol polymorphisms in the Venda. Hum. Toxicol. 1989, 8, 365–368. [Google Scholar] [CrossRef]
- Wennerholm, A.; Johansson, I.; Massele, A.Y.; Lande, M.; Alm, C.; Aden-Abdi, Y.; Dahl, M.L.; Ingelman-Sundberg, M.; Bertilsson, L.; Gustafsson, L.L. Decreased capacity for debrisoquine metabolism among black Tanzanians: Analyses of the CYP2D6 genotype and phenotype. Pharmacogenet. Genom. 1999, 9, 7823–7830. [Google Scholar] [CrossRef]
- Schuster, S.C.; Miller, W.; Ratan, A.; Tomsho, L.P.; Giardine, B.; Kasson, L.R.; Harris, R.S.; Petersen, D.C.; Zhao, F.; Qi, J.; et al. Complete Khoisan and Bantu genomes from southern Africa. Nature 2010, 463, 943. [Google Scholar] [CrossRef]
- Ramharter, M.; Kurth, F.M.; Bélard, S.; Bouyou-Akotet, M.K.; Mamfoumbi, M.M.; Agnandji, S.T.; Missinou, M.A.; Adegnika, A.A.; Issifou, S.; Cambon, N.; et al. Pharmacokinetics of two paediatric artesunate mefloquine drug formulations in the treatment of uncomplicated falciparum malaria in Gabon. J. Antimicrob. Chemother. 2007, 60, 1091–1096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byakika-Tusiime, J.; Chinn, L.W.; Oyugi, J.H.; Obua, C.; Bangsberg, D.R.; Kroetz, D.L. Steady state bioequivalence of generic and innovator formulations of stavudine, lamivudine, and nevirapine in HIV-infected Ugandan adults. PLoS ONE 2008, 3, e3981. [Google Scholar] [CrossRef]
- Decloedt, E.H.; Van Der Walt, J.S.; McIlleron, H.; Wiesner, L.; Maartens, G. The pharmacokinetics of lopinavir/ritonavir when given with isoniazid in South African HIV-infected individuals. Int. J. Tuberc. Lung Dis. 2015, 19, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Sowunmi, A.; Rashid, T.J.; Akinyinka, O.O.; Renwick, A.G. Ethnic differences in nifedipine kinetics: Comparisons between Nigerians, Caucasians and South Asians. Br. J. Clin. Pharmacol. 1995, 40, 489–493. [Google Scholar]
- Svensson, U.S.H.; Ashton, M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br. J. Clin. Pharmacol. 1999, 48, 528–535. [Google Scholar] [CrossRef] [PubMed]
- African Genome Database. African Genomic Medicine Portal Variant Drug Associations. Available online: https://agmp.afrigen-d.org/drug-phenotype-associations/CYP3A4/ (accessed on 16 September 2025).
- Kitzmiller, J.P.; Luzum, J.A.; Baldassarre, D.; Krauss, R.M.; Medina, M.W. CYP3A4z.ast22 and CYP3A5z.ast3 are associated with increased levels of plasma Simvastatin concentrations in the Cholesterol and Pharmacogenetics study cohort. Pharmacogenet Genom. 2014, 24, 486–491. [Google Scholar] [CrossRef]
- Mutagonda, R.F.; Kamuhabwa, A.A.; Minzi, O.M.; Massawe, S.N.; Asghar, M.; Homann, M.V.; Färnert, A.; Aklillu, E. Effect of pharmacogenetics on plasma lumefantrine pharmacokinetics and malaria treatment outcome in pregnant women. Malar. J. 2017, 16, 267. [Google Scholar] [CrossRef]
- Lorenzini, K.I.; Desmeules, J.; Rollason, V.; Bertin, S.; Besson, M.; Daali, Y.; Samer, C.F. CYP450 Genotype—Phenotype Concordance Using the Geneva Micrococktail in a Clinical Setting. Front. Pharmacol. 2021, 12, 730637. [Google Scholar] [CrossRef]
- Jung, K.C.; Sang, C.K. Environmental Effects on Gene Expression Phenotype Have Regional Biases in the Human Genome. Genetics 2007, 175, 1607. [Google Scholar] [CrossRef]
- Lootens, O.; De Boevre, M.; Gasthuys, E.; De Saeger, S.; Van Bocxlaer, J.; Vermeulen, A. Exploring the Impact of Efavirenz on Aflatoxin B1 Metabolism: Insights from a Physiologically Based Pharmacokinetic Model and a Human Liver Microsome Study. Toxins 2024, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, A.I.; Harris, M.; Montaner, J.S.; Moyle, G.; Gazzard, B.; Youle, M.; Johnson, M.; Kwakkelstein, M.O.; Carlier, H.; van Leeuwen, R.; et al. The Steady-State Pharmacokinetics of Efavirenz and Nevirapine When Used in Combination in Human Immunodeficiency Virus Type 1-Infected Persons on JSTOR. Available online: https://www.jstor.org/stable/30137137?seq=4#metadata_info_tab_contents (accessed on 21 December 2022).
- Gilks, C.F.; Walker, A.S.; Dunn, D.T.; Gibb, D.M.; Kikaire, B.; Reid, A.; Musana, H.; Mambule, I. Lopinavir/ritonavir monotherapy after 24 weeks of second-line antiretroviral therapy in Africa: A randomized controlled trial (SARA). Antivir. Ther. 2012, 17, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Le Heuzey, J.Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.J.; Begg, E.J. Pharmacogenetics, Drug-Metabolizing Enzymes, and Clinical Practice. Pharmacol. Rev. 2006, 58, 521–590. [Google Scholar] [CrossRef] [PubMed]
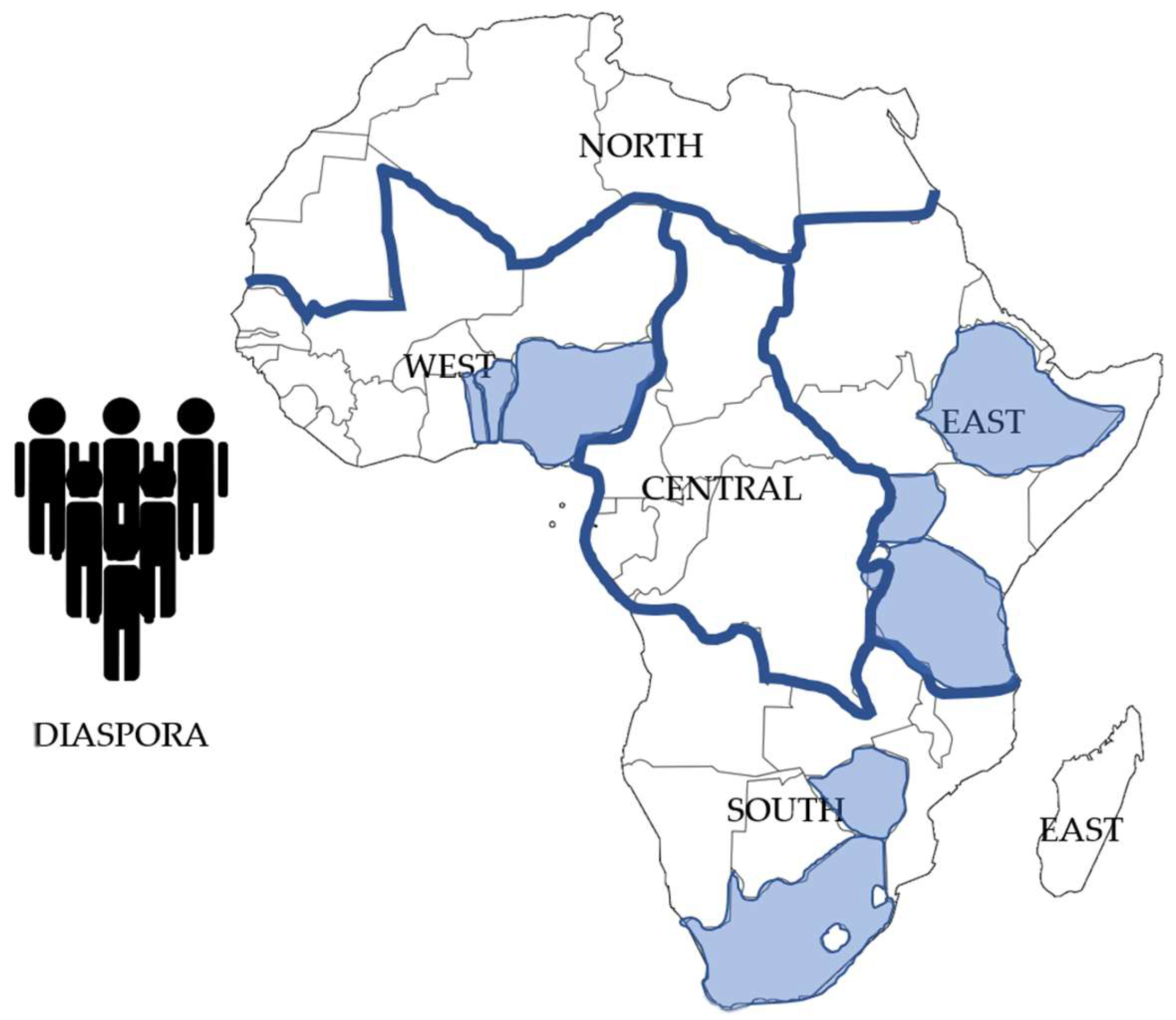
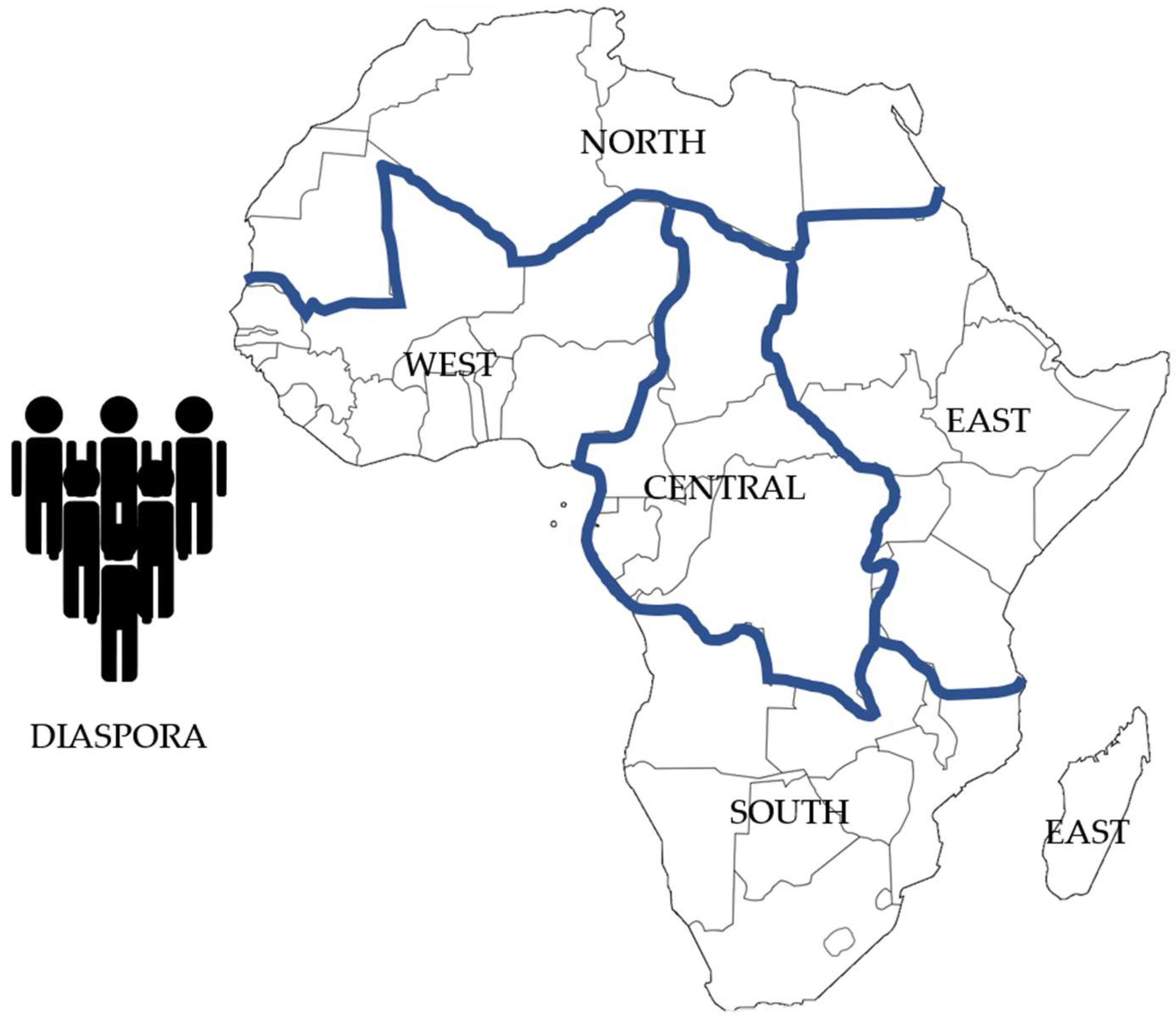
| Region | Countries Included | Included Population | Total Region Population | % Covered | % Missing |
|---|---|---|---|---|---|
| Central | Burundi | 13,200,000 | 180,619,000 | 7.30 | 92.7 |
| East | Ethiopia, Tanzania, Uganda | 242,500,000 | 426,371,900 | 56.9 | 43.1 |
| South | South Africa, Zimbabwe | 77,100,000 | 198,000,000 | 38.9 | 61.1 |
| West | Nigeria, Niger, Benin, Togo | 283,688,000 | 460,848,000 | 62.9 | 37.1 |
| CYP450 | Country | PM Frequency | EM Frequency | N | Reference |
|---|---|---|---|---|---|
| CYP2B6 | Ethiopia | 0.090 | 0.910 | 264 | [25] |
| Niger Delta Ethnic population | 0.440 | 0.560 | 50 | [26] * | |
| Nigeria/Benin/Togo | 0.180 | 0.820 | 226 | [27] | |
| South Africa | 0.130 | 0.870 | 81 | [28] | |
| South Africa | 0.220 | 0.780 | 80 | [29] | |
| South Africa | 0.120 | 0.880 | 163 | [27] | |
| South Africa | 0.230 | 0.770 | 60 | [30] | |
| Sub Saharan Africa | 0.675 | 0.325 | 961 | [31] * | |
| Tanzania | 0.150 | 0.850 | 153 | [28] | |
| Tanzania | 0.190 | 0.810 | 183 | [25] | |
| Zimbabwe | 0.140 | 0.860 | 100 | [28] | |
| Zimbabwe | 0.734 | 0.266 | 522 | [32] * | |
| CYP2C9 | Ethiopia | 0.043 | 0.957 | 69 | [33] |
| South Africa | 0.025 | 0.975 | 100 | [34] | |
| Zimbabwe | 0.170 | 0.830 | 522 | [32] * | |
| CYP2C19 | Nigeria | 0.048 | 0.952 | 126 | [35] |
| South Africa | 0.382 | 0.618 | 76 | [36] | |
| South Africa | 0.520 | 0.480 | 100 | [37] * | |
| South Africa | 0.480 | 0.520 | 75 | [37] * | |
| South Africa | 0.230 | 0.770 | 993 | [38] * | |
| Tanzania | 0.321 | 0.679 | 106 | [36] | |
| Uganda | 0.020 | 0.980 | 99 | [39] * | |
| Zimbabwe | 0.226 | 0.774 | 84 | [36] | |
| Zimbabwe | 0.048 | 0.952 | 84 | [40] | |
| CYP2D6 | Burundi | 0.050 | n.d. | 100 | [41] |
| South Africa | 0.057 | n.d. | 98 | [42] | |
| South Africa | 0.592 | 0.408 | 76 | [36] | |
| Tanzania | 0.462 | 0.538 | 106 | [36] | |
| Tanzania | 0.070 | n.d. | 106 | [43] | |
| Zimbabwe | 0.037 | 0.940 | 103 | [40] | |
| Zimbabwe | 0.554 | 0.446 | 114 | [36] | |
| CYP3A4/5 | Zimbabwe | 0.726 | 0.274 | 522 | [32] |
| Region | CYP2B6 | CYP2C9 | CYP2C19 | CYP2D6 | CYP3A4/5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PM | EM | PM | EM | PM | EM | PM | EM | PM | EM | |
| Central | 0.150 * | 0.850 * | 0.024 * | 0.976 * | 0.038 * | 0.962 * | 0.050 | 0.950 | 0.180 * | 0.820 * |
| East | 0.136 | 0.864 | 0.043 | 0.957 | 0.176 | 0.824 | 0.266 | 0.734 | 0.180 * | 0.820 * |
| South | 0.456 | 0.544 | 0.147 | 0.853 | 0.261 | 0.739 | 0.301 | 0.699 | 0.726 | 0.274 |
| West | 0.227 | 0.773 | 0.024 * | 0.976 * | 0.048 | 0.952 | 0.031 * | 0.969 * | 0.180 * | 0.820 * |
| SSA | 0.440 | 0.560 | 0.136 | 0.864 | 0.235 | 0.764 | 0.255 | 0.745 | 0.726 | 0.274 |
| CENTRAL (dihydroartemisinin) | |||
|---|---|---|---|
| Observed [45] | Predicted | Predicted/Observed | |
| Cmax (mg/L) | 0.812 ± 1.07 | 0.825 ± 0.370 | 1.02 |
| Tmax (h) | 1.50 * | 0.850 ± 0.130 | 0.570 |
| AUCss (mg/L × h) | 1.76 ± 1.86 | 1.25 ± 0.670 | 0.710 |
| EAST (lamivudine) | |||
| Observed [46] | Predicted | ||
| Cmax (mg/L) | 1.10 ± 0.500 | 1.60 ± 0.610 | 1.45 |
| Tmax (h) | 1.10 ± 0.800 | 1.27 ± 0.460 | 1.15 |
| AUCss (mg/L × h) | 5.60 ± 2.50 | 6.14 ± 1.89 | 1.10 |
| SOUTH (ritonavir) | |||
| Observed [47] | Predicted | ||
| Cmax (mg/L) | 13.7 ± 3.04 | 7.13 ± 6.76 | 0.520 |
| Tmax (h) | 4.00 ± 1.48 | 3.04 ± 0.620 | 0.760 |
| AUCss (mg/L × h) | 123 ± 36.7 | 71.2 ± 79.9 | 0.580 |
| WEST (nifedipine) | |||
| Observed [48] | Predicted | ||
| Cmax (mg/L) | 0.205 ± 0.149 | 0.194 ± 72.4 | 0.950 |
| Tmax (h) | 0.75 ± 2.59 | 0.300 ± 0.06 | 0.400 |
| AUCss (mg/L × h) | 0.605 ± 0.155 | 0.348 ± 0.202 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lootens, O.; De Boevre, M.; De Saeger, S.; Van Bocxlaer, J.; Vermeulen, A. Building Sub-Saharan African PBPK Populations Reveals Critical Data Gaps: A Case Study on Aflatoxin B1. Toxins 2025, 17, 493. https://doi.org/10.3390/toxins17100493
Lootens O, De Boevre M, De Saeger S, Van Bocxlaer J, Vermeulen A. Building Sub-Saharan African PBPK Populations Reveals Critical Data Gaps: A Case Study on Aflatoxin B1. Toxins. 2025; 17(10):493. https://doi.org/10.3390/toxins17100493
Chicago/Turabian StyleLootens, Orphélie, Marthe De Boevre, Sarah De Saeger, Jan Van Bocxlaer, and An Vermeulen. 2025. "Building Sub-Saharan African PBPK Populations Reveals Critical Data Gaps: A Case Study on Aflatoxin B1" Toxins 17, no. 10: 493. https://doi.org/10.3390/toxins17100493
APA StyleLootens, O., De Boevre, M., De Saeger, S., Van Bocxlaer, J., & Vermeulen, A. (2025). Building Sub-Saharan African PBPK Populations Reveals Critical Data Gaps: A Case Study on Aflatoxin B1. Toxins, 17(10), 493. https://doi.org/10.3390/toxins17100493








