Properties and Pharmacology of Scorpion Toxins and Their Biotechnological Potential in Agriculture and Medicine
Abstract
1. Introduction
2. Scorpion Venoms: An Overview of Composition and Diversity
3. Evolving Paradigms in Scorpion Toxin Classification
3.1. General Classification of Toxin Families
3.2. General Organization of Scorpion Peptide Genes
3.3. Scorpion Disulfide-Bridged Peptides (DBPs)
3.3.1. Cystine-Stabilized α/β (CSα/β) Scaffold
Long-Chain Scorpion Toxins (Protein Family PF14866)
Short-Chain Scorpion Toxins (Protein Family PF00451)
| KTx Subfamily | Length (Amino Acids) | Disulfide Bridges | Key Structural Features | Primary Target/Function | Noteworthy Characteristics | References |
|---|---|---|---|---|---|---|
| General Short KTxs | 23–64 | 3–4 | - | K+ channel blockade | - | [101] |
| α-KTx | 23–42 | 3 or 4 | CS α/β motif (α-helix + β-sheet) | K+ channel blockade | Largest subgroup of short scorpion toxins. | [63,120] |
| β-KTx | 50–75 | - | CS α/β motif | K+ channel blockade | Comprises longer chain peptides within the short toxin family. | [63,110] |
| γ-KTx | - | - | CS α/β motif | Human Ether-à-go-go-Related Gene (hERG) channels | Found in the genera Centruroides, Mesobuthus, and Buthus. | [112,121] |
| κ-KTx | - | 2 | Two parallel short α-helices connected by a β-turn | K+ channel blockade | Interaction with K+ channels similar to α-KTx, despite structural difference. | [115] |
| δ-KTx | 59–70 | 3 | Kunitz-type structural fold (double-stranded antiparallel β-sheet flanked by α-helix) | K+ channel blockade; Serine protease inhibition | Exerts antiprotease and K+ channel-blocking properties. | [68] |
| λ-KTx | 29–49 | 3 | Inhibitor cystine knot (ICK) motif (triple-stranded antiparallel β-sheet) | - | Related to calcins | [122,123] |
| ε-KTx | - | 4 | ICK motif (unique pattern) | - | Only two members (Ts11 and Ts12 from T. serrulatus venom); Ts11 shows <50% identity with other KTxs. | [23,107] |
3.3.2. Calcium Channel-Modulating Peptides (Calcins) (Protein Family PF08099)
3.3.3. Chloride Channel Toxins (Protein Subfamily PS51200)
| Species | Toxin Name | Molecular Mass (kDa) | Target(s) | Biological Activity | References |
|---|---|---|---|---|---|
| Leiurus quinquestriatus quintestriatus | Venom (peptide(s) not identified) | - | Slow conductance Cl− channels (ClC) | Venom caused voltage-dependent reversible blockade of small conductance Cl− channels from embryonic rat brain growth cones (200 μg/mL) and anion channels from rat colonic epithelial cells (enterocytes) (100 and 400 μg/mL) reconstituted in artificial membranes (stoichiometry of one toxin molecule per channel). Channels blocked in open state. Large conductance Cl− channels of blood cells (predominantly platelets and white blood cells) were unaffected by venom (200 μg/mL). Specific Cl− channel type not identified in these studies. | [143,144] |
| Chlorotoxin (ClTx) | ~5 (4.07) | Slow conductance Cl− channels (ClC) | Purified toxin (ClTx) reproduced the venom activity initially reported by DeBin and Strichartz [143] and blocked CIC with a KD of ~600 nM. ClTx caused progressive, reversible paralysis (recovery depended on venom dose) in crayfish and cockroaches, and accounted for ~4.3% of the venom protein content. Blockade seen only when ClTx was applied to the cytoplasmic surface. A later study reported that ClTx applied extracellularly did not block volume-regulated, CFTR (cAMP-regulated) and glioma-specific Cl− channels [147]. | [144,147] | |
| Ca2+-regulated Cl− channels | Ca2+-regulated Cl− channels in astrocytes were potently blocked by ClTx [146], whereas similar channels in a human colon carcinoma (T84) cell line were not blocked by ClTx [147]. | [146,147] | |||
| Leiurus quinquestriatus hebraeus | Venom (peptide(s) not identified) | ---- | ClC-2 Cl− channels | A peptide-enriched fraction of venom (obtained by filtering through 10 kDa-cutoff filters) caused concentration-dependent, progressive, reversible blockade of rabbit CIC-2 (but not Torpedo CIC-0 or human CIC-1) channels when applied extracellularly. Inhibition was unaffected by boiling the venom fraction, but was prevented by incubation with trypsin, indicating involvement of a heat-stable peptide. Blockade produced by modulating the channel gating mechanism (slower activation, but unaltered deactivation). Purified ClTx from L. q. quinquestriatus was inactive on CIC-2 channels. | [145] |
| GaTX2 (Gating modifier of anion channels 2) | 3.2 | ClC-2 Cl− channels | A continuation of the investigation reported in [145] identified a toxin with three disulfide bonds and a sequence unrelated to ClTx but identical to previously identified leiuropeptide II from this same venom. Structurally related to toxins that inhibit K+ channels (apamin-sensitive K+ channels, Ca2+-activated K+ channels and Kv1.2 channels). Causes voltage-dependent blockade of ClC-2 channels with an apparent KD of ~20 pM. Slows channel activation but does not block open channels. No blockade of CIC-1, CIC-3 and CIC-4 channels or transporters, nor of CFTR (when applied extracellularly or intracellularly). No effect on GABAC receptors when applied to the extracellular surface, or when applied to the cytosolic side of endogenous Ca2+-activated chloride Cl− channels. No effect on Kv1.2 channels. | [154] | |
| Venom (peptide(s) not identified) | ---- | Cystic fibrosis transmembrane conductance regulator (CFTR) | Initial experiments showed that venom reversibly inhibited the CFTR channel in a voltage-dependent manner via a pore-block mechanism. Rapid, all-or-none blockade involving high affinity interaction with the nucleotide binding site of the channel in an interburst closed state, with a reduction in channel burst duration and open probability. No effect on ATP-dependent macroscopic opening rate. Only active when applied to the cytoplasmic side of phosphorylated channels. Activity was unaffected by boiling but was abolished by incubating venom with trypsin, suggesting peptide involvement. No effect on Xenopus oocyte Ca2+-activated Cl− channels (ClCa) when added to the extracellular or cytosolic side. Purfied ClTx from L. q. quinquestriatus was inactive on CFTR. | [155,156] | |
| Georgia anion toxin 1 (GaTX1) | 3.67 | CFTR | Potent, state-dependent (closed channel), reversible blockade of CFTR from the cytosolic side, with KD = 41.5 nM and IC50 = 48 nM. Reduced the open probability and increased the closed time of the channel. Blockade was reduced by high [ATP]. Possibly acts as a non-competitive blocker of CFTR. Greater than 94% identity with cDNA-derived sequences of ClTx-a, -b and -c and >62% sequence identity with various other ClTx-like peptides. No effect when applied to the extracellular surface of CIC-1 and CIC-2 channels or the chloride/H+ exchanger (antiporter) CIC-3, or the cytoplasmic surface of CIC-2 channels. GaTX1 was therefore different from the venom peptide that inhibits CIC-2 channels (see above). At concentrations that affected CFTR, GaTX1 had no effect on GABAC receptors when applied to the extracellular surface, or when applied to the cytosolic side of endogenous Ca2+-activated chloride Cl− channels. Also did not affect the ABC transporters MRP1, MRP2, and MRP3. | [157] | |
| Lqh7-1 | 3.65 | Ca2+-regulated Cl− channels | Of three ClTx-related peptides identified (Lqh2-2, Lqh7-1 and Lqh8-6), Lqh7-1 blocked Ca2+-regulated Cl− channels in rat portal vein myocytes (IC50 63 ± 13 nM). Synthetic Lqh-1 caused similar blockade (IC50 49 ± 5 nM). Lqh2-2 caused only 50% blockade at 1 μM, whereas Lqh8-6 was inactive. Lqh7-1 had no effect on L-type and T-type voltage-dependent Ca2+ channels, on intracellular Ca2+ release via ryanodine-sensitive channels, or on Ca2+-activated and voltage-activated K+ currents. | [158] |
3.4. Non-Disulfide-Bridged Peptides (NDBPs)
3.5. Non-Channel-Modulating Peptides
3.6. Enzymes
4. Insecticidal Activity of Scorpion Venoms and Toxins
4.1. Insecticidal Potential
4.2. Practical Considerations Related to Scorpion Toxin-Based Insecticides
5. Therapeutic Applications of Scorpion Venoms and Toxins
5.1. Antibacterial Activity
5.2. Antiviral Activity
5.3. Antifungal Activity
5.4. Antiparasitic Activity
5.5. Autoimmune Diseases
5.6. Antidiabetic Activity
5.7. Anticancer Activity
5.8. Analgesic Activity
5.9. Therapeutic Potential of Scorpion Toxins in Neurological and Neurodegenerative Diseases
6. Challenges and Future Perspectives
- Mechanistic studies: A deeper understanding of the molecular mechanisms of scorpion toxin action in insects is essential for optimizing their insecticidal activity. This includes identifying specific target sites within insect neuronal channels and elucidating the interactions between toxins and these targets. Techniques such as electrophysiology, molecular modeling, and proteomics can be useful in reaching this goal. This should lead to the development of more specific toxins.
- Structure-activity relationship studies: Research on the structure-activity relationships of scorpion toxins is crucial for designing more potent and selective insecticides. By identifying the structural features responsible for insecticidal activity, it should be possible to develop modified toxins with enhanced efficacy and reduced off-target effects. This can be achieved through peptide synthesis and combinatorial chemistry.
- Development of novel delivery systems: Efficient and targeted delivery of scorpion toxin-based insecticides is essential for maximizing their efficacy and minimizing environmental impact. Novel delivery systems, such as nanoencapsulation, liposomes, and viral vectors, can be explored to achieve this goal. These systems can enhance toxin stability, improve target specificity, and reduce the amount of toxin required for pest control [264].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rein, J.O. The Scorpion Files (Version Apr 2025). In Catalogue of Life (Version 20/08/2025); Bánki, O., Roskov, Y., Döring, M., Ower, G., Hernández Robles, D.R., Plata Corredor, C.A., Stjernegaard Jeppesen, T., Örn, A., Pape, T., et al., Eds.; Catalogue of Life Foundation: Amsterdam, The Netherlands, 2025; Available online: https://www.catalogueoflife.org/data/dataset/1164 (accessed on 12 September 2025). [CrossRef]
- Lourenço, W.R. The evolution and distribution of noxious species of scorpions (Arachnida: Scorpiones). J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 10, 71–79. [Google Scholar] [CrossRef]
- Santos, M.S.; Silva, C.G.; Neto, B.S.; Grangeiro Júnior, C.R.; Lopes, V.H.; Teixeira Júnior, A.G.; Bezerra, D.A.; Luna, J.V.; Cordeiro, J.B.; Júnior, J.G.; et al. Clinical and epidemiological aspects of scorpionism in the world: A systematic review. Wilderness Environ. Med. 2016, 27, 504–518. [Google Scholar] [CrossRef]
- Baradaran, M.; Salabi, F.; Mahdavinia, M.; Mohammadi, E.; Vazirianzadeh, B.; Avella, I.; Kazemi, S.M.; Lüddecke, T. ScorpDb: A novel open-access database for integrative scorpion toxinology. Toxins 2024, 16, 497. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, E.; Miranda, F.; Rochat, H. Chemistry and pharmacology of Buthinae scorpion venoms. In Arthropod Venoms. Handbook of Experimental Physiology; Bettini, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1978; Volume 48, pp. 317–369. [Google Scholar]
- Possani, L.D. Structure of scorpion toxins. In Handbook of Natural Toxins: Insect Poisons, Allergens, and Other Invertebrate Venoms; Tu, A.T., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1984; Volume 2, pp. 513–550. [Google Scholar]
- Gwee, M.C.E.; Nirthanan, S.; Khoo, H.E.; Gopalakrishnakone, P.; Kini, R.M.; Cheah, L.S. Autonomic effects of some scorpion venoms and toxins. Clin. Exp. Pharmacol. Physiol. 2002, 29, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Possani, L.D. The unfulfilled promises of scorpion insectotoxins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Pashmforoosh, N.; Baradaran, M. Peptides with diverse functions from scorpion venom: A great opportunity for the treatment of a wide variety of diseases. Iran. Biomed. J. 2023, 27, 84–99. [Google Scholar] [CrossRef]
- Rodríguez de la Vega, R.C.; Schwartz, E.F.; Possani, L.D. Mining on scorpion venom biodiversity. Toxicon 2010, 56, 1155–1161. [Google Scholar] [CrossRef]
- Quintero-Hernández, V.; Ortiz, E.; Rendón-Anaya, M.; Schwartz, E.F.; Becerril, B.; Corzo, G.; Possani, L.D. Scorpion and spider venom peptides: Gene cloning and peptide expression. Toxicon 2011, 58, 644–663. [Google Scholar] [CrossRef]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Expert Rev. Proteom. 2020, 17, 67–83. [Google Scholar] [CrossRef]
- Mouchbahani-Constance, S.; Sharif-Naeini, R. Proteomic and transcriptomic techniques to decipher the molecular evolution of venoms. Toxins 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Vonk, F.J.; Bittenbinder, M.A.; Kerkkamp, H.M.I.; Grashof, D.G.B.; Archer, J.P.; Afonso, S.; Richardson, M.K.; Kool, J.; van der Meijden, A. A non-lethal method for studying scorpion venom gland transcriptomes, with a review of potentially suitable taxa to which it can be applied. PLoS ONE 2021, 16, e0258712. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.C.; Mendes-Silva, E.; Rodrigues-Ribeiro, L.; Bolais-Ramos, L.G.; Verano-Braga, T. Toxinology in the proteomics era: A review on arachnid venom proteomics. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, 20210034. [Google Scholar] [CrossRef]
- Solano-Godoy, J.A.; Betancourt-Osorio, M.; Orjuela-Rodriguez, M.; Guerrero-Vargas, J.A.; Sepulveda-Arias, J.C. Scorpion venom gland transcriptomics: A systematic review. Toxicon 2025, 267, 108563. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Dutra, A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.; Kjeldsen, F. Moving pieces in a venomic puzzle: Unveiling post-translationally modified toxins from Tityus serrulatus. J. Proteome Res. 2013, 12, 3460–3470. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion venom: Detriments and benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef]
- Xin, K.; Sun, R.; Xiao, W.; Lu, W.; Sun, C.; Lou, J.; Xu, Y.; Chen, T.; Wu, D.; Gao, Y. Short peptides from Asian scorpions: Bioactive molecules with promising therapeutic potential. Toxins 2025, 17, 114. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Cao, Z.; Li, W.; Wu, Y. Diverse structural features of potassium channels characterized by scorpion toxins as molecular probes. Molecules 2019, 24, 2045. [Google Scholar] [CrossRef] [PubMed]
- Tajti, G.; Wai, D.C.C.; Panyi, G.; Norton, R.S. The voltage-gated potassium channel Kv1.3 as a therapeutic target for venom-derived peptides. Biochem. Pharmacol. 2020, 181, 114146. [Google Scholar] [CrossRef]
- Mendes, L.C.; Viana, G.M.M.; Nencioni, A.L.A.; Pimenta, D.C.; Beraldo-Neto, E. Scorpion peptides and ion channels: An insightful review of mechanisms and drug development. Toxins 2023, 15, 238. [Google Scholar] [CrossRef]
- Rojas-Palomino, J.; Gómez-Restrepo, A.; Salinas-Restrepo, C.; Segura, C.; Giraldo, M.A.; Calderón, J.C. Electrophysiological evaluation of the effect of peptide toxins on voltage-gated ion channels: A scoping review on theoretical and methodological aspects with focus on the Central and South American experience. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230048. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Figueiredo-Rezende, F.; Melo, M.N.; Lautner, R.Q.; Gomes, E.R.; Mata-Machado, L.T.; Murari, A.; Rocha-Resende, C.; Lima, M.E.; Guatimosim, S.; et al. Structure-function studies of Tityus serrulatus hypotensin-I (TsHpt-I): A new agonist of B2 kinin receptor. Toxicon 2010, 56, 1162–1171. [Google Scholar] [CrossRef]
- Rocha-Resende, C.; Leão, N.M.; de Lima, M.E.; Santos, R.A.; Pimenta, A.M.C.; Verano-Braga, T. Moving pieces in a cryptomic puzzle: Cryptide from Tityus serrulatus Ts3 Nav toxin as potential agonist of muscarinic receptors. Peptides 2017, 98, 70–77. [Google Scholar] [CrossRef]
- Ferreira, L.A.; Alves, E.W.; Henriques, O.B. Peptide T, a novel bradykinin potentiator isolated from Tityus serrulatus scorpion venom. Toxicon 1993, 31, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Meki, A.R.; Nassar, A.Y.; Rochat, H. A bradykinin-potentiating peptide (peptide K12) isolated from the venom of Egyptian scorpion Buthus occitanus. Peptides 1995, 16, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Wang, S.; Nie, Y.; Zhang, L.; Luo, X. Characterization of BmKbpp, a multifunctional peptide from the Chinese scorpion Mesobuthus martensii Karsch: Gaining insight into a new mechanism for the functional diversification of scorpion venom peptides. Peptides 2012, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Undheim, E.A.; Chan, A.H.; Koludarov, I.; Muñoz-Gómez, S.A.; Antunes, A.; Fry, B.G. Evolution stings: The origin and diversification of scorpion toxin peptide scaffolds. Toxins 2013, 5, 2456–2487. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Pinheiro-Junior, E.L.; Zoccal, K.F.; Bordon, K.C.F.; Amorim, F.G.; Peigneur, S.; Vriens, K.; Thevissen, K.; Cammue, B.P.A.; et al. Non-disulfide-bridged peptides from Tityus serrulatus venom: Evidence for proline-free ACE-inhibitors. Peptides 2016, 82, 44–51. [Google Scholar] [CrossRef]
- Setayesh-Mehr, Z.; Asoodeh, A. The inhibitory activity of HL-7 and HL-10 peptide from scorpion venom (Hemiscorpius lepturus) on angiotensin converting enzyme: Kinetic and docking study. Bioorg. Chem. 2017, 75, 30–37. [Google Scholar] [CrossRef]
- Goudarzi, H.R.; Salehi Najafabadi, Z.; Movahedi, A.; Noofeli, M. Bradykinin-potentiating factors of venom from Iranian medically important scorpions. Arch. Razi Inst. 2019, 74, 385–394. [Google Scholar] [CrossRef]
- Hasan, H.F.; Mohmed, H.K.; Galal, S.M. Scorpion bradykinin potentiating factor mitigates lung damage induced by γ-irradiation in rats: Insights on AngII/ACE/Ang(1-7) axis. Toxicon 2021, 203, 58–65. [Google Scholar] [CrossRef]
- Sharma, A.; Balde, A.; Nazeer, R.A. A review on animal venom-based matrix metalloproteinase modulators and their therapeutic implications. Int. Immunopharmacol. 2025, 157, 114703. [Google Scholar] [CrossRef]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kamau, P.M.; Zhong, J.; Yao, B.; Lai, R.; Luo, L. Bioactive peptides from scorpion venoms: Therapeutic scaffolds and pharmacological tools. Chin. J. Nat. Med. 2023, 21, 19–35. [Google Scholar] [CrossRef]
- Fong-Coronado, P.A.; Ramirez, V.; Quintero-Hernández, V.; Balleza, D. A critical review of short antimicrobial peptides from scorpion venoms, their physicochemical attributes, and potential for the development of new drugs. J. Membr. Biol. 2024, 257, 165–205. [Google Scholar] [CrossRef] [PubMed]
- Hemajha, L.; Singh, S.; Biji, C.A.; Balde, A.; Benjakul, S.; Nazeer, R.A. A review on inflammation modulating venom proteins/peptide therapeutics and their delivery strategies: A review. Int. Immunopharmacol. 2024, 142, 113130. [Google Scholar] [CrossRef] [PubMed]
- Lafnoune, A.; Darkaoui, B.; Chbel, A.; Nait Irahal, I. Emerging therapeutic applications of scorpion venom peptides in the Middle East and North Africa: A comprehensive review. Toxicon 2025, 256, 108270. [Google Scholar] [CrossRef]
- Amorim-Carmo, B.; Parente, A.M.S.; Souza, E.S.; Silva-Junior, A.A.; Araújo, R.M.; Fernandes-Pedrosa, M.F. Antimicrobial peptide analogs from scorpions: Modifications and structure-activity. Front. Mol. Biosci. 2022, 9, 887763. [Google Scholar] [CrossRef]
- Rincón-Cortés, C.A.; Bayona-Rojas, M.A.; Reyes-Montaño, E.A.; Vega-Castro, N.A. Antimicrobial activity developed by scorpion venoms and its peptide component. Toxins 2022, 14, 740. [Google Scholar] [CrossRef]
- Nasr, S.; Borges, A.; Sahyoun, C.; Nasr, R.; Roufayel, R.; Legros, C.; Sabatier, J.M.; Fajloun, Z. Scorpion venom as a source of antimicrobial peptides: Overview of biomolecule separation, analysis and characterization methods. Antibiotics 2023, 12, 1380. [Google Scholar] [CrossRef]
- Panayi, T.; Diavoli, S.; Nicolaidou, V.; Papaneophytou, C.; Petrou, C.; Sarigiannis, Y. Short-chained linear scorpion peptides: A pool for novel antimicrobials. Antibiotics 2024, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xie, L.; Li, B.; Lv, X.; Zhang, H.; Cao, Z. Antimicrobial potential of scorpion-venom-derived peptides. Molecules 2024, 29, 5080. [Google Scholar] [CrossRef] [PubMed]
- El Hidan, M.A.; Laaradia, M.A.; El Hiba, O.; Draoui, A.; Aimrane, A.; Kahime, K. Scorpion-derived antiviral peptides with a special focus on medically important viruses: An update. Biomed Res. Int. 2021, 2021, 9998420. [Google Scholar] [CrossRef]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion venom peptides: Molecular diversity, structural characteristics, and therapeutic use from channelopathies to viral infections and cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef] [PubMed]
- Srairi-Abid, N.; Othman, H.; Aissaoui, D.; BenAissa, R. Anti-tumoral effect of scorpion peptides: Emerging new cellular targets and signaling pathways. Cell Calcium 2019, 80, 160–174. [Google Scholar] [CrossRef]
- Díaz-García, A.; Varela, D. Voltage-gated K+/Na+ channels and scorpion venom toxins in cancer. Front. Pharmacol. 2020, 11, 913. [Google Scholar] [CrossRef]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic anticancer properties of scorpion venom peptides: Rhopalurus princeps venom as an anticancer agent. Drug Des. Devel Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef]
- Boltman, T.; Meyer, M.; Ekpo, O. Diagnostic and therapeutic approaches for glioblastoma and neuroblastoma cancers using chlorotoxin nanoparticles. Cancers 2023, 15, 3388. [Google Scholar] [CrossRef]
- El-Qassas, J.; Abd El-Atti, M.; El-Badri, N. Harnessing the potency of scorpion venom-derived proteins: Applications in cancer therapy. Bioresour. Bioprocess. 2024, 11, 93. [Google Scholar] [CrossRef]
- Wang, X.; Luo, H.; Peng, X.; Chen, J. Spider and scorpion knottins targeting voltage-gated sodium ion channels in pain signaling. Biochem. Pharmacol. 2024, 227, 116465. [Google Scholar] [CrossRef]
- He, D.; Lei, Y.; Qin, H.; Cao, Z.; Kwok, H.F. Deciphering scorpion toxin-induced pain: Molecular mechanisms and ion channel dynamics. Int. J. Biol. Sci. 2025, 21, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Balde, A.; Benjakul, S.; Nazeer, R.A. A review on NLRP3 inflammasome modulation by animal venom proteins/peptides: Mechanisms and therapeutic insights. Inflammopharmacology 2025, 33, 1013–1031. [Google Scholar] [CrossRef]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic potential of peptides derived from animal venoms: Current views and emerging drugs for diabetes. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211006071. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Rocha-Resende, C.; Silva, D.M.; Ianzer, D.; Martin-Eauclaire, M.F.; Bougis, P.E.; de Lima, M.E.; Santos, R.A.; Pimenta, A.M. Tityus serrulatus hypotensins: A new family of peptides from scorpion venom. Biochem. Biophys. Res. Commun. 2008, 371, 515–520. [Google Scholar] [CrossRef]
- Machado, R.J.; Junior, L.G.; Monteiro, N.K.; Silva-Júnior, A.A.; Portaro, F.C.; Barbosa, E.G.; Braga, V.A.; Fernandes-Pedrosa, M.F. Homology modeling, vasorelaxant and bradykinin-potentiating activities of a novel hypotensin found in the scorpion venom from Tityus stigmurus. Toxicon 2015, 101, 11–18. [Google Scholar] [CrossRef]
- Krayem, N.; Gargouri, Y. Scorpion venom phospholipases A2: A minireview. Toxicon 2020, 184, 48–54. [Google Scholar] [CrossRef]
- Soltan-Alinejad, P.; Alipour, H.; Meharabani, D.; Azizi, K. Therapeutic potential of bee and scorpion venom phospholipase A2 (PLA2): A narrative review. Iran. J. Med. Sci. 2022, 47, 300–313. [Google Scholar]
- Bordon, K.C.F.; Wiezel, G.A.; Amorim, F.G.; Arantes, E.C. Arthropod venom hyaluronidases: Biochemical properties and potential applications in medicine and biotechnology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 43. [Google Scholar] [CrossRef]
- Psenicnik, A.; Ojanguren-Affilastro, A.A.; Graham, M.R.; Hassan, M.K.; Abdel-Rahman, M.A.; Sharma, P.P.; Santibáñez-López, C.E. Optimizing scorpion toxin processing through artificial intelligence. Toxins 2024, 16, 437. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Possani, L.D.; Becerril, B.; Delepierre, M.; Tytgat, J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999, 264, 287–300. [Google Scholar] [CrossRef]
- Froy, O.; Sagiv, T.; Poreh, M.; Urbach, D.; Zilberberg, N.; Gurevitz, M. Dynamic diversification from a putative common ancestor of scorpion toxins affecting sodium, potassium, and chloride channels. J. Mol. Evol. 1999, 48, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Goudet, C.; Chi, C.-W.; Tytgat, J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 2002, 40, 1239–1258. [Google Scholar] [CrossRef]
- Zhijian, C.; Feng, L.; Yingliang, W.; Xin, M.; Wenxin, L. Genetic mechanisms of scorpion venom peptide diversification. Toxicon 2006, 47, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, F.; Feng, J.; Yang, W.; Zeng, D.; Zhao, R.; Cao, Z.; Liu, M.; Li, W.; Jiang, L.; et al. Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins. PLoS ONE 2013, 8, e60201. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Zhu, S. Exon shuffling and origin of scorpion venom biodiversity. Toxins 2017, 9, 10. [Google Scholar] [CrossRef]
- Rates, B.; Ferraz, K.K.; Borges, M.H.; Richardson, M.; De Lima, M.E.; Pimenta, A.M. Tityus serrulatus venom peptidomics: Assessing venom peptide diversity. Toxicon 2008, 52, 611–618. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Zhu, S. A single-point mutation enhances dual functionality of a scorpion toxin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 179, 72–78. [Google Scholar] [CrossRef]
- Delgado-Prudencio, G.; Possani, L.D.; Becerril, B.; Ortiz, E. The dual α-amidation system in scorpion venom glands. Toxins 2019, 11, 425. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, C.; Yang, M.; Sun, J.; Lu, J.; Zhang, H.; Qin, J.; Zhang, W.; Jiang, Z. Unveiling the diversity and modifications of short peptides in Buthus martensii scorpion venom through liquid chromatography-high resolution mass spectrometry. Toxins 2024, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Dai, L.; Lan, Z.D.; Chi, C.W. Genomic organization of three neurotoxins active on small conductance Ca2+-activated potassium channels from the scorpion Buthus martensi Karsch. FEBS Lett. 1999, 452, 360–364. [Google Scholar] [CrossRef]
- Dai, L.; Wu, J.J.; Gu, Y.H.; Lan, Z.D.; Ling, M.H.; Chi, C.W. Genomic organization of three novel toxins from the scorpion Buthus martensi Karsch that are active on potassium channels. Biochem. J. 2000, 346, 805–809. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Zeng, D.-Y.; Hu, Y.-T.; Ya-Wen He, Y.-W.; Pan, N.; Ding, J.-P.; Cao, Z.-J.; Liu, M.-L.; Wen-Xin Li, W.-X.; Yi, H.; et al. Structural and functional diversity of acidic scorpion potassium channel toxins. PLoS ONE 2012, 7, e35154. [Google Scholar] [CrossRef]
- Cao, Z.; Yu, Y.; Wu, Y.; Hao, P.; Di, Z.; He, Y.; Chen, Z.; Yang, W.; Shen, Z.; He, X.; et al. The genome of Mesobuthus martensii reveals a unique adaptation model of arthropods. Nat. Commun. 2013, 4, 2602. [Google Scholar] [CrossRef]
- Saucedo, A.L.; Flores-Solis, D.; Rodríguez de la Vega, R.C.; Ramírez-Cordero, B.; Hernández-López, R.; Cano-Sánchez, P.; Noriega Navarro, R.; García-Valdés, J.; Coronas-Valderrama, F.; de Roodt, A.; et al. New tricks of an old pattern: Structural versatility of scorpion toxins with common cysteine spacing. J. Biol. Chem. 2012, 287, 12321–12330. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez de la Vega, R.C.; Vidal, N.; Possani, L.D. Scorpion peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 423–429. [Google Scholar]
- Gregory, A.J.; Voit-Ostricki, L.; Lovas, S.; Watts, C.R. Effects of selective substitution of cysteine residues on the conformational properties of chlorotoxin explored by molecular dynamics simulations. Int. J. Mol. Sci. 2019, 20, 1261. [Google Scholar] [CrossRef]
- Titaux-Delgado, G.; Lopez-Giraldo, A.E.; Carrillo, E.; Cofas-Vargas, L.F.; Carranza, L.E.; López-Vera, E.; García-Hernández, E.; Del Rio-Portilla, F. β-KTx14.3, a scorpion toxin, blocks the human potassium channel KCNQ1. Biochim. Biophys. Acta Proteins Proteom. 2023, 1871, 140906. [Google Scholar] [CrossRef]
- Ghosh, A.; Roy, R.; Nandi, M.; Mukhopadhyay, A. Scorpion venom-toxins that aid in drug development: A review. Int. J. Pept. Res. Ther. 2019, 25, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, H.H.; Martin, B.M.; Ramírez, A.N.; Fletcher, P.L.; Possani, L.D. Isolation and pharmacological characterization of four novel Na+ channel-blocking toxins from the scorpion Centruroides noxius Homann. J. Biochem. 1994, 116, 1383–1391. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhu, Y.; Zhang, Z.; Sun, M.; Cheng, J.; Xiao, Q.; Li, G.; Tao, J. Scorpion toxins from Buthus martensii Karsch (BmK) as potential therapeutic agents for neurological disorders: State of the art and beyond. In Medical Toxicology; Erkekoglu, P., Ogawa, T., Eds.; IntechOpen: London, UK, 2020; pp. 1–20. [Google Scholar] [CrossRef][Green Version]
- Wisedchaisri, G.; Gamal El-Din, T.M. Druggability of voltage-gated sodium channels—Exploring old and new drug receptor sites. Front. Pharmacol. 2022, 13, 858348. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Blömer, L.A.; Millet, H.; Montnach, J.; De Waard, M.; Canepari, M. Analysis of the effect of the scorpion toxin AaH-II on action potential generation in the axon initial segment. Sci. Rep. 2024, 14, 4967. [Google Scholar] [CrossRef]
- Sun, H.Y.; Zhu, H.F.; Ji, Y.H. BmK I, an α-like scorpion neurotoxin, specifically modulates isolated rat cardiac mechanical and electrical activity. Sheng Li Xue Bao. 2003, 55, 530–534. [Google Scholar] [PubMed]
- Rowe, A.H.; Xiao, Y.; Scales, J.; Linse, K.D.; Rowe, M.P.; Cummins, T.R.; Zakon, H.H. Isolation and characterization of CvIV4: A pain inducing α-scorpion toxin. PLoS ONE 2011, 6, e23520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gordon, D.; Heinemann, S.H. Modulation of cloned skeletal muscle sodium channels by the scorpion toxins Lqh II, Lqh III, and Lqh αIT. Pflug. Arch. 2000, 439, 423–432. [Google Scholar] [CrossRef]
- Cestèle, S.; Gordon, D.; Kopeyan, C.; Rochat, H. Toxin III from Leiurus quinquestriatus quinquestriatus: A specific probe for receptor site 3 on insect sodium channels. Insect Biochem. Mol. Biol. 1997, 27, 523–528. [Google Scholar] [CrossRef]
- Goyffon, M.; Tournier, J.-N. Scorpions: A presentation. Toxins 2014, 6, 2137–2148. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.F.; Bougis, P.E.; de Lima, M.E. Ts1 from the Brazilian scorpion Tityus serrulatus: A half-century of studies on a multifunctional β-like toxin. Toxicon 2018, 152, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Cologna, C.T.; Peigneur, S.; Rustiguel, J.K.; Nonato, M.C.; Tytgat, J.; Arantes, E.C. Investigation of the relationship between the structure and function of Ts2, a neurotoxin from Tityus serrulatus venom. FEBS J. 2012, 279, 1495–1504. [Google Scholar] [CrossRef]
- Sampaio, S.V.; Arantes, E.C.; Prado, W.A.; Riccioppo Neto, F.; Giglio, J.R. Further characterization of toxins T1IV (TsTX-III) and T2IV from Tityus serrulatus scorpion venom. Toxicon 1991, 29, 663–672. [Google Scholar] [CrossRef]
- Israel, M.R.; Tanaka, B.S.; Castro, J.; Thongyoo, P.; Robinson, S.D.; Zhao, P.; Deuis, J.R.; Craik, D.J.; Durek, T.; Brierley, S.M.; et al. Nav 1.6 regulates excitability of mechanosensitive sensory neurons. J. Physiol. 2019, 597, 3751–3768. [Google Scholar] [CrossRef]
- del Río-Portilla, F.; Hernández-Marín, E.; Pimienta, G.; Coronas, F.V.; Zamudio, F.Z.; Rodríguez de la Vega, R.C.; Wanke, E.; Possani, L.D. NMR solution structure of Cn12, a novel peptide from the Mexican scorpion Centruroides noxius with a typical β-toxin sequence but with α-like physiological activity. Eur. J. Biochem. 2004, 271, 2504–2516. [Google Scholar] [CrossRef]
- He, H.; Liu, Z.; Dong, B.; Zhang, J.; Shu, X.; Zhou, J.; Ji, Y. Localization of receptor site on insect sodium channel for depressant β-toxin BmK IT2. PLoS ONE 2011, 6, e14510. [Google Scholar] [CrossRef]
- Song, W.; Du, Y.; Liu, Z.; Luo, N.; Turkov, M.; Gordon, D.; Gurevitz, M.; Goldin, A.L.; Dong, K. Substitutions in the domain III voltage-sensing module enhance the sensitivity of an insect sodium channel to a scorpion β-toxin. J. Biol. Chem. 2011, 286, 15781–15788. [Google Scholar] [CrossRef]
- Housley, D.M.; Housley, G.D.; Liddell, M.J.; Jennings, E.A. Scorpion toxin peptide action at the ion channel subunit level. Neuropharmacology 2017, 127, 46–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, Z.; Sheng, J.; Wu, W.; Luo, F.; Sha, Y.; Mao, X.; Liu, H.; Jiang, D.; Li, W. Genomic sequence analysis and organization of BmKαTx11 and BmKαTx15 from Buthus martensii Karsch: Molecular evolution of α-toxin genes. J. Biochem. Mol. Biol. 2005, 38, 386–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez de la Vega, R.C.; Possani, L.D. Current views on scorpion toxins specific for K+-channels. Toxicon 2004, 43, 865–875. [Google Scholar] [CrossRef]
- Naseem, M.U.; Gurrola-Briones, G.; Romero-Imbachi, M.R.; Borrego, J.; Carcamo-Noriega, E.; Beltrán-Vidal, J.; Zamudio, F.Z.; Shakeel, K.; Possani, L.D.; Panyi, G. Characterization and chemical synthesis of Cm39 (α-KTx 4.8): A scorpion toxin that inhibits voltage-gated K+ channel KV1.2 and small- and intermediate-conductance Ca2+-activated K+ channels KCa2.2 and KCa3.1. Toxins 2023, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Q.; He, L.L.; Brône, B.; Martin-Eauclaire, M.F.; Van Kerkhove, E.; Zhou, Z.; Chi, C.W. A novel scorpion toxin blocking small conductance Ca2+ activated K+ channel. Toxicon 2004, 43, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gao, B.; Aumelas, A.; Rodríguez, M.C.; Lanz-Mendoza, H.; Peigneur, S.; Diego-Garcia, E.; Martin-Eauclaire, M.F.; Tytgat, J.; Possani, L.D. MeuTXKβ1, a scorpion venom-derived two-domain potassium channel toxin-like peptide with cytolytic activity. Biochim. Biophys. Acta 2010, 1804, 872–883. [Google Scholar] [CrossRef]
- Bajaj, S.; Han, J. Venom-derived peptide modulators of cation-selective channels: Friend, foe or frenemy. Front. Pharmacol. 2019, 10, 58. [Google Scholar] [CrossRef]
- Tytgat, J.; Chandy, K.G.; Garcia, M.L.; Gutman, G.A.; Martin-Eauclaire, M.F.; van der Walt, J.J.; Possani, L.D. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends Pharmacol. Sci. 1999, 20, 444–447. [Google Scholar] [CrossRef]
- Cremonez, C.M.; Maiti, M.; Peigneur, S.; Cassoli, J.S.; Dutra, A.A.A.; Waelkens, E.; Lescrinier, E.; Herdewijn, P.; de Lima, M.E.; Pimenta, A.M.C.; et al. Structural and functional elucidation of peptide Ts11 shows evidence of a novel subfamily of scorpion venom toxins. Toxins 2016, 8, 288. [Google Scholar] [CrossRef]
- Bergeron, Z.L.; Bingham, J.-P. Scorpion toxins specific for potassium (K+) channels: A historical overview of peptide bioengineering. Toxins 2012, 4, 1082–1119. [Google Scholar] [CrossRef]
- Luna-Ramirez, K.; Csoti, A.; McArthur, J.R.; Chin, Y.K.Y.; Anangi, R.; Najera, R.D.C.; Possani, L.D.; King, G.F.; Panyi, G.; Yu, H.; et al. Structural basis of the potency and selectivity of urotoxin, a potent Kv1 blocker from scorpion venom. Biochem. Pharmacol. 2020, 174, 113782. [Google Scholar] [CrossRef]
- Cerni, F.A.; Pucca, M.B.; Amorim, F.G.; Bordon, K.C.F.; Echterbille, J.; Quinton, L.; De Pauw, E.; Peigneur, S.; Tytgat, J.; Arantes, E.C. Isolation and characterization of Ts19 Fragment II, a new long-chain potassium channel toxin from Tityus serrulatus venom. Peptides 2016, 80, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, W.; Zeng, X.; Jiang, D.; Mao, X.; Liu, H. Molecular cloning and sequencing of two ‘short chain’ and two ‘long chain’ K+ channel-blocking peptides from the Chinese scorpion Buthus martensii Karsch. FEBS Lett. 1999, 457, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Vargas, J.M.; Restano-Cassulini, R.; Possani, L.D. Toxin modulators and blockers of hERG K+ channels. Toxicon 2012, 60, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Olamendi-Portugal, T.; Bartok, A.; Zamudio-Zuñiga, F.; Balajthy, A.; Becerril, B.; Panyi, G.; Possani, L.D. Isolation, chemical and functional characterization of several new K+-channel blocking peptides from the venom of the scorpion Centruroides tecomanus. Toxicon 2016, 115, 1–12. [Google Scholar] [CrossRef]
- Kalapothakis, Y.; Miranda, K.; Pereira, A.H.; Witt, A.S.A.; Marani, C.; Martins, A.P.; Leal, H.G.; Campos-Júnior, E.; Pimenta, A.M.C.; Borges, A.; et al. Novel components of Tityus serrulatus venom: A transcriptomic approach. Toxicon 2021, 189, 91–104. [Google Scholar] [CrossRef]
- Srinivasan, K.N.; Sivaraja, V.; Huys, I.; Sasaki, T.; Cheng, B.; Kumar, T.K.; Sato, K.; Tytgat, J.; Yu, C.; San, B.C.; et al. κ-Hefutoxin1, a novel toxin from the scorpion Heterometrus fulvipes with unique structure and function. Importance of the functional diad in potassium channel selectivity. J. Biol. Chem. 2002, 277, 30040–30047. [Google Scholar] [CrossRef]
- Chagot, B.; Pimentel, C.; Dai, L.; Pil, J.; Tytgat, J.; Nakajima, T.; Corzo, G.; Darbon, H.; Ferrat, G. An unusual fold for potassium channel blockers: NMR structure of three toxins from the scorpion Opisthacanthus madagascariensis. Biochem. J. 2005, 388, 263–271. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Hu, Y.T.; Yang, W.S.; He, Y.W.; Feng, J.; Wang, B.; Zhao, R.M.; Ding, J.P.; Cao, Z.J.; Li, W.X.; et al. Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J. Biol. Chem. 2012, 287, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.F.; Diego-Garcia, E.; Rodríguez de la Veja, R.C.; Possani, L.D. Transcriptome analysis of the venom gland of the Mexican scorpion Hadrurus gertschi (Arachnida: Scorpiones). BMC Genom. 2007, 8, 119. [Google Scholar] [CrossRef]
- Zhao, R.; Dai, H.; Qiu, S.; Li, T.; He, Y.; Ma, Y.; Chen, Z.; Wu, Y.; Li, W.; Cao, Z. SdPI, the first functionally characterized Kunitz-type trypsin inhibitor from scorpion venom. PLoS ONE 2011, 6, e27548. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Peigneur, S.; Cologna, C.T.; Cerni, F.A.; Zoccal, K.F.; Bordon, K.C.; Faccioli, L.H.; Tytgat, J.; Arantes, E.C. Electrophysiological characterization of the first Tityus serrulatus α-like toxin, Ts5: Evidence of a pro-inflammatory toxin on macrophages. Biochimie 2015, 115, 8–16. [Google Scholar] [CrossRef]
- Kalapothakis, Y.; Miranda, K.; Molina, D.A.M.; Conceição, I.M.C.A.; Larangote, D.; Op den Camp, H.J.M.; Kalapothakis, E.; Chávez-Olórtegui, C.; Borges, A. An overview of Tityus cisandinus scorpion venom: Transcriptome and mass fingerprinting reveal conserved toxin homologs across the Amazon region and novel lipolytic components. Int. J. Biol. Macromol. 2023, 225, 1246–1266. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Harvey, P.J.; Craik, D.J.; Ronjat, M.; De Waard, M.; Zhu, S. Functional evolution of scorpion venom peptides with an inhibitor cystine knot fold. Biosci. Rep. 2013, 33, e00047. [Google Scholar] [CrossRef]
- Smith, J.J.; Hill, J.M.; Little, M.J.; Nicholson, G.M.; King, G.F.; Alewood, P.F. Unique scorpion toxin with a putative ancestral fold provides insight into evolution of the inhibitor cystine knot motif. Proc. Natl. Acad. Sci. USA 2011, 108, 10478–10483. [Google Scholar] [CrossRef]
- Xiao, L.; Gurrola, G.B.; Zhang, J.; Valdivia, C.R.; SanMartin, M.; Zamudio, F.Z.; Zhang, L.; Possani, L.D.; Valdivia, H.H. Structure-function relationships of peptides forming the calcin family of ryanodine receptor ligands. J. Gen. Physiol. 2016, 147, 375–394. [Google Scholar] [CrossRef]
- Simeoni, I.; Rossi, D.; Zhu, X.; García, J.; Valdivia, H.H.; Sorrentino, V. Imperatoxin A (IpTxa) from Pandinus imperator stimulates [3H]ryanodine binding to RyR3 channels. FEBS Lett. 2001, 508, 5–10. [Google Scholar] [CrossRef]
- Fajloun, Z.; Kharrat, R.; Chen, L.; Lecomte, C.; Di Luccio, E.; Bichet, D.; El Ayeb, M.; Rochat, H.; Allen, P.D.; Pessah, I.N.; et al. Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca2+ release channel/ryanodine receptors. FEBS Lett. 2000, 469, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Shahbazzadeh, D.; Srairi-Abid, N.; Feng, W.; Ram, N.; Borchani, L.; Ronjat, M.; Akbari, A.; Pessah, I.N.; De Waard, M.; El Ayeb, M. Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem. J. 2007, 404, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.F.; Capes, E.M.; Diego-García, E.; Zamudio, F.Z.; Fuentes, O.; Possani, L.D.; Valdivia, H.H. Characterization of hadrucalcin, a peptide from Hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors. Br. J. Pharmacol. 2009, 157, 392–403. [Google Scholar] [CrossRef]
- Zamaleeva, A.I.; Collot, M.; Bahembera, E.; Tisseyre, C.; Rostaing, P.; Yakovlev, A.V.; Oheim, M.; de Waard, M.; Mallet, J.M.; Feltz, A. Cell-penetrating nanobiosensors for pointillistic intracellular Ca2+-transient detection. Nano Lett. 2014, 14, 2994–3001. [Google Scholar] [CrossRef]
- Luna-Ramírez, K.; Bartok, A.; Restano-Cassulini, R.; Quintero-Hernández, V.; Coronas, F.I.; Christensen, J.; Wright, C.E.; Panyi, G.; Possani, L.D. Structure, molecular modeling, and function of the novel potassium channel blocker urotoxin isolated from the venom of the Australian scorpion Urodacus yaschenkoi. Mol. Pharmacol. 2014, 86, 28–41. [Google Scholar] [CrossRef]
- Vargas-Jaimes, L.; Xiao, L.; Zhang, J.; Possani, L.D.; Valdivia, H.H.; Quintero-Hernández, V. Recombinant expression of intrepicalcin from the scorpion Vaejovis intrepidus and its effect on skeletal ryanodine receptors. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 936–946. [Google Scholar] [CrossRef]
- Zhijian, C.; Yun, X.; Chao, D.; Shunyi, Z.; Shijin, Y.; Yingliang, W.; Wenxin, L. Cloning and characterization of a novel calcium channel toxin-like gene BmCa1 from Chinese scorpion Mesobuthus martensii Karsch. Peptides 2006, 27, 1235–1240. [Google Scholar] [CrossRef]
- Zhu, S.; Darbon, H.; Dyason, K.; Verdonck, F.; Tytgat, J. Evolutionary origin of inhibitor cystine knot peptides. FASEB J. 2003, 17, 1765–1767. [Google Scholar] [CrossRef]
- Lan, Z.D.; Dai, L.; Zhou, X.L.; Feng, J.C.; Xu, K.; Chi, C.W. Gene cloning and sequencing of BmK AS and BmK AS-1, two novel neurotoxins from the scorpion Buthus martensi Karsch. Toxicon 1999, 37, 815–823. [Google Scholar] [CrossRef]
- McCarthy, S.; Robinson, J.; Thalassinos, K.; Tabor, A.B. A chemical biology approach to probing the folding pathways of the inhibitory cystine knot (ICK) peptide ProTx-II. Front. Chem. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef]
- Rufenach, B.; Van Petegem, F. Structure and function of STAC proteins: Calcium channel modulators and critical components of muscle excitation-contraction coupling. J. Biol. Chem. 2021, 297, 100874. [Google Scholar] [CrossRef]
- Haji-Ghassemi, O.; Chen, Y.S.; Woll, K.; Gurrola, G.B.; Valdivia, C.R.; Cai, W.; Li, S.; Valdivia, H.H.; Van Petegem, F. Cryo-EM analysis of scorpion toxin binding to ryanodine receptors reveals subconductance that is abolished by PKA phosphorylation. Sci Adv. 2023, 9, eadf4936. [Google Scholar] [CrossRef]
- Gurrola, G.B.; Arévalo, C.; Sreekumar, R.; Lokuta, A.J.; Walker, J.W.; Valdivia, H.H. Activation of ryanodine receptors by imperatoxin A and a peptide segment of the II-III loop of the dihydropyridine receptor. J. Biol. Chem. 1999, 274, 7879–7886. [Google Scholar] [CrossRef]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001; p. 814. [Google Scholar]
- Verkman, A.; Galietta, L. Chloride channels as drug targets. Nat. Rev. Drug. Discov. 2009, 8, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Pusch, M. CLC chloride channels and transporters: Structure, function, physiology, and disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef] [PubMed]
- DeBin, J.A.; Strichartz, G.R. Chloride channel inhibition by the venom of the scorpion Leiurus quinquestriatus. Toxicon 1991, 29, 1403–1408. [Google Scholar] [CrossRef]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993, 264, C361–C369. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.H.; Fields, D.M.; Olivetti, P.R.; Fuller, M.D.; Zhang, Z.R.; Kubanek, J.; McCarty, N.A. Inhibition of ClC-2 chloride channels by a peptide component or components of scorpion venom. J. Membr. Biol. 2005, 208, 65–76. [Google Scholar] [CrossRef]
- Dalton, S.; Gerzanich, V.; Chen, M.; Dong, Y.; Shuba, Y.; Simardi, J.M. Chlorotoxin-sensitive Ca2+-activated Cl- channel in type R2 reactive astrocytes from adult rat brain. Glia 2003, 42, 325–339. [Google Scholar] [CrossRef]
- Maertens, C.; Wei, L.; Tytgat, J.; Droogmans, G.; Nilius, B. Chlorotoxin does not inhibit volume-regulated, calcium-activated and cyclic AMP-activated chloride channels. Br. J. Pharmacol. 2000, 129, 791–801. [Google Scholar] [CrossRef]
- Ni, M.-M.; Jie-Yu Sun, J.-Y.; Li, Z.Q.; Qiu, J.C.; Wu, C.-F. Role of voltage-gated chloride channels in epilepsy: Current insights and future directions. Front. Pharmacol. 2025, 16, 1560392. [Google Scholar] [CrossRef]
- Xu, X.; Duan, Z.; Di, Z.; He, Y.; Li, J.; Li, Z.; Xie, C.; Zeng, X.; Cao, Z.; Wu, Y.; et al. Proteomic analysis of the venom from the scorpion Mesobuthus martensii. J. Proteom. 2014, 106, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Mille, B.G.; Peigneur, S.; Predel, R.; Tytgat, J. Trancriptomic approach reveals the molecular diversity of Hottentotta conspersus (Buthidae) venom. Toxicon 2015, 99, 73–79. [Google Scholar] [CrossRef]
- Daoudi, K.; Malosse, C.; Lafnoune, A.; Darkaoui, B.; Chakir, S.; Sabatier, J.M.; Chamot-Rooke, J.; Cadi, R.; Oukkache, N. Mass spectrometry-based top-down and bottom-up approaches for proteomic analysis of the Moroccan Buthus occitanus scorpion venom. FEBS Open Biol. 2021, 11, 1867–1892. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Lomonte, B. Proteomic analysis and lethality of the venom of Aegaeobuthus nigrocinctus, a scorpion of medical significance in the Middle East. Acta Trop. 2024, 255, 107230. [Google Scholar] [CrossRef]
- Arzamasov, A.A.; Vassilevski, A.A.; Grishin, E.V. Chlorotoxin and related peptides: Short insect toxins from scorpion venom. Russ. J. Bioorg. Chem. 2014, 40, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.H.; Olivetti, P.R.; Fuller, M.D.; Freeman, C.S.; McMaster, D.; French, R.J.; Pohl, J.; Kubanek, J.; McCarty, N.A. Isolation and characterization of a high affinity peptide inhibitor of ClC-2 chloride channels. J. Biol. Chem. 2009, 284, 26051–26062. [Google Scholar] [CrossRef]
- Fuller, M.D.; Zhang, Z.R.; Cui, G.; Kubanek, J.; McCarty, N.A. Inhibition of CFTR channels by a peptide toxin of scorpion venom. Am. J. Physiol. Cell Physiol. 2004, 287, C1328–C1341. [Google Scholar] [CrossRef]
- Fuller, M.D.; Zhang, Z.R.; Cui, G.; McCarty, N.A. The block of CFTR by scorpion venom is state-dependent. Biophys. J. 2005, 89, 3960–3975. [Google Scholar] [CrossRef]
- Fuller, M.D.; Thompson, C.H.; Zhang, Z.R.; Freeman, C.S.; Schay, E.; Szakács, G.; Bakos, E.; Sarkadi, B.; McMaster, D.; French, R.J.; et al. State-dependent inhibition of cystic fibrosis transmembrane conductance regulator chloride channels by a novel peptide toxin. J. Biol. Chem. 2007, 282, 37545–37555. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.-L.; Mokrzycki, N.; Lippens, G.; Drobecq, H.; Sautière, P.; Hugues, M. Characterization of a family of scorpion toxins modulating Ca2+-activated Cl current in vascular myocytes. Toxins 2022, 14, 780. [Google Scholar] [CrossRef]
- Wu, J.J.; Dai, L.; Lan, Z.D.; Chi, C.W. The gene cloning and sequencing of Bm-12, a chlorotoxin-like peptide from the scorpion Buthus martensi Karsch. Toxicon 2000, 38, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Li, W.X.; Zhu, S.Y.; Peng, F.; Zhu, Z.H.; Wu, K.L.; Yiang, F.H. Cloning and characterization of a cDNA sequence encoding the precursor of a chlorotoxin-like peptide from the Chinese scorpion Buthus martensii Karsch. Toxicon 2000, 38, 1009–1014. [Google Scholar] [CrossRef]
- Diego-García, E.; Caliskan, F.; Tytgat, J. The Mediterranean scorpion Mesobuthus gibbosus (Scorpiones, Buthidae): Transcriptome analysis and organization of the genome encoding chlorotoxin-like peptides. BMC Genom. 2014, 15, 295. [Google Scholar] [CrossRef]
- Lippens, G.; Najib, J.; Wodak, S.J.; Tartar, A. NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels. Biochemistry 1995, 34, 13–21. [Google Scholar] [CrossRef]
- Rjeibi, I.; Mabrouk, K.; Mosrati, H.; Berenguer, C.; Mejdoub, H.; Villard, C.; Laffitte, D.; Bertin, D.; Ouafik, L.; Luis, J.; et al. Purification, synthesis and characterization of AaCtx, the first chlorotoxin-like peptide from Androctonus australis scorpion venom. Peptides 2011, 32, 656–663. [Google Scholar] [CrossRef]
- Ali, S.A.; Alam, M.; Abbasi, A.; Undheim, E.A.; Fry, B.G.; Kalbacher, H.; Voelter, W. Structure-activity relationship of chlorotoxin-like peptides. Toxins 2016, 8, 36. [Google Scholar] [CrossRef]
- Dhawan, R.D.; Joseph, S.; Sethi, A.; Lala, A.K. Purification and characterization of a short insect toxin from the venom of the scorpion Buthus tamulus. FEBS Lett. 2002, 528, 261–266. [Google Scholar] [CrossRef]
- Wudayagiri, R.; Inceoglu, B.; Herrmann, R.; Derbel, M.; Choudary, P.V.; Hammock, B.D. Isolation and characterization of a novel lepidopteran-selective toxin from the venom of South Indian red scorpion, Mesobuthus tamulus. BMC Biochem. 2001, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, M.; Jalali, A.; Soorki, M.N.; Jokar, M.; Galehdari, H. Three new scorpion chloride channel toxins as potential anti-cancer drugs: Computational prediction of the interactions with Hmmp-2 by docking and steered molecular dynamics simulations. Iran. J. Pharm. Res. 2019, 18, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Soorki, M.N.; Jalali, A.; Galehdari, H. Molecular characterization and biodiversity of a putative chlorotoxin from the Iranian yellow scorpion Odontobuthus doriae. Iran. Biomed. J. 2017, 21, 342–346. [Google Scholar] [CrossRef]
- Megaly, A.M.A.; Nakamichi, R.; Wakayu, M.; Nakagawa, Y.; Abdel-Wahab, M.; Miyashita, M. Identification of a novel insecticidal chlorotoxin-like peptide from the venom of the Compsobuthus egyptiensis scorpion. Toxicon 2025, 267, 108556. [Google Scholar] [CrossRef]
- Ojeda, P.G.; Wang, C.K.; Craik, D.J. Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Pept. Sci. 2016, 106, 25–36. [Google Scholar] [CrossRef]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar]
- Soroceanu, L.; Manning, T.J., Jr.; Sontheimer, H. Modulation of glioma cell migration and invasion using Cl- and K+ ion channel blockers. J. Neurosci. 1999, 19, 5942–5954. [Google Scholar] [CrossRef]
- Fu, Y.J.; Yin, L.T.; Liang, A.H.; Zhang, C.F.; Wang, W.; Chai, B.F.; Yang, J.Y.; Fan, X.J. Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci. Lett. 2007, 412, 62–67. [Google Scholar] [CrossRef]
- Fan, S.; Sun, Z.; Jiang, D.; Dai, C.; Ma, Y.; Zhao, Z.; Liu, H.; Wu, Y.; Cao, Z.; Li, W. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010, 291, 158–166. [Google Scholar] [CrossRef]
- Xiang, Y.; Liang, L.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J. Control. Release 2011, 152, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; An, N.; Li, K.; Zheng, Y.; Liang, A. Chlorotoxin-conjugated nanoparticles as potential glioma-targeted drugs. J. Neurooncol. 2012, 107, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Burks, S.R.; Frank, J.A. Chlorotoxin—A multimodal imaging platform for targeting glioma tumors. Toxins 2018, 10, 496. [Google Scholar] [CrossRef]
- Khanyile, S.; Masamba, P.; Oyinloye, B.E.; Mbatha, L.S.; Kappo, A.P. Current biochemical applications and future prospects of chlorotoxin in cancer diagnostics and therapeutics. Adv. Pharm. Bull. 2019, 9, 510–520. [Google Scholar] [CrossRef]
- de Paula, G.A.; de Paula, M.C.; Dutra, J.A.P.; Carvalho, S.G.; Di Filippo, L.D.; Villanova, J.C.O.; Chorilli, M. Targeted polymeric nanoparticles as a strategy for the treatment of glioblastoma: A review. Curr. Drug Deliv. 2025, 22, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Farkas, S.; Cioca, D.; Murányi, J.; Hornyák, P.; Brunyánszki, A.; Szekér, P.; Boros, E.; Horváth, P.; Hujber, Z.; Rácz, G.Z.; et al. Chlorotoxin binds to both matrix metalloproteinase 2 and neuropilin 1. J. Biol. Chem. 2023, 299, 104998. [Google Scholar] [CrossRef] [PubMed]
- Othman, H.; Wieninger, S.A.; ElAyeb, M.; Nilges, M.; Srairi-Abid, N. In silico prediction of the molecular basis of ClTx and AaCTx interaction with matrix metalloproteinase-2 (MMP-2) to inhibit glioma cell invasion. J. Biomol. Struct. Dyn. 2017, 35, 2815–2829. [Google Scholar] [CrossRef]
- Zeng, X.-C.; Corzo, G.; Hahin, R. Scorpion venom peptides without disulfide bridges. IUBMB Life 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Almaaytah, A.; Albalas, Q. Scorpion venom peptides with no disulfide bridges: A review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.B.; de Souza, B.M.; Cocchi, F.K.; Chalkidis, H.M.; Dorce, V.A.C.; Palma, M.S. Profiling the short, linear, non-disulfide bond-containing peptidome from the venom of the scorpion Tityus obscurus. J. Proteom. 2018, 170, 70–79. [Google Scholar] [CrossRef]
- Cassini-Vieira, P.; Felipetto, M.; Prado, L.B.; Verano-Braga, T.; Andrade, S.P.; Santos, R.A.S.; Teixeira, M.M.; de Lima, M.E.; Pimenta, A.M.C.; Barcelos, L.S. Ts14 from Tityus serrulatus boosts angiogenesis and attenuates inflammation and collagen deposition in sponge-induced granulation tissue in mice. Peptides 2017, 98, 63–69. [Google Scholar] [CrossRef]
- Goméz-Mendoza, D.P.; Lemos, R.P.; Jesus, I.C.G.; Gorshkov, V.; McKinnie, S.M.K.; Vederas, J.C.; Kjeldsen, F.; Guatimosim, S.; Santos, R.A.; Pimenta, A.M.C.; et al. Moving pieces in a cellular puzzle: A cryptic peptide from the scorpion toxin Ts14 activates AKT and ERK signaling and decreases cardiac myocyte contractility via dephosphorylation of phospholamban. J. Proteome Res. 2020, 19, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Zhang, L.; Nie, Y.; Luo, X. Identification and molecular characterization of three new K+-channel specific toxins from the Chinese scorpion Mesobuthus martensii Karsch revealing intronic number polymorphism and alternative splicing in duplicated genes. Peptides 2012, 34, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Venancio, E.J.; Portaro, F.C.; Kuniyoshi, A.K.; Carvalho, D.C.; Pidde-Queiroz, G.; Tambourgi, D.V. Enzymatic properties of venoms from Brazilian scorpions of Tityus genus and the neutralisation potential of therapeutical antivenoms. Toxicon 2013, 69, 180–190. [Google Scholar] [CrossRef]
- Mendoza-Tobar, L.L.; Clement, H.; Arenas, I.; Sepulveda-Arias, J.C.; Vargas, J.A.G.; Corzo, G. An overview of some enzymes from buthid scorpion venoms from Colombia: Centruroides margaritatus, Tityus pachyurus, and Tityus n. sp. aff. metuendus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230063. [Google Scholar] [CrossRef]
- de Oliveira, I.S.; Alano-da-Silva, N.M.; Ferreira, I.G.; Cerni, F.A.; Sachett, J.A.G.; Monteiro, W.M.; Pucca, M.B.; Arantes, E.C. Understanding the complexity of Tityus serrulatus venom: A focus on high molecular weight components. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230046. [Google Scholar] [CrossRef]
- Díaz, C.; Lomonte, B.; Chang-Castillo, A.; Bonilla, F.; Alfaro-Chinchilla, A.; Triana, F.; Angulo, D.; Fernández, J.; Sasa, M. Venomics of scorpion Ananteris platnicki (Lourenco, 1993), a New World buthid that inhabits Costa Rica and Panama. Toxins 2024, 16, 327. [Google Scholar] [CrossRef]
- Wiezel, G.A.; Oliveira, I.S.; Reis, M.B.; Ferreira, I.G.; Cordeiro, K.R.; Bordon, K.C.F.; Arantes, E.C. The complex repertoire of Tityus spp. venoms: Advances on their composition and pharmacological potential of their toxins. Biochimie 2024, 220, 144–166. [Google Scholar] [CrossRef]
- Das, B.; Patra, A.; Mukherjee, A.K. Correlation of venom toxinome composition of Indian red scorpion (Mesobuthus tamulus) with clinical manifestations of scorpion stings: Failure of commercial antivenom to immune-recognize the abundance of low molecular mass toxins of this venom. J. Proteome Res. 2020, 19, 1847–1856. [Google Scholar] [CrossRef]
- Guerra-Duarte, C.; Horta, C.C.R.; Oliveira-Mendes, B.B.R.; Magalhães, B.F.; Costal-Oliveira, F.; Stransky, S.; de Freitas, C.F.; Campolina, D.; Pardal, P.P.O.; Lira-da-Silva, R.; et al. Determination of hyaluronidase activity in Tityus spp. scorpion venoms and its inhibition by Brazilian antivenoms. Toxicon 2019, 167, 134–143. [Google Scholar] [CrossRef]
- Abreu, C.B.; Bordon, K.C.F.; Cerni, F.A.; Oliveira, I.S.; Balenzuela, C.; Alexandre-Silva, G.M.; Zoccal, K.F.; Reis, M.B.; Wiezel, G.A.; Peigneur, S.; et al. Pioneering study on Rhopalurus crassicauda scorpion venom: Isolation and characterization of the major toxin and hyaluronidase. Front. Immunol. 2020, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Velázquez, L.L.; Cid-Uribe, J.; Romero-Gutierrez, M.T.; Olamendi-Portugal, T.; Jimenez-Vargas, J.M.; Possani, L.D. Transcriptomic and proteomic analyses of the venom and venom glands of Centruroides hirsutipalpus, a dangerous scorpion from Mexico. Toxicon 2020, 179, 21–32. [Google Scholar] [CrossRef]
- Magalhães, A.C.M.; de Santana, C.J.C.; Melani, R.D.; Domont, G.B.; Castro, M.S.; Fontes, W.; Roepstorff, P.; Júnior, O.R.P. Exploring the biological activities and proteome of Brazilian scorpion Rhopalurus agamemnon venom. J. Proteom. 2021, 237, 104119. [Google Scholar] [CrossRef] [PubMed]
- Ghezellou, P.; Jakob, K.; Atashi, J.; Ghassempour, A.; Spengler, B. Mass-spectrometry-based lipidome and proteome profiling of Hottentotta saulcyi (Scorpiones: Buthidae) venom. Toxins 2022, 14, 370. [Google Scholar] [CrossRef]
- Silva de França, F.; Tambourgi, D.V. Hyaluronan breakdown by snake venom hyaluronidases: From toxins delivery to immunopathology. Front. Immunol. 2023, 14, 1125899. [Google Scholar] [CrossRef] [PubMed]
- Pessini, A.C.; Takao, T.T.; Cavalheiro, E.C.; Vichnewski, W.; Sampaio, S.V.; Giglio, J.R.; Arantes, E.C. A hyaluronidase from Tityus serrulatus scorpion venom: Isolation, characterization and inhibition by flavonoids. Toxicon 2001, 39, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Khan, N.; Niazi, Z.R.; Rehman, F.U.; Akhtar, A.; Khan, M.M.; Khan, S.; Baloch, N.; Khan, S. Hyaluronidases: A therapeutic enzyme. Protein Pept. Lett. 2018, 25, 663–676. [Google Scholar] [CrossRef]
- De Oliveira-Mendes, B.B.R.; Miranda, S.E.M.; Sales-Medina, D.F.; de Magalhães, B.F.; Kalapothakis, Y.; deSouza, R.P.; Cardoso, V.N.; de Barros, A.L.B.; Guerra-Duarte, C.; Kalapothakis, E.; et al. Inhibition of Tityus serrulatus venom hyaluronidase affects venom biodistribution. PLoS Negl. Trop. Dis. 2019, 13, e0007048. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Lomonte, B. Lys49 myotoxins, secreted phospholipase A2-like proteins of viperid venoms: A comprehensive review. Toxicon 2023, 224, 107024. [Google Scholar] [CrossRef]
- Sampat, G.H.; Hiremath, K.; Dodakallanavar, J.; Patil, V.S.; Harish, D.R.; Biradar, P.; Mahadevamurthy, R.K.; Barvaliya, M.; Roy, S. Unraveling snake venom phospholipase A2: An overview of its structure, pharmacology, and inhibitors. Pharmacol. Rep. 2023, 75, 1454–1473. [Google Scholar] [CrossRef]
- Zambelli, V.O.; Picolo, G.; Fernandes, C.A.H.; Fontes, M.R.M.; Cury, Y. Secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins 2017, 9, 406. [Google Scholar] [CrossRef]
- Mamede, C.C.N.; Simamoto, B.B.S.; Pereira, D.F.C.; Costa, J.O.; Ribeiro, M.S.M.; de Oliveira, F. Edema, hyperalgesia and myonecrosis induced by Brazilian bothropic venoms: Overview of the last decade. Toxicon 2020, 187, 10–18. [Google Scholar] [CrossRef]
- Moreira, V.; Leiguez, E.; Janovits, P.M.; Maia-Marques, R.; Fernandes, C.M.; Teixeira, C. Inflammatory effects of Bothrops phospholipases A2: Mechanisms involved in biosynthesis of lipid mediators and lipid accumulation. Toxins 2021, 13, 868. [Google Scholar] [CrossRef]
- Tonello, F. Secretory phospholipases A2, from snakebite envenoming to a myriad of inflammation associated human diseases—What is the secret of their activity? Int. J. Mol. Sci. 2023, 24, 1579. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Cardoso, F.F.; Lomonte, B.; Fontes, M.R.M. Inhibitors and activators for myotoxic phospholipase A2-like toxins from snake venoms—A structural overview. Biochimie 2024, 227, 231–247. [Google Scholar] [CrossRef]
- Almeida, F.M.; Pimenta, A.M.C.; De Figueiredo, S.G.; Santoro, M.M.; Martin-Eauclaire, M.F.; Diniz, C.R.; De Lima, M.E. Enzymes with gelatinolytic activity can be found in Tityus bahiensis and Tityus serrulatus venoms. Toxicon 2002, 40, 1041–1045. [Google Scholar] [CrossRef]
- Brazón, J.; Guerrero, B.; D’Suze, G.; Sevcik, C.; Arocha-Piñango, C.L. Fibrin(ogen)olytic enzymes in scorpion (Tityus discrepans) venom. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 168, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Gómez, S.; Gomez-Rave, L.; Vargas-Muñoz, L.J.; van der Meijden, A. Characterizing the biological and biochemical profile of six different scorpion venoms from the Buthidae and Scorpionidae family. Toxicon 2017, 130, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gutiérrez, M.T.; Santibáñez-López, C.E.; Jiménez-Vargas, J.M.; Batista, C.V.F.; Ortiz, E.; Possani, L.D. Transcriptomic and proteomic analyses reveal the diversity of venom components from the vaejovid scorpion Serradigitus gertschi. Toxins 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.M.; Sabatier, J.-M. Venoms of Iranian scorpions (Arachnida, Scorpiones) and their potential for drug discovery. Molecules 2019, 24, 2670. [Google Scholar] [CrossRef]
- Rojas-Azofeifa, D.; Sasa, M.; Lomonte, B.; Diego-García, E.; Ortiz, N.; Bonilla, F.; Murillo, R.; Tytgat, J.; Díaz, C. Biochemical characterization of the venom of Central American scorpion Didymocentrus krausi Francke, 1978 (Diplocentridae) and its toxic effects in vivo and in vitro. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 217, 54–67. [Google Scholar] [CrossRef]
- Rami, A.; Damizadeh, B.; Behdani, M.; Kazemi-Lomedasht, F. Insights into the evolutionary dynamics: Characterization of disintegrin and metalloproteinase proteins in the venom gland transcriptome of the Hemiscorpius lepturus scorpion. Protein Pept. Lett. 2024, 31, 639–656. [Google Scholar] [CrossRef]
- Salabi, F.; Jafari, H.; Mahdavinia, M.; Azadnasab, R.; Shariati, S.; Baghal, M.L.; Tebianian, M.; Baradaran, M. First transcriptome analysis of the venom glands of the scorpion Hottentotta zagrosensis (Scorpions: Buthidae) with focus on venom lipolysis activating peptides. Front. Pharmacol. 2024, 15, 1464648. [Google Scholar] [CrossRef] [PubMed]
- Alano-da-Silva, N.M.; Oliveira, I.S.; Cardoso, I.A.; Bordon, K.C.F.; Arantes, E.C. Exploring high molecular weight components in Tityus serrulatus venom. Toxicon 2025, 255, 108240. [Google Scholar] [CrossRef]
- Fletcher, P.L., Jr.; Fletcher, M.D.; Weninger, K.; Anderson, T.E.; Martin, B.M. Vesicle associated membrane protein (VAMP) cleavage by a new metalloprotease from the Brazilian scorpion Tityus serrulatus. J. Biol. Chem. 2010, 285, 7405–7416. [Google Scholar] [CrossRef] [PubMed]
- Zornetta, I.; Scorzeto, M.; Mendes dos Reis, P.V.; de Lima, M.E.; Montecucco, C.; Megighian, A.; Rossetto, O. Electrophysiological characterization of the antarease metalloprotease from Tityus serrulatus venom. Toxins 2017, 9, 81. [Google Scholar] [CrossRef]
- Carmo, A.O.; Oliveira-Mendes, B.B.R.; Horta, C.C.R.; Magalhães, B.F.; Dantas, A.E.; Chaves, L.M.; Chávez-Olórtegui, C.; Kalapothakis, E. Molecular and functional characterization of metalloserrulases, new metalloproteases from the Tityus serrulatus venom gland. Toxicon 2014, 90, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cajado-Carvalho, D.; Kuniyoshi, A.K.; Duzzi, B.; Iwai, L.K.; Oliveira, Ú.C.; Junqueira-de-Azevedo, I.L.; Kodama, R.T.; Portaro, F.V. Insights into the hypertensive effects of Tityus serrulatus scorpion venom: Purification of an angiotensin-converting enzyme-like peptidase. Toxins 2016, 8, 348. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef]
- Luo, P.; Ji, Y.; Liu, X.; Zhang, W.; Cheng, R.; Zhang, S.; Qian, X.; Huang, C. Affected inflammation-related signaling pathways in snake envenomation: A recent insight. Toxicon 2023, 234, 107288. [Google Scholar] [CrossRef]
- Rao, S.; Reghu, N.; Nair, B.G.; Vanuopadath, M. The role of snake venom proteins in inducing inflammation post-evenomation: An overview on mechanistic insights and treatment strategies. Toxins 2024, 16, 519. [Google Scholar] [CrossRef]
- Arias, A.S.; Rucavado, A.; Gutiérrez, J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon 2017, 132, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, P.A.; Romanelli, M.A.; Cesar, M.O.; Nogueira-Souza, P.D.; Monteiro-Machado, M.; Oliveira, S.S.C.; Santos, A.L.S.; Melo, P.A.; Lara, L.S. Doxycycline treatment reestablishes renal function of Wistar rats in experimental envenomation with Bothrops jararacussu venom. Toxicon 2021, 99, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Clare, R.H.; Dawson, C.A.; Westhorpe, A.; Albulescu, L.O.; Woodley, C.M.; Mosallam, N.; Chong, D.J.W.; Kool, J.; Berry, N.G.; O’Neill, P.M.; et al. Snakebite drug discovery: High-throughput screening to identify novel snake venom metalloproteinase toxin inhibitors. Front. Pharmacol. 2024, 14, 1328950. [Google Scholar] [CrossRef]
- Smith, C.F.; Modahl, C.M.; Galindo, D.C.; Larson, K.Y.; Maroney, S.P.; Bahrabadi, L.; Brandehoff, N.P.; Perry, B.W.; McCabe, M.C.; Petras, D.; et al. Assessing target specificity of the small molecule inhibitor MARIMASTAT to snake venom toxins: A novel application of thermal proteome profiling. Mol. Cell. Proteom. 2024, 23, 100779. [Google Scholar] [CrossRef] [PubMed]
- Arens, D.K.; Rose, M.A.; Salazar, E.M.; Harvey, M.A.; Huh, E.Y.; Ford, A.A.; Thompson, D.W.; Sanchez, E.E.; Hwang, Y.Y. Doxycycline-mediated inhibition of snake venom phospholipase and metalloproteinase. Mil. Med. 2024, 189, e2430–e2438. [Google Scholar] [CrossRef]
- Kadler, R.; Morrison, B.; Yanagihara, A.A. Assessing the utility of broad-acting inhibitors as therapeutics in diverse venoms. Toxins 2025, 17, 188. [Google Scholar] [CrossRef]
- Rudresha, G.V.; Khochare, S.; Casewell, N.R.; Sunagar, K. Preclinical evaluation of small molecule inhibitors as early intervention therapeutics against Russell’s viper envenoming in India. Commun. Med. 2025, 5, 226. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Casewell, N.R.; Laustsen, A.H. Progress and challenges in the field of snakebite envenoming therapeutics. Annu. Rev. Pharmacol. Toxicol. 2025, 6, 465–485. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Change 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Gula, L.T. Researchers Helping Protect Crops From Pests; The National Institute of Food and Agriculture. U.S. Department of Agriculture: Washington, DC, USA, 2023; pp. 1–5. Available online: https://www.nifa.usda.gov/about-nifa/blogs/researchers-helping-protect-crops-pests (accessed on 1 July 2005).
- Pu, J.; Chung, H. New and emerging mechanisms of insecticide resistance. Curr. Opin. Insect Sci. 2024, 63, 101184. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.E.; Campos, L.; Borges, B.T.; Santos, S.; Barreto, Y.C.; de Assis, D.R.; Hyslop, S.; de Souza, V.Q.; Vinadé, L.; Dal Belo, C.A. Fipronil affects cockroach behavior and olfactory memory. J. Exp. Biol. 2023, 226, jeb245239. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.E.; Oliveira, R.S.; Barbosa, R.F.; Hyslop, S.; Dal Belo, C.A. Recent advances on the influence of fipronil on insect behavior. Curr. Opin. Insect Sci. 2024, 65, 101251. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Rosa, M.E.; Rodrigues, L.G.S.; Dias, D.R.C.; Guimarães, M.P.; Valsecchi, C.; Queiroz de Souza, V.; Barbosa, R.F.; Vinadé, L.H.; Hyslop, S.; et al. Disruption of exploratory behavior and olfactory memory in cockroaches exposed to sublethal doses of the neonicotinoid thiamethoxam. Pestic. Biochem. Physiol. 2024, 205, 106167. [Google Scholar] [CrossRef]
- Whalon, M.; Mota-Sanchez, D.; Hollingworth, R. Analysis of global pesticide resistance in arthropods. In Global Pesticide Resistance in Arthropods; Whalon, M.E., Ed.; CABI: Wallingford, UK, 2008; pp. 5–31. [Google Scholar]
- Hafeez, M.; Li, X.; Zhang, Z.; Huang, J.; Wang, L.; Zhang, J.; Shah, S.; Khan, M.M.; Xu, F.; Fernández-Grandon, G.M.; et al. De novo transcriptomic analyses revealed some detoxification genes and related pathways responsive to Noposion Yihaogong® 5% EC (Lambda-Cyhalothrin 5%) exposure in Spodoptera frugiperda third-instar larvae. Insects 2021, 12, 132. [Google Scholar] [CrossRef]
- Kariyanna, B.; Sowjanya, M. Unravelling the use of artificial intelligence in management of insect pests. Smart Agric. Technol. 2024, 8, 100517. [Google Scholar] [CrossRef]
- Zlotkin, E. The insect voltage-gated sodium channel as target of insecticides. Annu. Rev. Entomol. 1999, 44, 429–455. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Omran, M.A.A.; Abdel-Nabi, I.M.; Nassier, O.A.; Schemerhorn, B.J. Neurotoxic and cytotoxic effects of venom from different populations of the Egyptian Scorpio maurus palmatus. Toxicon 2010, 55, 298–306. [Google Scholar] [CrossRef]
- Huang, L.; Xu, J.; Duan, K.; Bao, T.; Cheng, Y.; Zhang, H.; Zhang, Y.; Lin, Y.; Li, F. Scorpion venom heat-resistant peptide alleviates mitochondrial dynamics imbalance induced by PM2.5 exposure by downregulating the PGC-1α/SIRT3 signaling pathway. Toxicol. Res. 2023, 12, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.S.; Carvalho, E.L.; de Lima, J.C.; Breda, R.V.; Oliveira, R.S.; de Freitas, T.C.; Salamoni, S.D.; Domingues, M.F.; Piovesan, A.R.; Boldo, J.T.; et al. Bothriurus bonariensis scorpion venom activates voltage-dependent sodium channels in insect and mammalian nervous systems. Chem. Biol. Interact. 2016, 258, 1–9. [Google Scholar] [CrossRef]
- Deng, S.Q.; Chen, J.T.; Li, W.W.; Chen, M.; Peng, H.J. Application of the scorpion neurotoxin AaIT against insect pests. Int. J. Mol. Sci. 2019, 20, 3467. [Google Scholar] [CrossRef]
- Zilberberg, N.; Zlotkin, E.; Gurevitz, M. The cDNA sequence of a depressant insect selective neurotoxin from the scorpion Buthotus judaicus. Toxicon 1991, 29, 1155–1158. [Google Scholar] [CrossRef]
- Oren, D.A.; Froy, O.; Amit, E.; Kleinberger-Doron, N.; Gurevitz, M.; Shaanan, B. An excitatory scorpion toxin with a distinctive feature: An additional a helix at the C terminus and its implications for interaction with insect sodium channels. Structure 1998, 6, 1095–1103. [Google Scholar] [CrossRef]
- Zlotkin, E. Insect selective toxins derived from scorpion venoms: An approach to insect neuropharmacology. Insect Biochem. 1983, 13, 219–236. [Google Scholar] [CrossRef]
- Bermúdez-Guzmán, M.J.; Buenrostro-Nava, M.T.; Valdez-Velázquez, L.; Lino-López, G.J.; García-Villalvazo, P.E.; Orozco-Santos, M.; Michel-López, C.Y. Scorpion venom insectotoxins: A sustainable alternative for pest control in agriculture. Phytoparasitica 2024, 52, 83. [Google Scholar] [CrossRef]
- Riaz, N.; Zubair, F.; Amjad, N.; Ashraf, S.; Asghar, S.; Awan, M.Z.; Javaid, S. Acetylcholinesterase inhibitory potential of scorpion venom in Aedes aegypti (Diptera: Culicidae). Braz. J. Biol. 2022, 84, e259506. [Google Scholar] [CrossRef]
- Bosmans, F.; Tytgat, J. Voltage-gated sodium channel modulation by scorpion α-toxins. Toxicon 2007, 49, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Cestèle, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Pedraza Escalona, M.; Possani, L.D. Scorpion β-toxins and voltage-gated sodium channels: Interactions and effects. Front. Biosci. 2013, 18, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Zhorov, B.S.; Du, Y.; Song, W.; Luo, N.; Gordon, D.; Gurevitz, M.; Dong, K. Mapping the interaction surface of scorpion β-toxins with an insect sodium channel. Biochem. J. 2021, 478, 2843–2869. [Google Scholar] [CrossRef]
- Zlotkin, E.; Rochat, H.; Kopeyan, C.; Miranda, F.; Lissitzky, S. Purification and properties of the insect toxin from the venom of the scorpion Androctonus australis Hector. Biochimie 1971, 53, 1073–1078. [Google Scholar] [CrossRef]
- Zlotkin, E.; Fishman, Y.; Elazar, M. AaIT: From neurotoxin to insecticide. Biochimie 2000, 82, 869–881. [Google Scholar] [CrossRef]
- Darbon, H.; Weber, C.; Brawn, W. Two dimensional 1H nuclear magnetic resonance study of AaH IT, an anti-insect toxin from the scorpion Androctonus australis Hector. Sequential resonance assignments and folding of the polypeptide chain. Biochemistry 1991, 30, 1836–1844. [Google Scholar] [CrossRef]
- Ji, S.J.; Liu, F.; Li, E.Q.; Zhu, Y.X. Recombinant scorpion insectotoxin AaIT kills specifically insect cells but not human cells. Cell Res. 2002, 12, 143–150. [Google Scholar] [CrossRef]
- Valdez-Velázquez, L.L.; Romero-Gutierrez, M.T.; Delgado-Enciso, I.; Dobrovinskaya, O.; Melnikov, V.; Quintero-Hernández, V.; Ceballos-Magaña, S.G.; Gaitan-Hinojosa, M.A.; Coronas, F.I.; Puebla-Perez, A.M.; et al. Comprehensive analysis of venom from the scorpion Centruroides tecomanus reveals compounds with antimicrobial, cytotoxic, and insecticidal activities. Toxicon 2016, 118, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Guzmán, M.J.; Jiménez-Vargas, J.M.; Possani, L.D.; Zamudio, F.; Orozco-Gutiérrez, G.; Oceguera Contreras, E.; Enríquez-Vara, J.N.; Vazquez-Vuelvas, O.F.; García-Villalvazo, P.E.; Valdez-Velázquez, L.L. Biochemical characterization and insecticidal activity of isolated peptides from the venom of the scorpion Centruroides tecomanus. Toxicon 2022, 206, 90–102. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B.; Peigneur, S.; Tytgat, J. How a scorpion toxin selectively captures a prey sodium channel: The molecular and evolutionary basis uncovered. Mol. Biol. Evol. 2020, 37, 3149–3164. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Lee, Y.M.; Lehmberg, E.; Herrmann, R.; Herrmann, R.; Moskowitz, H.; Jones, A.D.; Hammock, B.D. Anti-insect toxin 5 (Aalts) from Androctonus australis. Eur. J. Biochem. 1997, 246, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Bel Haj Rhouma, R.; Cérutti-Duonor, M.; Benkhadir, K.; Goudey-Perrière, F.; El Ayeb, M.; Lopez-Ferber, M.; Karoui, H. Insecticidal effects of Buthus occitanus tunetanus BotIT6 toxin expressed in Escherichia coli and baculovirus/insect cells. J. Insect Physiol. 2005, 51, 1376–1383. [Google Scholar] [CrossRef]
- Liu, S.M.; Li, J.; Zhu, J.Q.; Wang, X.W.; Wang, C.S.; Liu, S.S.; Chen, X.X.; Li, S. Transgenic plants expressing the AaIT/GNA fusion protein show increased resistance and toxicity to both chewing and sucking pests. Insect Sci. 2016, 23, 265–276. [Google Scholar] [CrossRef]
- Coelho, V.A.; Cremonez, C.M.; Anjolette, F.A.; Aguiar, J.F.; Varanda, W.A.; Arantes, E.C. Functional and structural study comparing the C-terminal amidated β-neurotoxin Ts1 with its isoform Ts1-G isolated from Tityus serrulatus venom. Toxicon 2014, 83, 15–21. [Google Scholar] [CrossRef]
- Peigneur, S.; Cologna, C.T.; Cremonez, C.M.; Mille, B.G.; Pucca, M.B.; Cuypers, E.; Arantes, E.C.; Tytgat, J. A gamut of undiscovered electrophysiological effects produced by Tityus serrulatus toxin 1 on NaV-type isoforms. Neuropharmacology 2015, 95, 269–277. [Google Scholar] [CrossRef]
- Tibery, D.V.; Campos, L.A.; Mourão, C.B.F.; Peigneur, S.; Cruz e Carvalho, A.; Tytgat, J.; Schwartz, E.F. Electrophysiological characterization of Tityus obscurus β toxin 1 (To1) on Na+-channel isoforms. Biochim. Biophys. Acta Biomembr. 2019, 1861, 142–150. [Google Scholar] [CrossRef]
- Pimenta, A.M.; Martin-Eauclaire, M.; Rochat, H.; Figueiredo, S.G.; Kalapothakis, E.; Afonso, L.C.; De Lima, M.E. Purification, amino-acid sequence and partial characterization of two toxins with anti-insect activity from the venom of the South American scorpion Tityus bahiensis (Buthidae). Toxicon 2001, 39, 1009–1019. [Google Scholar] [CrossRef]
- Salazar, M.H.; Arenas, I.; Corrales-García, L.L.; Miranda, R.; Vélez, S.; Sánchez, J.; Mendoza, K.; Cleghorn, J.; Zamudio, F.Z.; Castillo, A.; et al. Venoms of Centruroides and Tityus species from Panama and their main toxic fractions. Toxicon 2018, 141, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.H.; Ortíz, M.H.; Encarnación, S.; Zamudio, F.; Possani, L.D.; Cleghorn, J.; Morán, M.; Acosta, H.; Corzo, G. A proteomic overview of the major venom components from Tityus championi from Panama. Toxicon 2023, 227, 107082. [Google Scholar] [CrossRef]
- Rincón-Cortés, C.A.; Olamendi-Portugal, T.; Carcamo-Noriega, E.N.; Santillán, E.G.; Zuñiga, F.Z.; Reyes-Montaño, E.A.; Castro, N.A.V.; Possani, L.D. Structural and functional characterization of toxic peptides purified from the venom of the Colombian scorpion Tityus macrochirus. Toxicon 2019, 169, 5–11. [Google Scholar] [CrossRef]
- Díaz, P.; D’Suze, G.; Salazar, V.; Sevcik, C.; Shannon, J.D.; Sherman, N.E.; Fox, J.W. Antibacterial activity of six novel peptides from Tityus discrepans scorpion venom. A fluorescent probe study of microbial membrane Na+ permeability changes. Toxicon 2009, 54, 802–817. [Google Scholar] [CrossRef]
- Diego-García, E.; Batista, C.V.; García-Gómez, B.I.; Lucas, S.; Candido, D.M.; Gómez-Lagunas, F.; Possani, L.D. The Brazilian scorpion Tityus costatus Karsch: Genes, peptides and function. Toxicon 2005, 45, 273–283. [Google Scholar] [CrossRef]
- Estrada-Gómez, S.; Vargas-Muñoz, L.J.; Saldarriaga-Córdoba, M.M.; van der Meijden, A. MS/MS analysis of four scorpion venoms from Colombia: A descriptive approach. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200173. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Vargas, J.A.; Mourão, C.B.F.; Quintero-Hernández, V.; Possani, L.D.; Schwartz, E.F. Identification and phylogenetic analysis of Tityus pachyurus and Tityus obscurus novel putative Na+-channel scorpion toxins. PLoS ONE 2012, 7, e30478. [Google Scholar] [CrossRef] [PubMed]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Herzig, V.; King, G.F.; Alewood, P.F. The insecticidal potential of venom peptides. Cell. Mol. Life. Sci. 2013, 70, 3665–3693. [Google Scholar] [CrossRef]
- Saez, N.J.; Herzig, V. Versatile spider venom peptides and their medical and agricultural applications. Toxicon 2019, 158, 109–126. [Google Scholar] [CrossRef]
- Fassolo, E.M.; Guo, S.; Wang, Y.; Rosa, S.; Herzig, V. Genetically encoded libraries and spider venoms as emerging sources for crop protective peptides. J. Pept. Sci. 2024, 30, e3600. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhang, K.; Wu, S. Functional diversity of sodium channel variants in common eastern bumblebee, Bombus impatiens. Arch. Insect Biochem. Physiol. 2023, 114, e22052. [Google Scholar] [CrossRef]
- Endersby-Harshman, N.M.; Schmidt, T.L.; Hoffmann, A.A. Diversity and distribution of sodium channel mutations in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2024, 61, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Alzabib, A.A.; Al-Sarar, A.S.; Abobakr, Y.; Saleh, A.A. Investigating the molecular mechanisms of deltamethrin resistance in Musca domestica populations from Saudi Arabia. Parasites Vectors 2025, 18, 55. [Google Scholar] [CrossRef]
- Mashlawi, A.M.; Al-Nazawi, A.M.; Noureldin, E.M.; Alqahtani, H.; Mahyoub, J.A.; Saingamsook, J.; Debboun, M.; Kaddumukasa, M.; Al-Mekhlafi, H.M.; Walton, C. Molecular analysis of knockdown resistance (kdr) mutations in the voltage-gated sodium channel gene of Aedes aegypti populations from Saudi Arabia. Parasites Vectors 2022, 15, 375. [Google Scholar] [CrossRef]
- Nascimento, G.J.; Cosme, L.V.; Torres, A.L.Q.; Lima, J.B.P.; Martins, A.J. High voltage-gated sodium channel gene diversity in Aedes albopictus across Brazil. Sci. Rep. 2025, 15, 20832. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wu, L.; Luo, C.; Guo, X.; Yang, R.; Zhang, Y. Population genetic structure and evolutionary genetics of Anopheles sinensis based on knockdown resistance (kdr) mutations and mtDNA-COII gene in China-Laos, Thailand-Laos, and Cambodia-Laos borders. Parasites Vectors 2022, 15, 229. [Google Scholar] [CrossRef]
- Zang, C.; Wang, X.; Cheng, P.; Liu, L.; Guo, X.; Wang, H.; Lou, Z.; Lei, J.; Wang, W.; Wang, Y.; et al. Evaluation of the evolutionary genetics and population structure of Culex pipiens pallens in Shandong province, China based on knockdown resistance (kdr) mutations and the mtDNA-COI gene. BMC Genom. 2023, 24, 145. [Google Scholar] [CrossRef]
- Seydi-Gazafi, K.; Seidi, S.; Ahmadabad, A.E.; Belkahia, H.; Tavassoli, M.; Said, M.B. Phylogenetic analysis and pyrethroid resistance mutation profiling of Haematopinus tuberculatus in buffaloes from Naqadeh, Iran. Parasitol. Int. 2025, 109, 103101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, K.; Wang, Z.; Xian, L.; Liu, K.; Wu, S. Functional diversity of voltage-gated sodium channel in Drosophila suzukii (Matsumura). Pest Manag. Sci. 2024, 80, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Prieto da Silva, A.R.B.; Pompeia, C.; Tytgat, J.; de Sá Junior, P.L. Toxin bioportides: Exploring toxin biological activity and multifunctionality. Cell. Mol. Life Sci. 2017, 74, 647–661. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Gláucia-Silva, F.; Rocha Soares, K.S.; de Souza, L.B.F.C.; Damasceno, I.Z.; Santos-Silva, E.D.; Lacerda, A.F.; Chaves, G.M.; Silva-Júnior, A.A.D.; Fernandes-Pedrosa, M.F. Biodegradable cross-linked chitosan nanoparticles improve anti-Candida and anti-biofilm activity of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109830. [Google Scholar] [CrossRef]
- Rebbouh, F.; Martin-Eauclaire, M.F.; Laraba-Djebari, F. Chitosan nanoparticles as a delivery platform for neurotoxin II from Androctonus australis hector scorpion venom: Assessment of toxicity and immunogenicity. Acta Trop. 2020, 205, 105353. [Google Scholar] [CrossRef]
- Gláucia-Silva, F.; Torres, J.V.P.; Torres-Rêgo, M.; Daniele-Silva, A.; Furtado, A.A.; Ferreira, S.S.; Chaves, G.M.; Xavier-Júnior, F.H.; Rocha Soares, K.S.; Silva-Júnior, A.A.D.; et al. . Tityus stigmurus-venom-loaded cross-linked chitosan nanoparticles improve antimicrobial activity. Int. J. Mol. Sci. 2024, 25, 9893. [Google Scholar] [CrossRef]
- Herzig, V.; Ahabh, A.; Jones, A.; King, G.F. Shining a light on the photochemical stability of peptidic bioinsecticides. Toxicon 2025, 262, 108381. [Google Scholar] [CrossRef]
- Xue, Q.; Swevers, L.; Taning, C.N.T. Drosophila X virus-like particles as delivery carriers for improved oral insecticidal efficacy of scorpion Androctonus australis peptide against the invasive fruit fly, Drosophila suzukii. Insect Sci. 2024, 31, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Bilgo, E.; Lovett, B.; Fang, W.; Bende, N.; King, G.F.; Diabate, A.; St Leger, R.J. Improved efficacy of an arthropod toxin expressing fungus against insecticide-resistant malaria-vector mosquitoes. Sci. Rep. 2017, 7, 3433. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Suroshe, S.S.; Yadav, P.R.; Kumar, A.; Saini, G.K. Bioefficacy of engineered Beauveria bassiana with scorpion neurotoxin, LqqIT1 against cotton mealybug, Phenacoccus solenopsis and cowpea aphid, Aphis craccivora. Peer J. 2023, 11, e16030. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, E.; Fishman, L.; Shapiro, J.P. Oral toxicity to flesh flies of a neurotoxic polypeptide. Arch. Insect Biochem. Physiol. 1992, 21, 41–52. [Google Scholar] [CrossRef]
- Wu, J.; Luo, X.; Wang, Z.; Tian, Y.; Liang, A.; Sun, Y. Transgenic cotton expressing synthesized scorpion insect toxin AaHIT gene confers enhanced resistance to cotton bollworm (Heliothis armigera) larvae. Biotechnol. Lett. 2008, 30, 547–554. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Du, J.; Sun, Y.; Liang, A. Novel insect resistance in Brassica napus developed by transformation of chitinase and scorpion toxin genes. Plant Cell Rep. 2005, 24, 549–555. [Google Scholar] [CrossRef]
- Yao, B.; Fan, Y.; Zeng, Q.; Zhao, R. Insect-resistant tobacco plants expressing insect-specific neurotoxin AaIT. Chin. J. Biotechnol. 1996, 12, 67–72. [Google Scholar]
- Trung, N.P.; Fitches, E.; Gatehouse, J.A. A fusion protein containing a lepidopteran-specific toxin from the South Indian red scorpion (Mesobuthus tamulus) and snowdrop lectin shows oral toxicity to target insects. BMC Biotechnol. 2006, 6, 18. [Google Scholar] [CrossRef]
- Fitches, E.C.; Bell, H.A.; Powell, M.E.; Back, E.; Sargiotti, C.; Weaver, R.J.; Gatehouse, J.A. Insecticidal activity of scorpion toxin (ButaIT) and snowdrop lectin (GNA) containing fusion proteins towards pest species of different orders. Pest Manag. Sci. 2010, 6, 74–83. [Google Scholar] [CrossRef]
- Nezamiha, F.K.; Imani, S.; Mianroodi, R.A.; Tirgari, S.; Shahbazzadeh, D. OdTx12/GNA, a chimeric variant of a β excitatory toxin from Odontobuthus doriae, reveals oral toxicity towards Locusta migratoria and Tenebrio molitor. Toxicon 2021, 202, 13–19. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Chen, J.; Xia, Y. The fusion protein of scorpion neurotoxin BjαIT and Galanthus nivalis agglutinin (GNA) enhanced the injection insecticidal activity against silkworms, but only has lethal activity against newly hatched larva when administered orally. World J. Microbiol. Biotechnol. 2024, 40, 326. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Chen, J.; Xia, Y. Scorpion insect neurotoxin LqhIT2 is a promising oral biopesticide: High-level preparation in Pichia pastoris and bioactivity assays. Pest Manag. Sci. 2025, 81, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- Tobassum, S.; Tahir, H.M.; Arshad, M.; Zahid, M.T.; Ali, S.; Ahsan, M.M. Nature and applications of scorpion venom: An overview. Toxin Rev. 2020, 39, 214–225. [Google Scholar] [CrossRef]
- Koç, H.; Avşar, C.; Bayrakci, Y. Antimicrobial activity of hemolymph and venom obtained from some scorpion species. Hacet. J. Biol. Chem. 2020, 48, 349–354. [Google Scholar] [CrossRef]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: Structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins 2015, 7, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Zhou, M.; Wang, L.; Chen, T.; Walker, B.; Shaw, C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: Biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides 2012, 35, 291–299. [Google Scholar] [CrossRef]
- Song, C.; Wen, R.; Zhou, J.; Zeng, X.; Kou, Z.; Zhang, J.; Wang, T.; Chang, P.; Lv, Y.; Wu, R. Antibacterial and antifungal properties of a novel antimicrobial Peptide GK-19 and its application in skin and soft tissue infections induced by MRSA or Candida albicans. Pharmaceutics 2022, 14, 1937. [Google Scholar] [CrossRef]
- Zerouti, K.; Khemili, D.; Laraba-Djebari, F.; Hammoudi-Triki, D. Nontoxic fraction of scorpion venom reduces bacterial growth and inflammatory response in a mouse model of infection. Toxin Rev. 2021, 40, 310–324. [Google Scholar] [CrossRef]
- Alajmi, R.; Al-ghamdi, S.; Barakat, I.; Mahmoud, A.; Abdon, N.; Al-Ahidib, M.; AbdelGaber, R. Antimicrobial activity of two novel venoms from Saudi Arabian scorpions (Leiurus quinquestriatus and Androctonus crassicauda). Int. J. Pept. Res. Therapeut. 2020, 26, 67–74. [Google Scholar] [CrossRef]
- Salama, W.; Geasa, N. Investigation of the antimicrobial and hemolytic activity of venom of some Egyptian scorpion. J. Microbiol. Antimicrob. 2014, 6, 21–28. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Alamri, M.A.; Almasoudi, A.S.; Abbasmanthiri, R.; Mahfoud, M. Evaluation of the in vitro antimicrobial activity of selected Saudi scorpion venoms tested against multidrug-resistant micro-organisms. J. Glob. Antimicrob. Resist. 2017, 10, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, E.; Zargan, J. Investigating the antibacterial effects of 3 novel peptides isolated from the venom of Iranian Odontobuthus doriae and Buthotus saulcyi scorpions on Escherichia coli (UTI89) and Enterococcus faecalis causing urinary tract infection. Int. J. Pept. Res. Ther. 2023, 29, 55. [Google Scholar] [CrossRef]
- Conde, R.; Zamudio, F.Z.; Rodríguez, M.H.; Possani, L.D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.M.; Bertelsen, M.; Abdel-Rahman, M.A.; Strong, P.N.; Miller, K. Scorpion venom antimicrobial peptides induce siderophore biosynthesis and oxidative stress responses in Escherichia coli. mSphere 2021, 6, e00267-21. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Strong, P.N.; Tawfik, M.M.; Miller, K. Characterisation of three α-helical antimicrobial peptides from the venom of Scorpio maurus palmatus. Toxicon 2016, 117, 30–36. [Google Scholar] [CrossRef]
- Gagandeep, K.R.; Narasingappa, R.B.; Vyas, G.V. Unveiling mechanisms of antimicrobial peptide: Actions beyond the membranes disruption. Heliyon 2024, 10, e38079. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, Y.; Qu, D.; Xie, Z.; Guo, X.; Zhu, Z.; Chen, Z.; Zhang, L.; Li, W.; Cao, Z.; et al. Ion channel modulation by scorpion hemolymph and its defensin ingredients highlights origin of neurotoxins in telson formed in Paleozoic scorpions. Int. J. Biol. Macromol. 2020, 148, 351–363. [Google Scholar] [CrossRef]
- Di, Z.; Qiao, S.; Liu, X.; Xiao, S.; Lei, C.; Li, Y.; Li, S.; Zhang, F. Transcriptome sequencing and comparison of venom glands revealed intraspecific differentiation and expression characteristics of toxin and defensin genes in Mesobuthus martensii populations. Toxins 2022, 14, 630. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, A.; Mekni-Toujani, M.; Ferchichi, A.; Gharsallah, C.; Malosse, C.; Chamot-Rooke, J.; ElAyeb, M.; Ghram, A.; Srairi-Abid, N.; Daoud, S. BotCl, the first chlorotoxin-like peptide inhibiting Newcastle disease virus: The emergence of a new scorpion venom AMPs family. Molecules 2023, 28, 4355. [Google Scholar] [CrossRef]
- Mahnam, K.; Lotfi, M.; Shapoorabadi, F.A. Examining the interactions scorpion venom peptides (HP1090, Meucin-13, and Meucin-18) with the receptor binding domain of the coronavirus spike protein to design a mutated therapeutic peptide. J. Mol. Graph. Model. 2021, 107, 107952. [Google Scholar] [CrossRef]
- Soorki, M.N. In silico antiviral effect assessment of some venom gland peptides from Odontobuthus doriae scorpion against SARS-CoV-2. Toxicon 2025, 255, 108229. [Google Scholar] [CrossRef]
- El-Bitar, A.M.H.; Sarhan, M.M.H.; Aoki, C.; Takahara, Y.; Komoto, M.; Deng, L.; Moustafa, M.A.; Hotta, H. Virocidal activity of Egyptian scorpion venoms against hepatitis C virus. Virol. J. 2015, 12, 47. [Google Scholar] [CrossRef]
- El-Bitar, A.M.H.; Sarhan, M.; Abdel-Rahman, M.A.; Quintero-Hernandez, V.; AokiUtsubo, C.; Moustafa, M.A.; Possani, L.D.; Hotta, H. Smp76, a scorpine-like peptide isolated from the venom of the scorpion Scorpio maurus palmatus, with a potent antiviral activity against hepatitis C virus and dengue virus. Int. J. Pept. Res. Therapeut. 2020, 26, 811–821. [Google Scholar] [CrossRef]
- Keikha, M.; Kamali, H.; Ghazvini, K.; Karbalaei, M. Antimicrobial peptides: Natural or synthetic defense peptides against HBV and HCV infections. Virusdisease 2022, 33, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Agbadamashi, D.J.; Price, C.L. Novel strategies for preventing fungal infections—Outline. Pathogens 2025, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Guilhelmelli, F.; Vilela, N.; Smidt, K.S.; de Oliveira, M.A.; Álvares, A.C.M.; Rigonatto, M.C.; Costa, P.H.S.; Tavares, A.H.; de Freitas, S.M.; Nicola, A.M.; et al. Activity of scorpion venom-derived antifungal peptides against planktonic cells of Candida spp. and Cryptococcus neoformans and Candida albicans biofilms. Front. Microbiol. 2016, 7, 1844. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Kang, D.D.; Park, Y. Exploring the therapeutic potential of scorpion-derived Css54 peptide against Candida albicans. J. Microbiol. 2024, 62, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ehret-Sabatier, L.; Loew, D.; Goyffon, M.; Fehlbaum, P.; Hoffmann, J.A.; van Dorsselaer, A.; Bulet, P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol. Chem. 1996, 271, 29537–29544. [Google Scholar] [CrossRef]
- Rostamkolaie, L.K.; Hamidinejat, H.; Jalali, M.H.R.; Varzi, H.N.; Abadshapouri, M.R.S.; Jafari, H. Inhibitory effect of Hemiscorpius lepturus scorpion venom fractions on tachyzoites of Toxoplasma gondii. Iran. J. Parasitol. 2022, 17, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, A.; Anwar, F.A.S.; Mohamed, S.A.-A.; Abdelsater, N. Antihelimentic effect of Androctonus crassicauda scorpion venom against Trichuris arvicolae isolated from Psammomys obesus in Egypt. Saudi J. Biol. Sci. 2023, 30, 103713. [Google Scholar] [CrossRef]
- Al-Malki, E.S.; Abdelsater, N. In vitro scolicidal effects of Androctonus crassicauda (Olivier, 1807) venom against the protoscolices of Echinococcus granulosus. Saudi J. Biol. Sci. 2020, 27, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Xu, J.; Rodriguez, M.C.; Lanz-Mendoza, H.; Hernandez-Rivas, R.; Du, W.; Zhu, S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 2010, 92, 350–359. [Google Scholar] [CrossRef]
- Jafari, H.; Nemati, M.; Molayan, P.H.; Rostamkolaie, L.K.; Nejat, H. Scolicidal activity of Mesobuthus eupeus venom against the protoscolices of Echinococcus granulosus. Arch. Razi Inst. 2019, 74, 183–189. [Google Scholar] [CrossRef]
- Casella-Martins, A.; Ayres, L.R.; Burin, S.M.; Morais, F.R.; Pereira, J.C.; Faccioli, L.H.; Sampaio, S.V.; Arantes, E.C.; Castro, F.A.; Pereira-Crott, L.S. Immunomodulatory activity of Tityus serrulatus scorpion venom on human T lymphocytes. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 46. [Google Scholar] [CrossRef]
- Chiang, E.Y.; Li, T.; Jeet, S.; Peng, I.; Zhang, J.; Lee, W.P.; DeVoss, J.; Caplazi, P.; Chen, J.; Warming, S.; et al. Potassium channels Kv1.3 and KCa3.1 cooperatively and compensatorily regulate antigen-specific memory T cell functions. Nat. Commun. 2017, 8, 14644. [Google Scholar] [CrossRef]
- Varga, Z.; Gurrola-Briones, G.; Papp, F.; Rodríguez de la Veja, R.C.; Pedraza-Alva, G.; Tajhya, R.B.; Gaspar, R.; Cardenas, L.; Rosenstein, Y.; Beeton, C.; et al. Vm24, a natural immunosuppressant peptide potently and selectively blocks Kv1.3 potassium channels of human T cells. Mol. Pharmacol. 2012, 82, 372–382. [Google Scholar] [CrossRef]
- Balozet, L. Scorpionism in the Old World. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E., Eds.; Academic Press: New York, NY, USA, 1971; Volume 3, pp. 349–371. [Google Scholar]
- Bouafir, Y.; Ait-Lounis, A.; Laraba-Djebari, F. Improvement of function and survival of pancreatic beta-cells in streptozotocin-induced diabetic model by the scorpion venom fraction F1. Toxin Rev. 2017, 36, 101–108. [Google Scholar] [CrossRef][Green Version]
- Salama, W.M.; El-Naggar, S.A.; Tabl, G.A.E.; El Shefiey, L.M.M.; El-Desouki, N.I. Treatment with Leiurus quinquestratus scorpion venom ameliorates the histopathological changes of type-2 diabetic rats’ splenic tissues. J. Biosci. Appl. Res. 2023, 9, 356–365. [Google Scholar] [CrossRef]
- Salama, W.M.; El-Naggar, S.A.; Tabl, G.A.; El-Desouki, N.I.; El Shefiey, L.M. Leiurus quinquestratus venom promotes beta islets regeneration and restores glucose level in streptozotocin induced type 2 diabetes mellitus in rats. Sci. Rep. 2025, 15, 11841. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bharadvaja, N. Venom-derived bioactive compounds as potential anticancer agents: A review. Int. J. Pept. Res. Ther. 2021, 27, 129–147. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Lafnoune, A.; Chakir, S.; Darkaoui, B.; Cadi, R.; Oukkache, N. Moroccan Naja haje venom and its peptides: In vivo toxicity and in vitro antiproliferative effect on hepatocellular carcinoma HepG2 cells. Int. J. Pept. Res. Ther. 2024, 30, 62. [Google Scholar] [CrossRef]
- Lafnoune, A.; Chbel, A.; Darkaoui, B.; Irahal, I.N.; Oukkache, N. Cobra venom: From envenomation syndromes to therapeutic innovations. Int. J. Pept. Res. Ther. 2024, 30, 71. [Google Scholar] [CrossRef]
- Mlayah-Bellalouna, S.; Aissaoui-Zid, D.; Chantome, A.; Jebali, J.; Souid, S.; Ayedi, E.; Mejdoub, H.; Belghazi, M.; Marrakchi, N.; Essafi-Benkhadir, K.; et al. Insights into the mechanisms governing P01 scorpion toxin effect against U87 glioblastoma cells oncogenesis. Front. Pharmacol. 2023, 14, 1203247. [Google Scholar] [CrossRef]
- Aissaoui-Zid, D.; Saada, M.C.; Moslah, W.; Potier-Cartereau, M.; Lemettre, A.; Othman, H.; Gaysinski, M.; Abdelkafi-Koubaa, Z.; Souid, S.; Marrakchi, N.; et al. AaTs-1: A tetrapeptide from Androctonus australis scorpion venom, inhibiting U87 glioblastoma cells proliferation by p53 and FPRL-1 up-regulations. Molecules 2021, 26, 7610. [Google Scholar] [CrossRef]
- Moslah, W.; Aissaoui-Zid, D.; Aboudou, S.; Abdelkafi-Koubaa, Z.; Potier-Cartereau, M.; Lemettre, A.; ELBini-Dhouib, I.; Marrakchi, N.; Gigmes, D.; Vandier, C.; et al. Strengthening anti-glioblastoma effect by multi-branched dendrimers design of a scorpion venom tetrapeptide. Molecules 2022, 27, 806. [Google Scholar] [CrossRef]
- Béchohra, L.; Laraba-Djebari, F.; Hammoudi-Triki, D. Cytotoxic activity of Androctonus australis hector venom and its toxic fractions on human lung cancer cell line. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 29. [Google Scholar] [CrossRef]
- Issaad, N.; Ait-Lounis, A.; Laraba-Djebari, F. Cytotoxicity and actin cytoskeleton damage induced in human alveolar epithelial cells by Androctonus australis hector venom. Toxin Rev. 2018, 37, 67–74. [Google Scholar] [CrossRef]
- Caliskan, F.; Ergene, E.; Sogut, I.; Hatipoglu, I.; Basalp, A.; Sivas, H.; Kanbak, G. Biological assays on the effects of Acra3 peptide from Turkish scorpion Androctonus crassicauda venom on a mouse brain tumor cell line (BC3H1) and production of specific monoclonal antibodies. Toxicon 2013, 76, 350–361. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion venom causes upregulation of p53 and downregulation of Bcl-xL and BID protein expression by modulating signaling proteins Erk1/2 and STAT3, and DNA damage in breast and colorectal cancer cell lines. Integr. Cancer Ther. 2018, 17, 271–281. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. J. Evid. Based Integr. Med. 2018, 23, 2156587217751796. [Google Scholar] [CrossRef]
- Lafnoune, A.; Lee, S.-Y.; Heo, J.-Y.; Daoudi, K.; Darkaoui, B.; Chakir, S.; Cadi, R.; Mounaji, K.; Shum, D.; Seo, H.-R.; et al. Anti-cancer activity of Buthus occitanus venom on hepatocellular carcinoma in 3D cell culture. Molecules 2022, 27, 2219. [Google Scholar] [CrossRef]
- Khamessi, O.; Ben Mabrouk, H.; ElFessi-Magouri, R.; Kharrat, R. RK1, the first very short peptide from Buthus occitanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem. Biophys. Res. Commun. 2018, 499, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kayhan, N.Y.; Ocal, I.Ç.; Akdeniz, M. Investigation the cytotoxic and antiproliferative effects of crude venom of Euscorpius mingrelicus (Scorpiones: Euscorpiidae) scorpion. Eur. J. Biol. 2022, 81, 117–124. [Google Scholar] [CrossRef]
- Rezaei, A.; Asgari, S.; Komijani, S.; Sadat, S.N.; Sabatier, J.M.; Nasrabadi, D.; Pooshang Bagheri, K.; Shahbazzadeh, D.; Akbari Eidgahi, M.R.; De Waard, M.; et al. Discovery of leptulipin, a new anticancer protein from the Iranian scorpion, Hemiscorpius lepturus. Molecules 2022, 27, 2056. [Google Scholar] [CrossRef] [PubMed]
- Nosouhian, M.; Rastegari, A.A.; Shahanipour, K.; Ahadi, A.M.; Sajjadieh, M.S. Anticancer potentiality of Hottentotta saulcyi scorpion curd venom against breast cancer: An in vitro and in vivo study. Sci. Rep. 2024, 14, 24607. [Google Scholar] [CrossRef] [PubMed]
- Dezianian, S.; Zargan, J.; Goudarzi, H.R.; Noormohamadi, A.H.; Mousavi, M.; Alikhani, H.K.; Johari, B. In vitro study of Hottentotta schach crude venom anticancer effects on MCF-7 and Vero cell lines. Iran. J. Pharm. Res. 2020, 19, 192–202. [Google Scholar] [CrossRef]
- Zidan, S.A.; Ghany, S.A.; Abdel-Hakeem, M.A. Leiurus quinquestriatus venom gold nanoparticle conjugates: A novel approach for modulating ion channel gene expression in liver cancer. Egypt. J. Basic Appl. Sci. 2025, 12, 1–17. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma multiforme targeted therapy: The chlorotoxin story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Badr-Eldin, S.M.; Caruso, G.; Fahmy, U.A.; Alharbi, W.S.; Almehmady, A.M.; Alghamdi, S.A.; Alhakamy, N.A.; Mohamed, A.I.; Aldawsari, H.M.; et al. Colon targeted Eudragit coated beads loaded with optimized fluvastatin-scorpion venom conjugate as a potential approach for colon cancer therapy: In vitro anticancer activity and in vivo colon imaging. J. Pharmaceut. Sci. 2022, 111, 3304–3317. [Google Scholar] [CrossRef]
- Asfour, H.Z.; Fahmy, U.A.; Alharbi, W.S.; Almehmady, A.M.; Alamoudi, A.J.; Tima, S.; Mansouri, R.A.; Omar, U.M.; Ahmed, O.A.A.; Zakai, S.A.; et al. Phyto-phospholipid conjugated scorpion venom nanovesicles as promising carrier that improves efficacy of thymoquinone against adenocarcinoma human alveolar basal epithelial cells. Pharmaceutics 2021, 13, 2144. [Google Scholar] [CrossRef]
- Gandomkari, M.S.; Ayat, H.; Ahadi, A.M. Recombinantly expressed MeICT, a new toxin from Mesobuthus eupeus scorpion, inhibits glioma cell proliferation and downregulates Annexin A2 and FOXM1 genes. Biotechnol. Lett. 2022, 44, 703–712. [Google Scholar] [CrossRef]
- Alikhani, H.K.; Bidmeshkipour, A.; Zargan, J. Cytotoxic and apoptotic induction effects of the venom of Iranian scorpion (Odontobuthus bidentatus) in the hepatocellular carcinoma cell line (HepG2). Int. J. Pept. Res. Therapeut. 2020, 26, 2475–2484. [Google Scholar] [CrossRef]
- Alikhani, H.K.; Zargan, J.; Bidmeshkipour, A.; Zamani, E.; Mosavi, M.; Heidari, A.; Hajinoormohammadi, A. Iranian scorpion (Odontobuthus bidentatus) crude venom change the redox potential of MCF-7 breast cancer cell line and induce apoptosis. Mod. Med. Lab. J. 2021, 4, 28–35. [Google Scholar] [CrossRef]
- Zargan, J.; Sajad, M.; Umar, S.; Naime, M.; Ali, S.; Khan, H.A. Scorpion (Odontobuthus doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol. Cell. Biochem. 2011, 348, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zargan, J.; Umar, S.; Sajad, M.; Naime, M.; Ali, S.; Khan, H.A. Scorpion venom (Odontobuthus doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7). Toxicol. In Vitro 2011, 25, 1748–1756. [Google Scholar] [CrossRef]
- Dogan, T.; Igci, N.; Biber, A.; Gerekci, S.; Husnugil, H.; Izbirak, A.; Ozen, C. Peptidomic characterization and bioactivity of Protoiurus kraepelini (Scorpiones: Iuridae) venom. Turk. J. Biol. 2018, 42, 490–497. [Google Scholar] [CrossRef]
- Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion peptide Smp24 exhibits a potent antitumor effect on human lung cancer cells by damaging the membrane and cytoskeleton in vivo and in vitro. Toxins 2022, 14, 438. [Google Scholar] [CrossRef]
- Nguyen, T.; Guo, R.; Chai, J.; Wu, J.; Liu, J.; Chen, X.; Abdel-Rahman, M.A.; Xia, H.; Xu, X. Smp24, a scorpion-venom peptide, exhibits potent antitumor effects against hepatoma HepG2 cells via multi-mechanisms in vivo and in vitro. Toxins 2022, 14, 717. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, Y.; Nguyen, T.; Chai, J.; Wu, J.; Li, J.; Abdel-Rahman, M.A.; Xu, X.; Chen, X. The potent antitumor activity of Smp43 against non-small-cell lung cancer A549 cells via inducing membranolysis and mitochondrial dysfunction. Toxins 2023, 15, 347. [Google Scholar] [CrossRef]
- Chai, J.; Yang, W.; Gao, Y.; Guo, R.; Peng, Q.; Abdel-Rahman, M.A.; Xu, X. Antitumor effects of scorpion peptide Smp43 through mitochondrial dysfunction and membrane disruption on hepatocellular carcinoma. J. Nat. Prod. 2021, 84, 3147–3160. [Google Scholar] [CrossRef]
- Bucaretchi, F.; Fernandes, L.C.; Fernandes, C.B.; Branco, M.M.; Prado, C.C.; Vieira, R.J.; De Capitani, E.M.; Hyslop, S. Clinical consequences of Tityus bahiensis and Tityus serrulatus scorpion stings in the region of Campinas, southeastern Brazil. Toxicon 2014, 89, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, C.; Gnanathasan, A. Management of scorpion envenoming: A systematic review and meta-analysis of controlled clinical trials. Syst. Rev. 2017, 6, 74. [Google Scholar] [CrossRef]
- Amr, Z.S.; Abu Baker, M.A.; Al-Saraireh, M.; Warrell, D.A. Scorpions and scorpion sting envenoming (scorpionism) in the Arab countries of the Middle East. Toxicon 2021, 191, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Vaucel, J.A.; Larréché, S.; Paradis, C.; Courtois, A.; Pujo, J.M.; Elenga, N.; Résière, D.; Caré, W.; de Haro, L.; Gallart, J.C.; et al. French scorpionism (mainland and oversea territories): Narrative review of scorpion species, scorpion venom, and envenoming management. Toxins 2022, 14, 719. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.; Erickson, A.; Brierley, S.M.; Vetter, I. Pain-causing venom peptides: Insights into sensory neuron pharmacology. Toxins 2017, 10, 15. [Google Scholar] [CrossRef]
- Diochot, S. Pain-related toxins in scorpion and spider venoms: A face to face with ion channels. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20210026. [Google Scholar] [CrossRef]
- Geron, M.; Hazan, A.; Priel, A. Animal toxins providing insights into TRPV1 activation mechanism. Toxins 2017, 9, 326. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Manchope, M.F.; Bertozzi, M.M.; Saraiva-Santos, T.; Andrade, K.C.; Franciosi, A.; Zaninelli, T.H.; Bagatim-Souza, J.; Borghi, S.M.; Cândido, D.M.; et al. Tityus serrulatus scorpion venom-induced nociceptive responses depend on TRPV1, immune cells, and pro-inflammatory cytokines. Toxins 2025, 17, 332. [Google Scholar] [CrossRef]
- Haddad, L.; Chender, A.; Roufayel, R.; Accary, C.; Borges, A.; Sabatier, J.M.; Fajloun, Z.; Karam, M. Effect of Hottentotta judaicus scorpion venom on nociceptive response and inflammatory cytokines in mice using experimental hyperalgesia. Molecules 2025, 30, 2750. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, J.; Liu, H.; Sun, J.; Yu, Y.; Su, Y.; Cui, Y.; Zhao, M.; Zhang, J. Scorpion toxins targeting voltage-gated sodium channels associated with pain. Curr. Pharm. Biotechnol. 2018, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.F.M.; Cavalcante, J.S.; Angstmam, D.G.; Almeida, C.; Soares, G.S.; Pucca, M.B.; Ferreira Junior, R.S. Unveiling the pain relief potential: Harnessing analgesic peptides from animal venoms. Pharmaceutics 2023, 15, 2766. [Google Scholar] [CrossRef]
- Bagheri-Ziari, S.; Bagheri, K.P. From discovery to the future medical applications of venom-derived analgesic peptides for the treatment of peripheral pains. Curr. Pharm. Des. 2025; in press. [Google Scholar] [CrossRef]
- Wang, D.; Herzig, V.; Dekan, Z.; Rosengren, K.J.; Payne, C.D.; Hasan, M.M.; Zhuang, J.; Bourinet, E.; Ragnarsson, L.; Alewood, P.F.; et al. Novel scorpion toxin ω-buthitoxin-Hf1a selectively inhibits calcium influx via CaV3.3 and CaV3.2 and alleviates allodynia in a mouse model of acute postsurgical pain. Int. J. Mol. Sci. 2024, 25, 4745. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.N.; Vo, H.D.; Vo, N.P.; Kudryashova, K.S.; Nekrasova, O.V.; Feofanov, A.V.; Kirpichnikov, M.P.; Andreeva, T.V.; Serebryakova, M.V.; Tsetlin, V.I.; et al. Vietnamese Heterometrus laoticus scorpion venom: Evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 2014, 77, 40–48. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.-F.; Abbas, H.; Sauze, N.; Mercier, L.; Berge-Lefranc, J.-L.; Condo, J.; Bougis, P.E.; Guieu, R. Involvement of endogenous opioid system in scorpion toxin-induced antinociception in mice. Neurosci Lett. 2010, 482, 45–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Wang, Z.; Zhang, X.; Liang, X.; Civelli, O. BmK-YA, an enkephalin-like peptide in scorpion venom. PLoS ONE 2012, 7, e40417. [Google Scholar] [CrossRef]
- Aliakbari, F.; Rahmati, S.; Ghanbari, A.; Madanchi, H.; Rashidy-Pour, A. Identification and designing an analgesic opioid cyclic peptide from Defensin 4 of Mesobuthus martensii Karsch scorpion venom with more effectiveness than morphine. Biomed. Pharmacother. 2025, 188, 118139. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Wu, W.; Wang, Y. Peptides with therapeutic potential in the venom of the scorpion Buthus martensii Karsch. Peptides 2019, 115, 43–50. [Google Scholar] [CrossRef]
- Zheng, Y.; Wen, Q.; Huang, Y.; Guo, D. The significant therapeutic effects of Chinese scorpion: Modern scientific exploration of ion channels. Pharmaceuticals 2024, 17, 1735. [Google Scholar] [CrossRef]
- Shoukry, N.M.; Salem, M.L.; Teleb, W.K.; Abdel Daim, M.M.; Abdel-Rahman, M.A. Antinociceptive, antiinflammatory, and antipyretic effects induced by the venom of Egyptian scorpion Androctonus amoreuxi. J. Basic Appl. Zool. 2020, 81, 56. [Google Scholar] [CrossRef]
- Abbas, N.; Gaudioso-Tyzra, C.; Bonnet, C.; Gabriac, M.; Amsalem, M.; Lonigro, A.; Padilla, F.; Crest, M.; Martin-Eauclaire, M.F.; Delmas, P. The scorpion toxin Amm VIII induces pain hypersensitivity through gain-of-function of TTX-sensitive Na+ channels. Pain 2013, 154, 1204–1215. [Google Scholar] [CrossRef]
- Maatoug, R.; Jebali, J.; Guieu, R.; De Waard, M.; Kharrat, R. BotAF, a new Buthus occitanus tunetanus scorpion toxin, produces potent analgesia in rodents. Toxicon 2018, 149, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Ziari, S.; Shahbazzadeh, D.; Sardari, S.; Sabatier, J.-M.; Pooshang Bagheri, K. Discovery of a new analgesic peptide, leptucin, from the Iranian scorpion, Hemiscorpius lepturus. Molecules 2021, 26, 2580. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Ming, H.; Deng, L.; Zhu, H.; Liu, Z.; Zhang, J.; Song, Y. A novel expression vector for the improved solubility of recombinant scorpion venom in Escherichia coli. Biochem. Biophys. Res. Commun. 2017, 482, 120–125. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Z.; Zhang, Q.; Li, C.; Jin, W.; Liu, L.; Zhang, J.; Zhang, J. Investigation of binding modes and functional surface of scorpion toxins ANEP to sodium channels 1.7. Toxins 2017, 9, 387. [Google Scholar] [CrossRef]
- Mao, Q.; Ruan, J.; Cai, X.; Lu, W.; Ye, J.; Yang, J.; Yang, Y.; Sun, X.; Cao, J.; Cao, P. Antinociceptive effects of analgesic-antitumor peptide (AGAP), a neurotoxin from the scorpion Buthus martensii Karsch, on formalin-induced inflammatory pain through a mitogen-activated protein kinases-dependent mechanism in mice. PLoS ONE 2013, 8, e78239. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-P.; Mao, Q.-H.; Lu, W.-G.; Cai, X.-T.; Chen, J.; Li, Q.; Fu, Q.; Yan, H.-J.; Cao, J.L.; Cao, P. Inhibition of spinal MAPKs by scorpion venom peptide BmK AGAP produces a sensory-specific analgesic effect. Mol. Pain 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Richard, S.A.; Kampo, S.; Sackey, M.; Hechavarria, M.E.; Buunaaim, A.D.B. The pivotal potentials of scorpion Buthus martensii Karsch-analgesic-antitumor peptide in pain management and cancer. Evid. Based Complement. Alternat. Med. 2020, 2020, 4234273. [Google Scholar] [CrossRef]
- Kampo, S.; Cui, Y.; Yu, J.; Anabah, T.W.; Falagán, A.A.; Bayor, M.T.; Wen, Q.P. Scorpion venom peptide, AGAP inhibits TRPV1 and potentiates the analgesic effect of lidocaine. Heliyon 2021, 7, e08560. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, X.H.; Shi, J.; Tan, Z.Y.; Bai, Z.T.; Liu, T.; Ji, Y.H. The anti-nociceptive effect of BmK AS, a scorpion active polypeptide, and the possible mechanism on specifically modulating voltage-gated Na+ currents in primary afferent neurons. Peptides 2006, 27, 2182–2192. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Mi, Z.M.; Cheng, G.F.; Shen, W.Q.; Xiao, X.; Liu, X.M.; Liang, X.T.; Yu, D.Q. Purification and characterization of a new peptide with analgesic effect from the scorpion Buthus martensi Karch. J. Pept. Res. 2004, 64, 33–41. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zhu, Y.; Zhang, L.; Ji, J.; Gui, M.; Li, C.; Song, Y. Study of anti-inflammatory and analgesic activity of scorpion toxins DKK-SP1/2 from scorpion Buthus martensii Karsch (BmK). Toxins 2021, 13, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, X.; Hu, X.; Zhao, Y.; Zhao, M.; Zhang, J.; Cui, Y. Recombinant expression, functional characterization of two scorpion venom toxins with three disulfide bridges from the Chinese scorpion Buthus martensii Karsch. Protein Pept. Lett. 2017, 24, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Song, Y.; Cao, Y.; Ban, M.; Zhang, Z.; Sun, Y.; Feng, Y.; Li, C. Scorpion neurotoxin Syb-prII-1 exerts analgesic effect through Nav1.8 channel and MAPKs pathway. Int. J. Mol. Sci. 2022, 23, 7065–7088. [Google Scholar] [CrossRef]
- Rigo, F.K.; Bochi, G.V.; Pereira, A.L.; Adamante, G.; Ferro, P.R.; Dal-Toé De Prá, S.; Milioli, A.M.; Damiani, A.P.; da Silveira Prestes, G.; Dalenogare, D.P.; et al. TsNTxP, a non-toxic protein from Tityus serrulatus scorpion venom, induces antinociceptive effects by suppressing glutamate release in mice. Eur. J. Pharm. 2019, 855, 65–74. [Google Scholar] [CrossRef]
- Ma, R.; Cui, Y.; Zhou, Y.; Bao, Y.M.; Yang, W.Y.; Liu, Y.F.; Wu, C.F.; Zhang, J.H. Location of the analgesic domain in scorpion toxin BmK AGAP by mutagenesis of disulfide bridges. Biochem. Biophys. Res. Commun. 2010, 394, 330–334. [Google Scholar] [CrossRef]
- Montero-Dominguez, P.A.; Restano-Cassulini, R.; Magaña-Ávila, L.C.; Almanza, A.; Mercado, F.; Corzo, G. Design of antinociceptive peptide by grafting domains between scorpion β-neurotoxins. Bioorg. Chem. 2025, 162, 108592. [Google Scholar] [CrossRef]
- Lima, L.S.; Loyola, V.; Bicca, J.V.M.L.; Faro, L.; Vale, C.L.C.; Denucci, B.L.; Mortari, M.R. Innovative treatments for epilepsy: Venom peptides, cannabinoids, and neurostimulation. J. Neurosci. Res. 2022, 100, 1969–1986. [Google Scholar] [CrossRef]
- Veiseh, M.; Gabikian, P.; Bahrami, S.-B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor paint: A chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2005, 102, 9860–9865. [Google Scholar] [CrossRef]
- Guan, R.J.; Wang, M.; Wang, D.; Wang, D.C. A new insect neurotoxin AngP1 with analgesic effect from the scorpion Buthus martensii Karsch: Purification and characterization. J. Pept. Res. 2001, 58, 27–35. [Google Scholar] [CrossRef]
- Sui, A.R.; Piao, H.; Xiong, S.T.; Zhang, P.; Guo, S.Y.; Kong, Y.; Gao, C.Q.; Wang, Z.X.; Yang, J.; Ge, B.Y.; et al. Scorpion venom heat-resistant synthesized peptide ameliorates epileptic seizures and imparts neuroprotection in rats mediated by NMDA receptors. Eur. J. Pharmacol. 2024, 978, 176704. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, R.; Yin, L.; Fu, Y.; Cai, Y.; Zhang, Z.; Liang, A. BmK CT enhances the sensitivity of temozolomide-induced apoptosis of malignant glioma U251 cells in vitro through blocking the AKT signaling pathway. Oncol. Lett. 2018, 15, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zou, X.; Li, S.; He, J.; Xi, C.; Tang, Q.; Wang, Y.; Cao, Z. BmK NSPK, a potent potassium channel inhibitor from scorpion Buthus martensii Karsch, promotes neurite outgrowth via NGF/TrkA signaling pathway. Toxins 2021, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M. The scorpion envenoming syndrome. Toxicon 1995, 33, 825–858. [Google Scholar] [CrossRef]

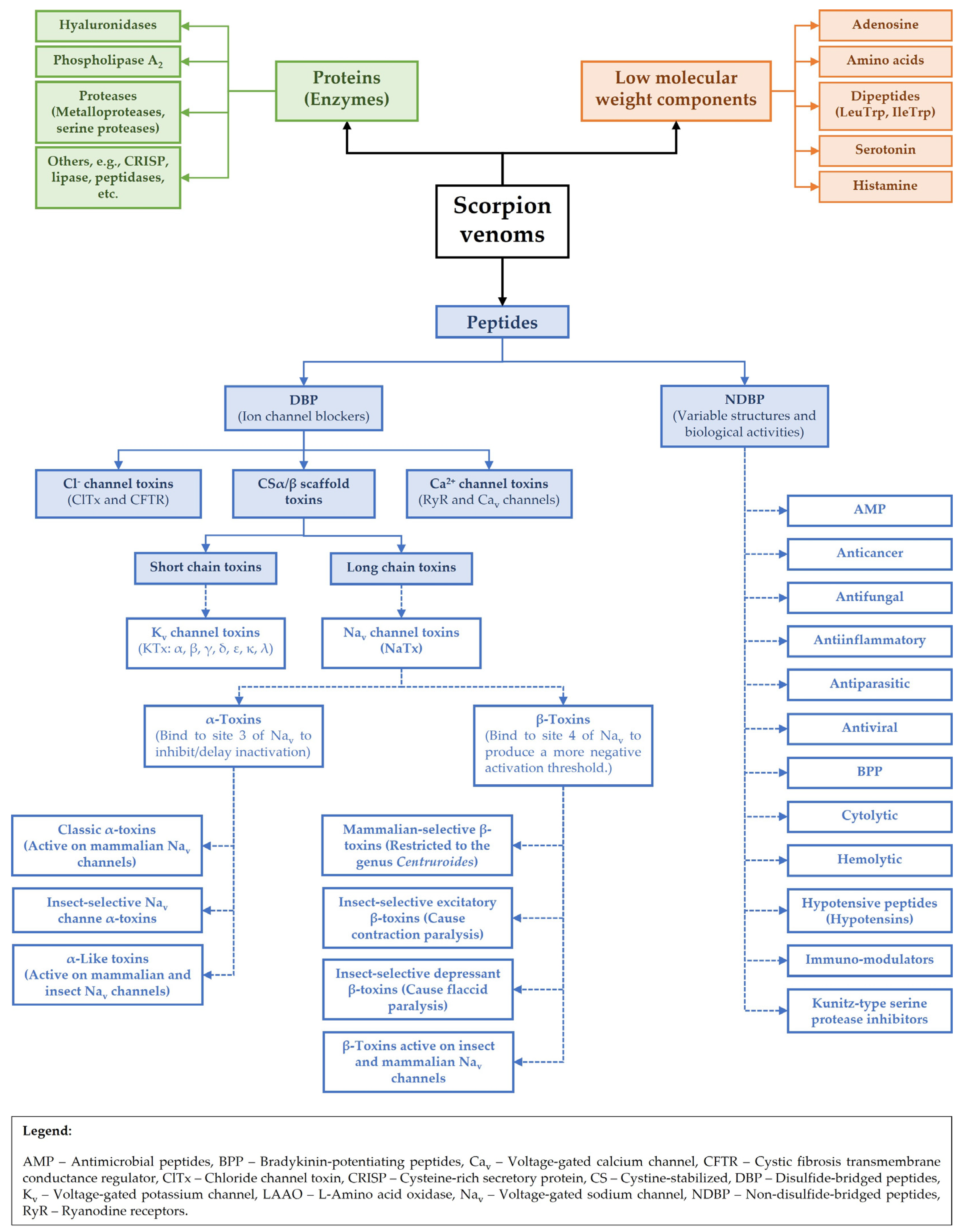
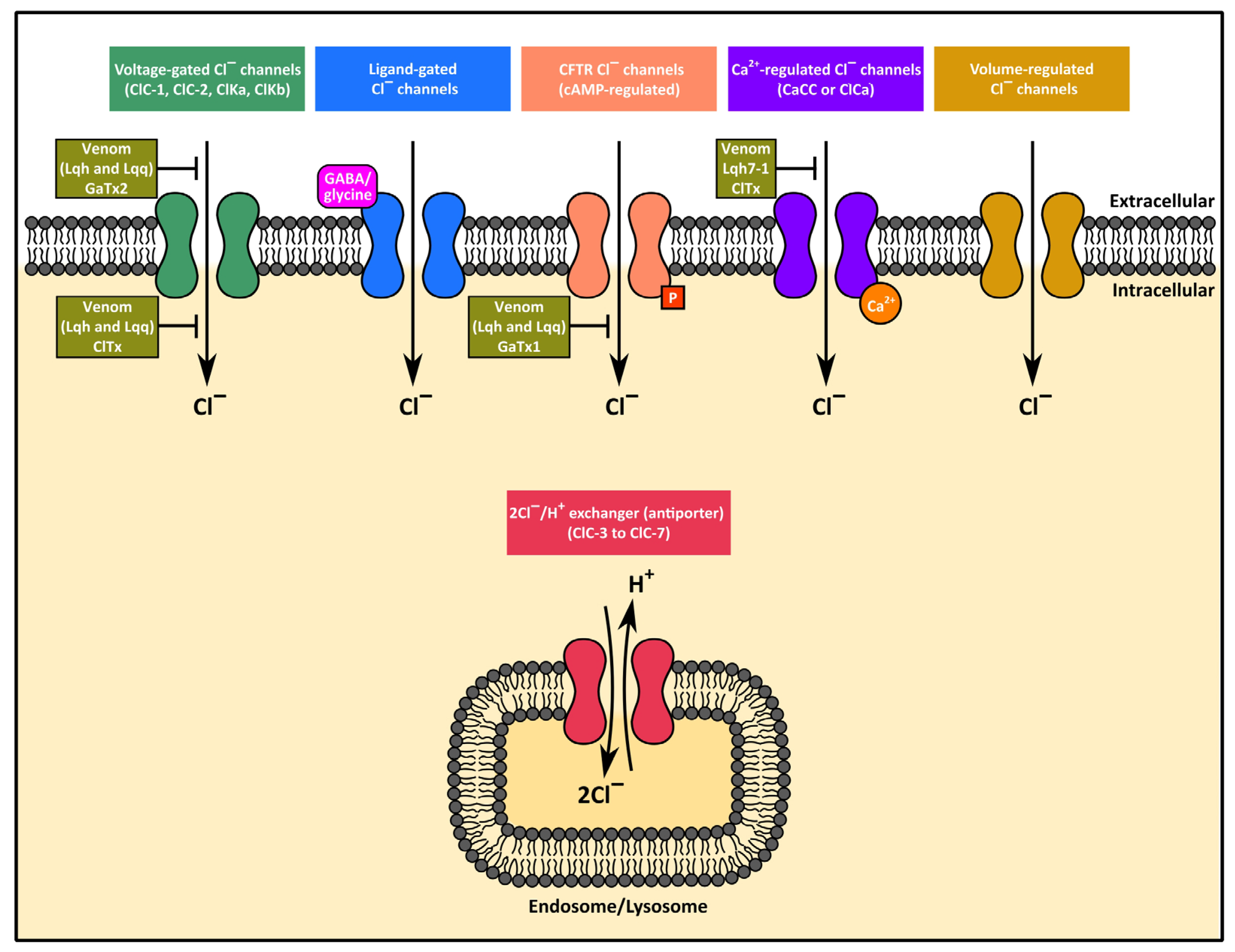

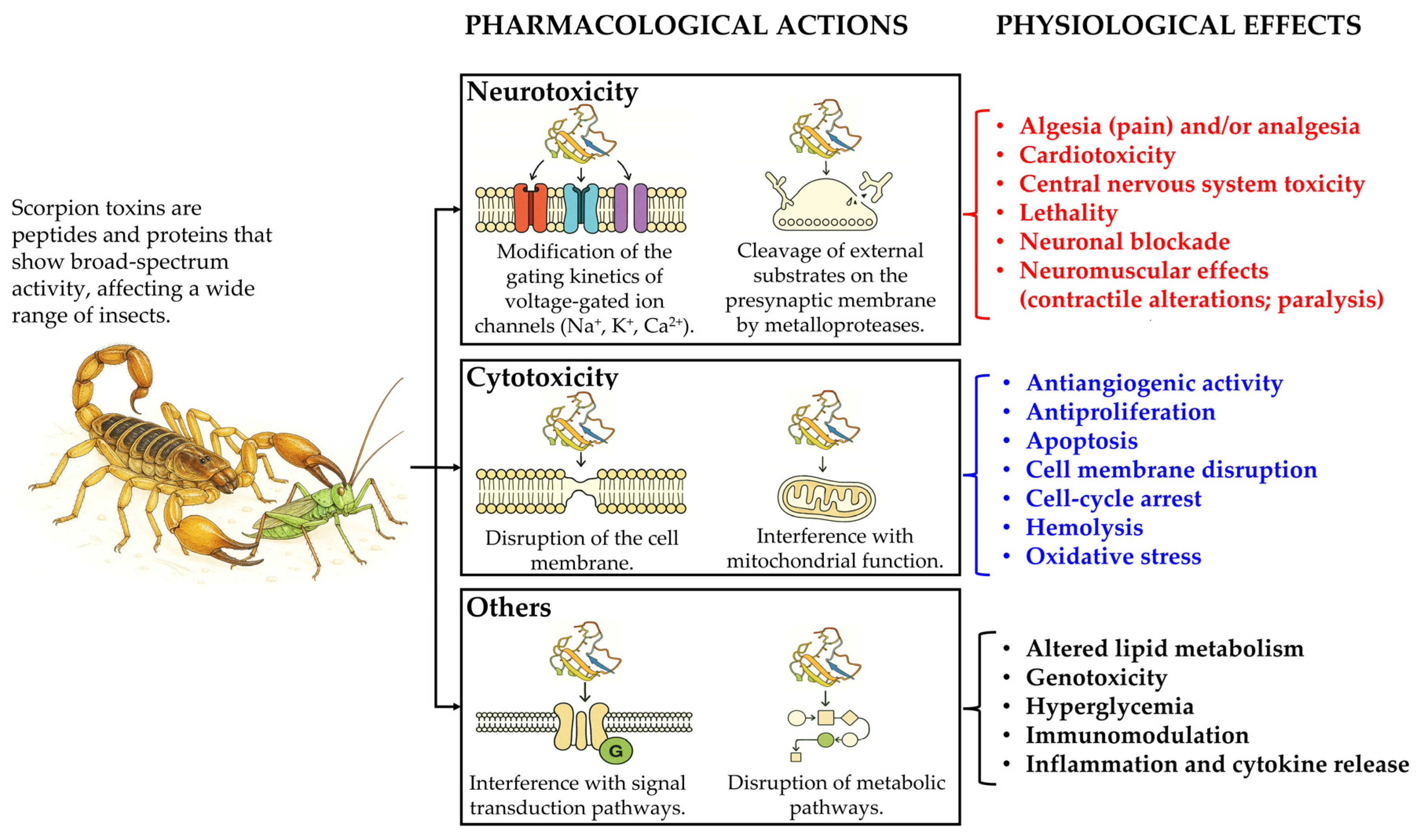
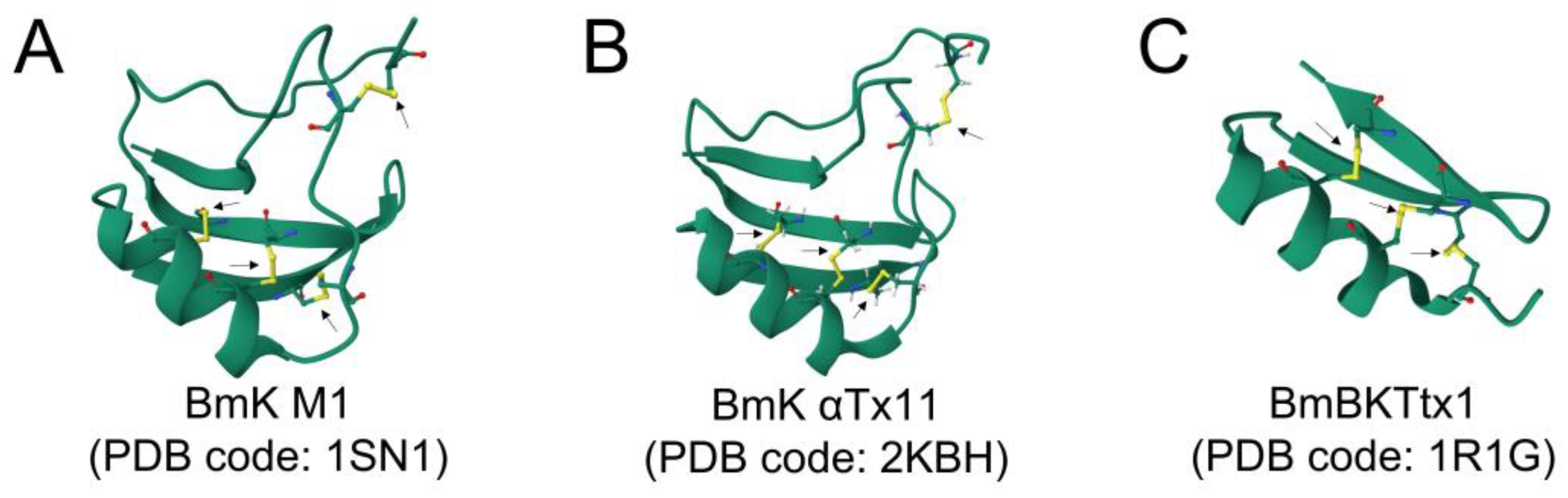
| Species | Origin | Molecule(s) | Nature | Activity | References |
|---|---|---|---|---|---|
| Aegaeobuthus gibbosus | Turkey | Venom | Venom | Venom was active against Bacillus sphaericus, Bacillus subtilis, Bacillus cereus and Staphylococcus aureus (all Gram-positive) and Escherichia coli (Gram-negative), but not against Enterococcus faecalis and Micrococcus luteus (both Gram-positive) | [316] |
| Androctonus aeneas | North Africa | AaeAP1, AaeAP2 | Antimicrobial peptides | Both peptides inhibited S. aureus (MIC: 16 μg/mL), but were much less effective against E. coli (MIC: >512 μg/mL). | [317] |
| Androctonus amoreuxi | North Africa | AamAP1, AamAP2 | Antimicrobial peptides | AamP1 and AamAP2 inhibited S. aureus (MIC: 20 and 48 μM, respectively) more effectively than E. coli (MIC: 120 and 150 μM, respectively). | [318] |
| GK-19 (AamAP1 derivative) | Antimicrobial peptide | Broad-spectrum antibacterial activity against E. coli, E. faecalis, K. pneumoniae, P. aeruginosa and S. aureus (MIC: 3–10 μM). Disrupted bacterial membranes. | [319] | ||
| Androctonus australis | Algeria | Venom and peptide G-TI | Sodium channel inhibitor | Venom was active against B. cereus, E. coli, Microcossus spp. and S. aureus (MIC: 75, 150, 125, and 125 μg/mL, respectively). Disrupted bacterial membranes, leading to cell death. The peptide G-TI was active against B. cereus. | [320] |
| Androctonus crassicauda | Saudi Arabia | Venom | Venom | Inhibited the growth of E. coli, Salmonella spp., S. aureus, and Paenibacillus larvae. | [321] |
| Turkey | Venom | Venom | Venom was active against Bacillus cereus, Bacillus sphaericus, Bacillus subtilis, Escherichia coli, and Micrococcus luteus, but not against Enterococcus faecalis and Staphylococcus aureus. | [316] | |
| Hottentotta saulcyi | Turkey | Venom | Venom | Venom was active against Bacillus cereus, Bacillus sphaericus, Bacillus subtilis, Enterococcus faecalis and Staphylococcus aureus, but not against Escherichia coli or Micrococcus luteus. | [316] |
| Leiurus abdullahbayrami | Turkey | Venom | Venom | Venom was active against Bacillus sphaericus, but not against Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, Escherichia coli, Micrococcus luteus or Staphylococcus aureus. | [316] |
| Leiurus quinquestriatus | Egypt | Venom | Venom | Inhibited B. subtilis and C. freundii; no effect on B. cereus and K. pneumoniae. | [322] |
| Saudi Arabia | Venom | Venom | Inhibited the growth of E. coli, Salmonella spp., S. aureus, and Paenibacillus larvae. More effective than A. crassicauda venom | [321] | |
| Saudi Arabia | Venom | Venom | Concentration-dependent inhibition of A. baumannii, E. coli, E. faecalis, K. pneumoniae, P. aeruginosa, and S. aureus. | [323] | |
| Odontobuthus doriae | Iran | Peptide 3 | Venom fraction | Inhibited Enterococcus faecalis and Escherichia coli UT189 (IC50: 160 and 80 μg/mL, respectively). | [324] |
| Pandinus imperator | North Africa | Scorpine | Antimicrobial peptide | Inhibited B. subtilis and K. pneumoniae (MIC: 1 and 10 μM, respectively). | [325] |
| Protoiurus kraepelini | Turkey | Venom | Venom | High bactericidal activity, with inhibition zones of 9–20 mm against Bacillus sphaericus, Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, Escherichia coli, Micrococcus luteus and Staphylococcus aureus. | [316] |
| Scorpio maurus palmatus | Egypt | Smp24, Smp43 | Antimicrobial peptides | Disrupted bacterial membranes, with activity against Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus and Staphylococcus epidermidis. Smp43 also interfered with B. subtilis DNA synthesis. Caused pore formation and induced oxidative stress in E. coli (MIC: 4–128 μg/mL). Greater efficacy against Gram-positive bacteria. | [326,327] |
| Species | Origin | Molecule(s) | Nature | Activity | References |
|---|---|---|---|---|---|
| Buthus occitanus tunetanus | Tunisia | BotCl | Chlorotoxin-like peptide | Concentration-dependent inhibition of Newcastle disease virus in avian species, with an IC50 of 0.69 μM. Mechanism involves direct disruption of viral particle structure, preventing cellular entry and proliferation. | [331] |
| Mesobuthus eupeus | Iran | Mucin 13, Mucin 18 | Antimicrobial peptides | Potential antiviral activity against SARS-CoV-2 by targeting the receptor-binding domain (RBD) of the spike protein. Mucin-18 shows stronger binding affinity, with the A9T mutation enhancing its effectiveness. | [332] |
| Odontobuthus doriae | Iran | ODAMP2, ODAMP5 | Antimicrobial peptides | Computational studies suggest high binding affinities to the SARS-CoV-2 spike protein’s receptor binding domain, with binding energies of −59.2 and −51.8 kcal/mol for ODAMP2 and ODAMP5, respectively, indicating potential efficacy. | [333] |
| Scorpio maurus palmatus | Egypt | Venom | Venom | Significant activity against hepatitis C virus (HCV), interfering with viral entry. IC50 of 6.3 μg/mL, selectivity index of 15.8. Activity is independent of enzymatic processes and specific to Flaviviridae viruses such as HCV and Dengue virus (DENV). | [334] |
| Egypt | Smp76 | Scorpine-like peptide | Potent antiviral activity against HCV and DENV, inhibiting early infection stages, likely Via direct viral particle interaction. IC50 of 0.01 μg/mL for both viruses. No cytotoxic or hemolytic effects at concentrations exceeding 1000 times the antiviral dose. | [335] |
| Species | Origin | Molecule(s) | Nature | Activity | References |
|---|---|---|---|---|---|
| Androctonus aeneas | North Africa | AaeAP1, AaeAP2 | Antimicrobial peptides | Inhibits Candida albicans (MIC: 32 μg/mL). | [317] |
| Androctonus amoreuxi | North Africa | GK-19 | Derived from AamAP1 | Shows strong antifungal activity against Candida albicans, Candida glabrata and Candida krusei (MIC: 5–10 µM). Disrupts fungal membranes, causing structural damage. | [319] |
| Androctonus australis | North Africa | AamAP1, AamP2 | Antimicrobial peptides | Inhibits Candida albicans (MIC: 64 µM). | [42] |
| North Africa | Androctonin | Cysteine-rich antimicrobial peptide | Exhibits potent antifungal activity against various fungal species, particularly Verticillium torelis and Fusarium oxysporum (MIC: <4 µM). Completely inhibits Neurospora crassa spore growth at ≥12 µM, without regrowth. | [340] | |
| Leiurus quinquestriatus | Saudi Arabia | Venom | Venom | Reduces growth and survival of Candida albicans (by 31.2%) and Candida glabrata (by 39.0%). | [323] |
| Species | Origin | Molecule(s) | Nature | Activity | References |
|---|---|---|---|---|---|
| Androctonus crassicauda | Egypt | Venom | Venom | Antihelminthic activity against Trichuris arvicolae that involved significant ultrastructural changes. Potential use in the treatment of gastrointestinal nematodes resistant to conventional drugs. | [342] |
| Saudi Arabia | Venom | Venom | Complete destruction of Echinococcus granulosus protoscolices after 4 h of exposure at 100 μg/mL. The damage involved apoptosis and structural alterations. Potential use in the non-surgical treatment for hydatidosis, a significant public health problem. | [343] | |
| Hemiscorpius lepturus | Iran | Fraction 5 | Venom peptide fraction (<10 kDa) | Reduced the viability of Toxoplasma gondii tachyzoites at 100 μg after 2 h. | [341] |
| Mesobuthus eupeus | Iran | Mucin 24, Mucin 25 | Antimicrobial peptides | Inhibited Plasmodium falciparum without harming human cells (erythrocytes and GC-2 cells), and prevented P. berghei ookinete growth at 10–20 μM, possibly via membrane disruption. | [344] |
| Mesobuthus eupeus | Iran | Fraction 8 | Venom peptide fraction (<10 kDa) | Scolicidal activity against Echinococcus granulosus protoscolices within 30 min of exposure to venom fraction. | [345] |
| Pandinus imperator | North Africa | Scorpine | Antimicrobial peptide | Activity against Plasmodium berghei, involving disruption of sexual stage development in mosquito midguts. Inhibition of fertilization and ookinete formation with ED50 of 10 μM and 0.7 μM, respectively, an effect attributed to membrane disruption. | [325] |
| Species | Origin | Molecule(s) | Activity | References |
|---|---|---|---|---|
| Androctonus australis hector | Algeria | Venom Fraction F1 | Treatment with Fraction F1 (10 mg/kg, i.p.; daily for 11 days) restored the body weight gain, attenuated the diabetes-induced hyperglycemia and improved the glucose tolerance in mice with streptozotocin-induced diabetes. Fraction F1 improved β-cell function and survival and increased the mitotic activity of these cells. Characterization of Fraction F1 revealed the presence of hyaluronidase and peptides, potentially contributing to glucose homeostasis and β-cell survival. | [350] |
| Leiurus quinquestriatus | Egypt | Venom | Venom (daily injection of 1/10 of the lethal dose for eight weeks) normalized the blood glucose levels and body weight in rats with streptozotocin-induced diabetes. The venom prevented the histopathological and immunohistochemical changes caused by diabetes in splenic tissues. | [351] |
| Egypt | Venom | Venom (daily injection of 1/40 of the sublethal dose for eight weeks) prevented body weight loss, normalized hematological parameters, blood cell counts and blood glucose levels, reduced C-peptide levels to slightly below normal, normalized indicators of hepatic function and indicators of oxidative stress, and promoted β islets regeneration in rats with streptozotocin-induced diabetes. | [352] |
| Species | Origin | Molecule(s) | Nature | Activity | References |
|---|---|---|---|---|---|
| Androctonus australis | Tunisia | P01 | K+ channel toxin | Inhibits proliferation, adhesion, and migration of U87 glioblastoma cells. | [357] |
| Tunisia | AaTs-1 | Tetrapeptide | Inhibits glioblastoma U87 cell proliferation, modulates kinase expression, and enhances p53 and FPRL-1 expression. | [358] | |
| Tunisia | AaTs-1-4B | Dendrimer multi-branched molecules | Inhibits U87 cell proliferation and migration, enhances ERK1/2 and AKT phosphorylation, and increases p53 expression. | [359] | |
| Androctonus australis hector | Algeria | F3 fraction | Venom fraction | Induces apoptosis in lung cancer cells (NCI-H358) via the mitochondrial pathway. | [360] |
| Algeria | Venom | Venom | Alters alveolar epithelial cell (A549) integrity, disrupts cytoskeleton, and reduces cell migration. | [361] | |
| Androctonus crassicauda | Turkey | Acra3 | Na+ channel toxin | Cytotoxic to BC3H1 cells and induces necrosis. | [362] |
| Androctonus crassicauda, Androctonus bicolor, Leiurus quinquestriatus | Saudi Arabia | Venom | Venom | Inhibits cell proliferation, motility, and colony formation in colorectal and breast cancer cells. | [363,364] |
| Buthus occitanus | Morocco | α-Insect toxin Lqq3, α-Like toxin Bom4 | Na+ channel toxin | Inhibits hepatocellular carcinoma cell proliferation (Huh 7.5 cells in 3D culture). | [365] |
| Buthus occitanus tunetanus | Tunisia | RK1 | Short peptide (14 amino acids) | Inhibits cell proliferation, migration, and angiogenesis in U87 and IGR39 cells. | [366] |
| Euscorpius mingrelicus | Turkey | Venom | Venom | Cytotoxicity towards breast and lung cancer cells by inducing apoptosis and necrosis. | [367] |
| Hemiscorpius lepturus | Iran | Leptulipin | Phospholipase A2 | Inhibits proliferation, alters morphology, induces DNA fragmentation, and cell cycle arrest in HT-29 and MDA-MB-231 cells. | [368] |
| Hottentotta saulcyi | Iran | Venom | Venom | Induces apoptosis in MCF-7 cells, reduces tumor density in vivo, and upregulates pro-apoptotic genes. | [369] |
| Hottentotta Schach | Iran | Venom | Venom | Antiproliferative activity in MCF-7 cells by inducing oxidative stress leading to apoptosis. | [370] |
| Leiurus quinquestriatus | Egypt | GNPs-V | Venom conjugated with gold nanoparticles | Anticancer activity against liver cancer cell lines by inhibiting migration, inducing cell cycle arrest, and promoting apoptosis. | [371] |
| Israel | Chlorotoxin | Cl− channel toxin | Targets glioblastoma multiform cells, inhibits angiogenesis, and binds to specific channels. | [372] | |
| Saudi Arabia | FLV-SV | Fluvastatin-scorpion venom peptide nano-conjugate | Antiproliferative activity in colorectal adenocarcinoma cells. | [373] | |
| Saudi Arabia | THQ–PL–SV | Thymoquinone-phospholipon-scorpion venom peptide nanovesicles | Antiproliferative activity in lung cancer cells through modulation of gene expression. | [374] | |
| Mesobuthus eupeus | Iran | meuCl14 | Chlorotoxin | Inhibits hMMP-2 and limits tumor progression. | [167] |
| Iran | MeICT | Cl− channel toxin | Inhibits glioma cell proliferation and migration; downregulates Annexin A2 and FOXM1. | [375] | |
| Odontobuthus bidentatus | Iran | Venom | Venom | Induces apoptosis in HepG2 cells via increased nitric oxide levels. Antiproliferative activity in MCF-7 cells. | [376,377] |
| Odontobuthus doriae | Iran | Venom | Venom | Apoptotic and antiproliferative activity in human neuroblastoma (SH-SY5Y) and human breast cancer (MCF-7) cells | [378,379] |
| Protoiurus kraepelini | Turkey | Venom | Venom | Concentration-dependent cytotoxicity in Jurkat cells. | [380] |
| Scorpio maurus palmatus | Egypt | Smp24 | Cationic antimicrobial peptide | Suppresses lung cancer cell growth, and induces apoptosis, cell cycle arrest, and autophagy in HepG2 cells. | [381,382] |
| Egypt | Smp43 | Cationic antimicrobial peptide | Inhibits lung cancer cell proliferation, causes membrane rupture and mitochondrial dysfunction, and alters apoptosis, cell cycle, and autophagy. Inhibits hepatocellular carcinoma cells. | [383,384] |
| Species | Origin | Molecule(s) | Toxin Class | Activity | References |
|---|---|---|---|---|---|
| Androctonus amoreuxi | Egypt | Venom | - | Dose-dependent reduction in acetic acid-induced writhing (peripheral pain) and increased latency in the tail flick test (central pain). | [404] |
| Androctonus mauretanicusmauretanicus | Morocco | anatoxin Amm VIII | NaTx | Dose-dependent analgesia in hot plate and tail flick tests. Antinociception mediated by blockade of Nav1.2, activation of endogenous opioid system, and activation of mechanisms involving diffuse noxious inhibitory controls (DNIC). | [399,405] |
| Buthus occitanus tunetanus | Tunisia | BotAF | NaTx | Non-toxic β-toxin-like peptide with no effect on motor activity. Reversible low blockade (≤20%) of Na+ channels. Peripheral or spinal mechanisms involved in attenuating the pain associated with the acetic acid writhing, formalin, hot plate, and tail-flick assays. Analgesic activity independent of opioid system. More potent than β-endorphin or morphine in these tests. Effective when given i.p., but not when given i.v. or i.c.v. Low activity on TTX-S Na+ channels of DRG and does not bind to rat brain synaptosomes. Stimulates lumbar spinal cord c-fos/c-jun mRNA up regulation. | [406] |
| Hemiscorpius lepturus | Iran | Leptucin | ND | Analgesic effect against acute thermal pain (hot plate and tail-flick tests) at doses of 0.32 and 0.64 mg/kg, i.p., with similar or greater activity than morphine. No cytotoxicity or hemolysis. No histopathological alterations in heart, kidney or liver. LD50 (mice) > 4 mg/kg. | [407] |
| Heterometrus laoticus | Vietnam | Venom | KTx | Venom showed analgesic activity (9.5 and 19 mg/kg) in the tail immersion (55 °C water) and tail flick assays that was less potent than morphine (5 mg/kg). A short K+ channel toxin (Hetlaxin) that blocks Kv1.1 and Kv1.3, with high affinity for the latter (Ki = 59 nM), was isolated from the venom, but its analgesic activity in the assays indicated above was not tested. | [398] |
| Leiurus q. quinquestriatus | Sudan | LqqIT2 | NaTx | Dose-dependent analgesia in hot plate and tail flick tests. Antinociception mediated by blockade of Nav1.2, activation of endogenous opioid system, and activation of mechanisms involving diffuse noxious inhibitory controls (DNIC). | [398] |
| Mesobuthus martensii | China | ANEP (Anti-neuroexcitation peptide) | NaTx | β-Toxin that blocks Nav1.7. Analgesic activity in the mouse acetic acid writhing test and hot plate test. Recombinant peptide has same activity as native peptide. Several mutants showed greater activity than the recombinant peptide. | [408,409] |
| China | BmK-YA 8 | Short-chain NDBP | Structurally related to enkephalin. Activates μ, κ and δ opioid receptors, with selectivity for δ opioid receptors ~7- and 12-fold greater than for µ and κ receptors, respectively. Full agonist at δ opioid receptors, and partial agonist with lower efficacy and potency on μ and κ receptors. Activity at δ opioid receptors antagonized by naloxone. | [400] | |
| BmK AGAP (Analgesic-antitumor peptide) | NaTx | Blockade of Nav1.4, Nav1.5, Nav1.7, Nav1.8, TRPV1, and KCNQ2/3 currents. Analgesic activity in the writhing, hot-plate and formalin tests. Attenuation of pain in the formalin-induced spontaneous nociceptive behavior assay involved inhibition of the expression of peripheral and spinal mitogen-activated protein kinases (MAPK), including p-p38, p-ERK, p-JNK and spinal Fos. Intrathecal administration of AGAP inhibited and reversed pain from chronic constrictive injury (CCI) of the sciatic nerve, from thermal hyperalgesia, and mechanical allodynia. Potentiated the analgesic effect of lidocaine. | [410,411,412,413] | ||
| China | BmK AS | NaTx | Blockade of TTX-R (Nav1.8, 1.9) and TTX-S (Nav1.3), with no effect on voltage-dependent IK and KCl or caffeine-induced Ca2+ influx in neurons. Decreased the number of action potentials in DRG neurons by ~50% at 0.5 μM. Analgesic activity in formalin and carrageenan nociceptive assays. | [414] | |
| China | BmK AngM1 | NaTx | Little or no toxicity at doses up to 50 mg/kg, i.v. Irreversible (by washing) blockade of Nav and delayed rectifier K+ (IK) currents, but no effect on transient K+ currents (IA) in rat hippocampal pyramidal neurons. Analgesic effect in acetic acid writhing assay in mice (63% inhibition at 0.8 mg/kg, compared to 83% with morphine 0.2 mg/kg, i.v.). | [415] | |
| China | DKK-SP2 | NaTx | Blocked Nav1.7 channels expressed in Chinese hamster ovary cells and reduced the expression of this channel in trigeminal neurons. Reduced writhing in the acetic acid test in mice and pain associated with chronic constriction injury of the infraorbital nerve in rats was also attenuated. At the highest doses tested, the potency in both tests was similar to or better than morphine. | [416] | |
| China | BmKBTx, BmNaL-3SS2 | NaTx | Blockade of Nav1.7. BmKBTx and BmNal-3SS2 reducing writhing in the acetic acid test in mice and show similar potency to morphine (reductions of 43% and 63% at 1 mg/kg i.p., respectively, compared to 49% with morphine 1.5 mg/kg i.p.). | [417] | |
| China | Syb-prII | NaTx | β-Neurotoxin that blocks Nav1.8, but not Nav1.9. Attenuated pain associated with chronic constriction injury of the infraorbital nerve, probably by attenuating MAPK-activated pathways. Efficacy similar to morphine. | [418] | |
| Tityus serrulatus | Brazil | TsNTxP | NaTx | Non-toxic peptide structurally related to toxins TsVII (Ts1 or toxin-γ) and Ts3 (TsIV or tityustoxin). Non-toxic to mammals. Antinociceptive effect in tail-flick thermal test and intraplantar capsaisin injection. Attenuates neuropathic pain caused by constriction injury to sciatic nerve and paclitaxel administration. Caused reduced glutamate release from mouse spinal cord synaptosomes. | [419] |
| Species | Toxin | Toxin Characteristics | Therapeutic Potential |
|---|---|---|---|
| Androctonus australis | AaTx | One of a family of peptides that primarily targets Na+ channels | Shows immunomodulatory activity, including specific targeting of Kv1.3 channels involved in T cell-mediated autoimmune responses [424]. As oxidative stress is a major contributor to dopaminergic neuron loss in Parkinson´s disease and amyloid pathology in Alzheimer´s disease, AaTx could represent a potentially useful lead compound for developing therapeutic drugs for these neurodegenerative diseases. |
| B. martensii Karsch | BmK AngP1 | A 62-amino acid peptide with high sequence homology to neurotoxins that target ion channels, but it exerts non-toxic and neurotrophic effects | Promotes angiogenesis and neurogenesis in hippocampal neurons through modulation of pathways related to VEGF expression and PI3K/Akt signaling, both relevant to neuronal survival. In rodent models, this peptide reduces oxidative stress, neuroinflammation, and amyloid-β accumulation, suggesting a protective role against neurodegeneration [425,426]. |
| B. martensii Karsch | BmK CT | A small peptide (~36–40 amino acids) structurally related to chlorotoxin (chlorotoxin-like peptide) and capable of crossing the Blood–Brain Barrier (BBB). | Inhibits matrix metalloproteinase 2 (MMP-2), an enzyme overexpressed in neuroinflammation and tumors. Exhibits anti-inflammatory properties in models of neuroinflammation by suppressing IL-6 and TNF-α [427]. As chronic neuroinflammation is driven by cytokines and MMPs contributes to neurodegeneration, BmK CT could mitigate these pathways. |
| B. martensii Karsch | BmK NSPK (Neuroprotective scorpion peptide Karsch | A low-molecular-weight peptide the structure of which has yet to be solved. | Shows CNS bioactivity without the neurotoxicity of classic neurotoxins. As part of its protective mechanism of action, BmK NSPK improves mitochondrial membrane potential and ATP production in primary cortical neurons, and inhibits Aβ-induced neuronal apoptosis by regulating Bcl-2/Bax ratios and caspase-3 activity [428]. |
| B. martensii Karsch | BmK AGAP (Analgesic-antitumor peptide) | A 66-amino acid peptide originally isolated for its analgesic and anti-tumor effects. | Suppresses microglial activation and reduces secretion of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6). Inhibits p38 MAPK and ERK1/2 signaling pathways, key regulators of inflammatory and apoptotic signaling in activated microglia [413]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Belo, C.A.; Hyslop, S.; Carlini, C.R. Properties and Pharmacology of Scorpion Toxins and Their Biotechnological Potential in Agriculture and Medicine. Toxins 2025, 17, 497. https://doi.org/10.3390/toxins17100497
Dal Belo CA, Hyslop S, Carlini CR. Properties and Pharmacology of Scorpion Toxins and Their Biotechnological Potential in Agriculture and Medicine. Toxins. 2025; 17(10):497. https://doi.org/10.3390/toxins17100497
Chicago/Turabian StyleDal Belo, Cháriston André, Stephen Hyslop, and Célia Regina Carlini. 2025. "Properties and Pharmacology of Scorpion Toxins and Their Biotechnological Potential in Agriculture and Medicine" Toxins 17, no. 10: 497. https://doi.org/10.3390/toxins17100497
APA StyleDal Belo, C. A., Hyslop, S., & Carlini, C. R. (2025). Properties and Pharmacology of Scorpion Toxins and Their Biotechnological Potential in Agriculture and Medicine. Toxins, 17(10), 497. https://doi.org/10.3390/toxins17100497






