Toxic Alexandrium Treatment in Western Australia: Investigating the Efficacy of Modified Nano Clay
Abstract
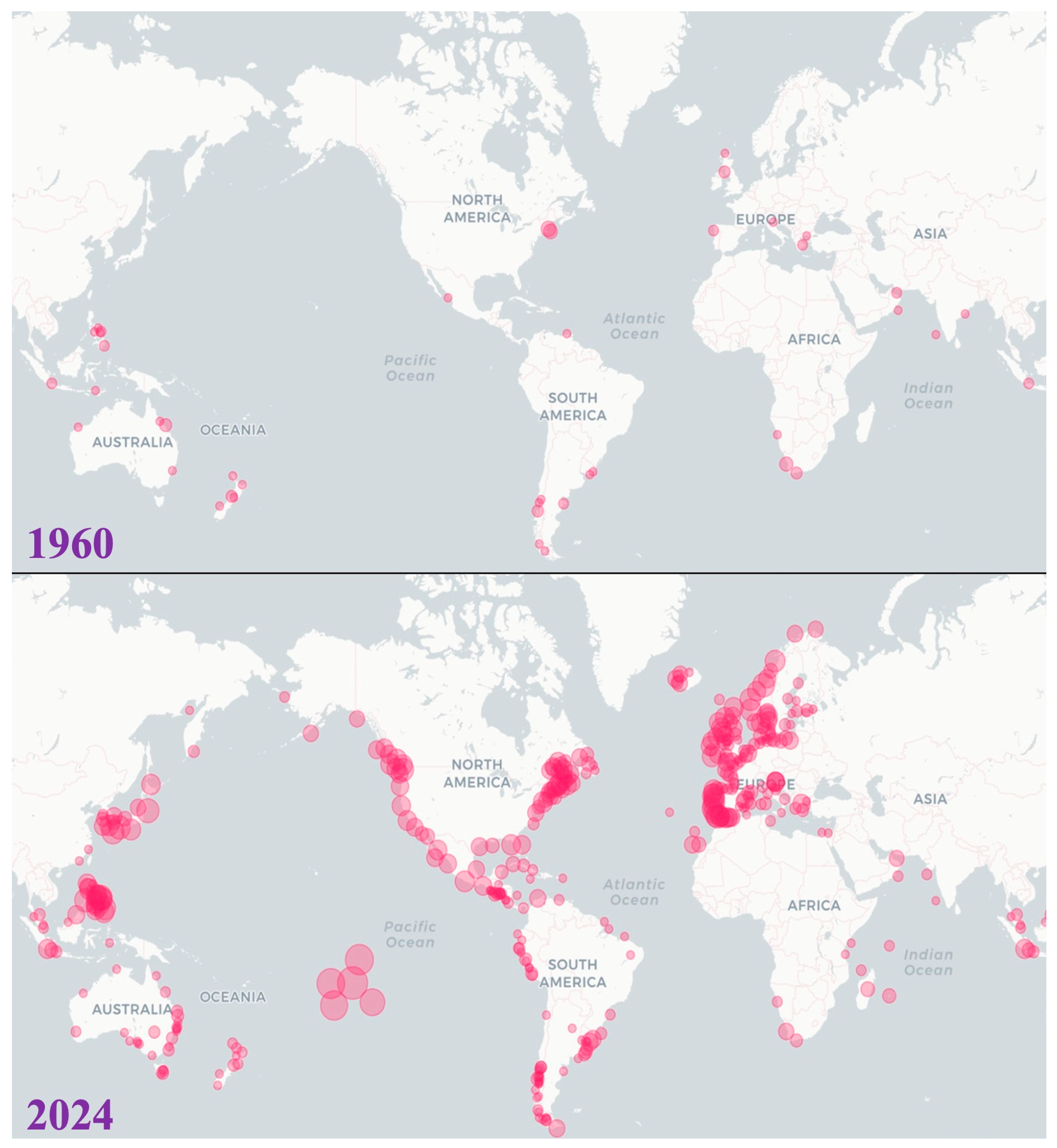
1. Introduction
2. Socio-Economic Impacts of Alexandrium Blooms
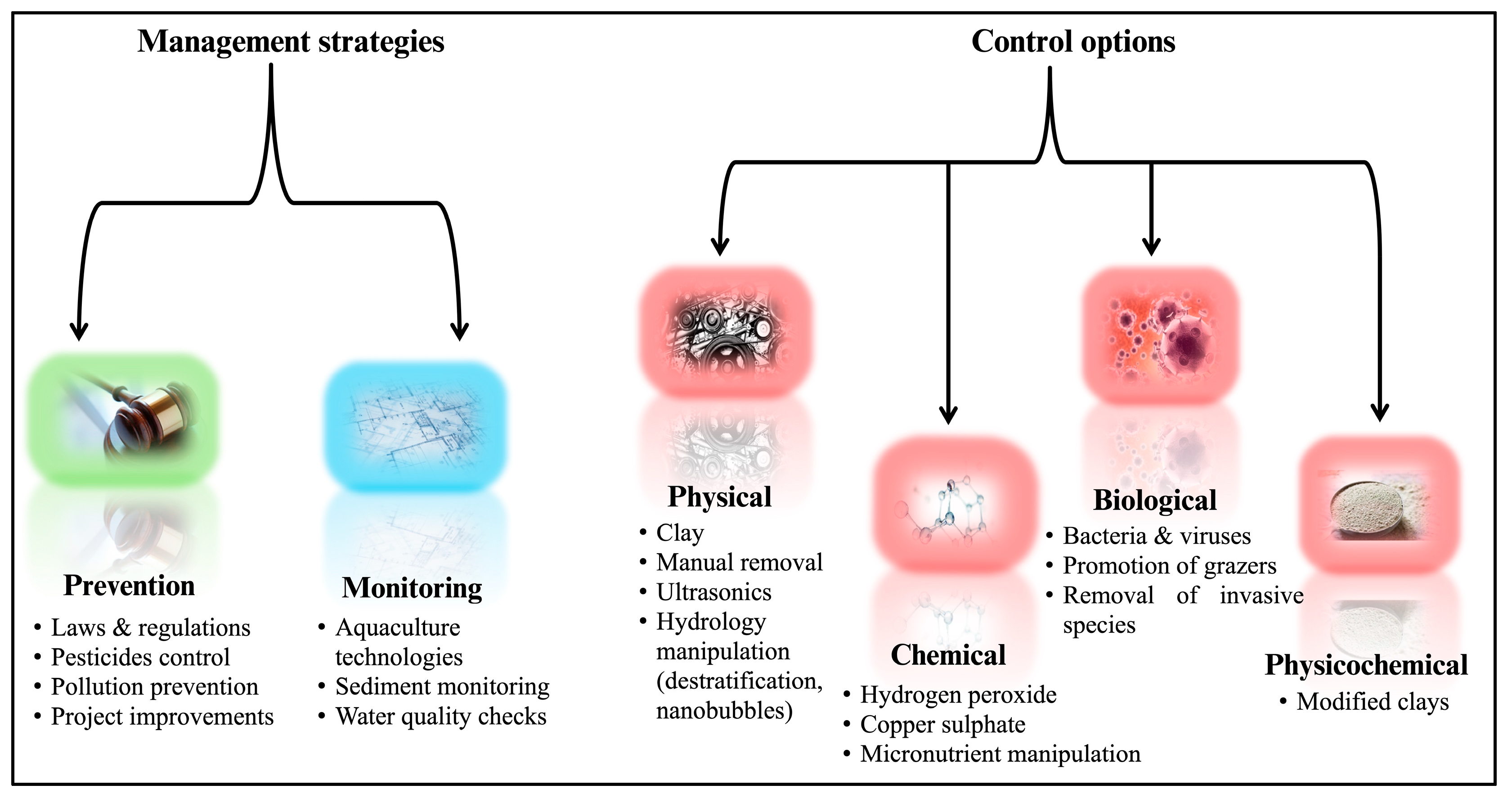
3. Management of Harmful Algal Blooms
3.1. Prevention
3.2. Treatment
| Category | Method | Removal Technique | Effectiveness & Scalability | Environmental Trade-Offs |
|---|---|---|---|---|
| Biological | Algicidal bacteria | Species-specific targeting; Cell destruction via enzymes [90]. | Time-consuming to isolate; Requires high-yield production; Expensive. | May affect non-target species; Environmental risks from biological release [91]. |
| Algicidal viruses | Infect and lyse algal cells [80]. | Naturally abundant; High host specificity; Easy to apply [80]. | Limited practical application; Potential non-target effects [91]. | |
| Allelochemicals | Inhibit or alter algal growth and reproduction [92,93]. | Low cost; Limited field data; Biodegradable; Variable effectiveness [80,84]. | May affect biodiversity; Non-targeted species impacts [94]. | |
| Protozoan grazers | Predate and feed on HABs can be species-specific [95]. | Experimental; Potential for large-scale use. | Non-specific feeding; Risk of trophic imbalance; May increase toxicity [63]. | |
| Chemical | Algicides (i.e., H2O2, CUSO4) | Rapid oxidative damage to algal cells and cysts [63]. | Effective for large-scale use; Short-lived effects; Risk of bloom recurrence [38]. | Fish behavioural changes; Oxygen depletion; Human health and water risks [62,63,89,95]. |
| Engineered Nanoparticles (e.g., TiO2, silver, magnetic NPs) | Adsorption via electrostatic interaction [96]. | Expensive; Limited scalability [80,95]. | Potential toxicity to environment and organisms [95]. | |
| Physical | Ultraviolet radiation | Damages algal metabolism and cell integrity [80]. | Not suitable for large-scale use; Equipment and manpower intensive effectiveness; light penetration limitations [95,97]. Not suitable for large-scale use; Equipment and manpower intensive effectiveness; Light penetration limitations [95,97]. | May disrupt aquatic organisms and microbial community; toxin release trade-off [95,97]. May disrupt aquatic organisms and microbial community; toxin Toxin release trade-off [95,97]. |
| Ultrasonication | Cell lysis and photosynthesis inhibition [98]. | High removal efficiency; Not scalable; High energy demand [80]. | Water quality impacts; Harm to non-target organisms; Toxin release risk [80,99,100]. | |
| Physicochemical | Modified clays | Flocculation and sedimentation of algal cells [81]. | Scalable; Low cost; Low energy demand high removal efficiency [13,101]. | Low doses minimal to no environmental impacts; Potential long term impacts on benthic and non-target aquatic organisms [81,101]. |
| Sophorolipid (Biosurfactant) | Disrupt algal cell membranes [102]. | Large scale applicability; selective action; high cost; low toxicity at low dosage [102]. Large scale applicability; selective action; High cost; Low toxicity at low dosage [102]. | Potential non-target effects; Industrial-scale development still under development [102]. |
4. Treatment of HABs Using Nano Clays
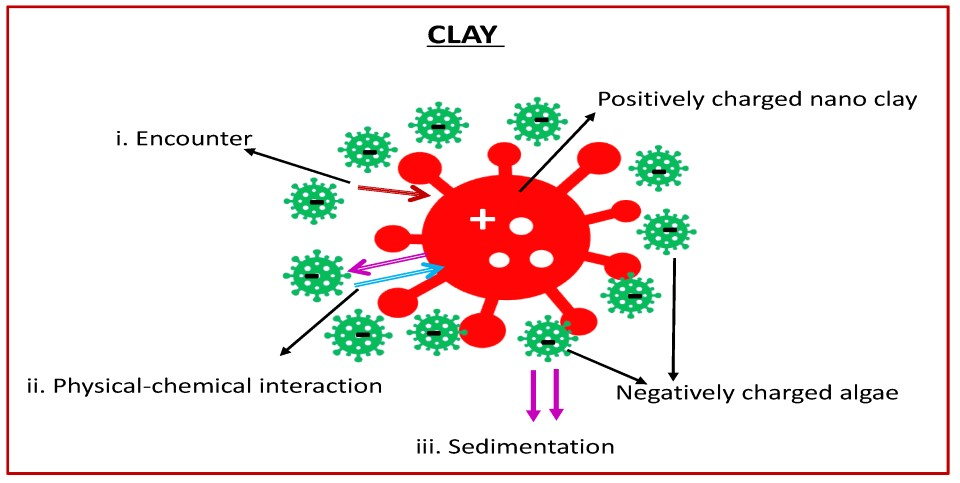
4.1. How Clay Works and Factors That Influence Binding Efficiency
4.2. Nano Clays and Alexandrium spp.
4.3. The Differential Removal of Alexandrium spp. by Aggregation of Nanoclays
4.4. Kaolinite-Alexandrium Adhesion Mechanism
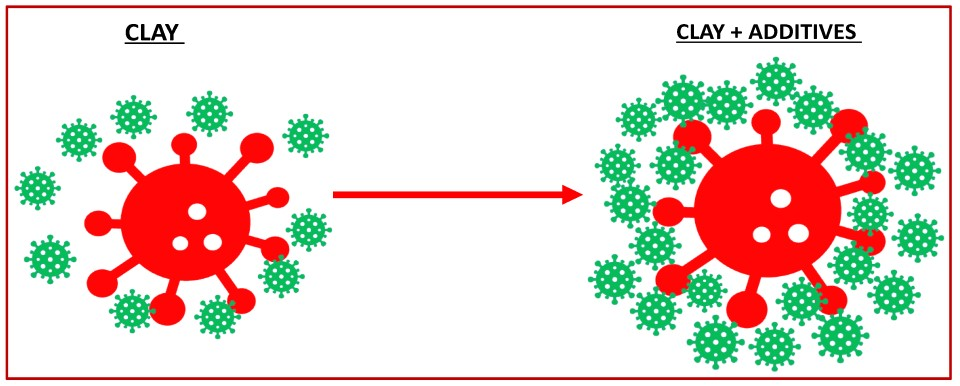
5. Improving the Efficiency of Nano Clays in Controlling Alexandrium spp.
6. Challenges and Opportunities
6.1. Environmental Implications and Risk Assessment
6.2. Reducing HAB Toxicity
6.3. Stability
6.4. Cost/Sustainability
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MC | Modified clay |
| PACl | polyaluminum hydroxy chloride |
| PMPS-MC | potassium peroxymonosulfate modified clay |
| RE | Removal efficiency |
| WA | Western Australia |
| PAC | Polyaluminum chloride |
| HAB | Harmful algal bloom |
| PST | Paralytic shellfish toxin |
| NaCl | Sodium chloride |
| NaOH | Sodium hydroxide |
| LCA | Life cycle assessments |
References
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, O.; Akselmann, R.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Komárek, J.; Larsen, J.; Lundholm, N.; Zingone, A. IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Intergovernmental Oceanographic Commission of UNESCO. 2009. Available online: http://www.marinespecies.org/hab (accessed on 25 January 2025).
- Leftley, J. Harmful marine algal blooms, proceedings of the sixth international conference on toxic marine phytoplankton, October 1993, Nantes, France: Edited by P. Lassus, G. Arzul, E. Erard-Le Denn, P. Gentien and C. Marcaillou-Le Baut; Lavoisier Publishing, ISBN 2-85206-972-5 Paris and Intercept Ltd. ISBN 1-898298-11-4, Andover, UK, 1995; 878 pp.; GBP about 106.00, FF 800.00. J. Exp. Mar. Biol. Ecol. 1996, 202, 260–262. [Google Scholar] [CrossRef]
- Sunda, W.G.; Graneli, E.; Gobler, C.J. Positive feedback and the development and persistenceof ecosystem disruptive algal blooms. J. Hycology 2006, 42, 963–974. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. United Kingd. 2016, 96, 61–91. [Google Scholar] [CrossRef]
- Davidson, K.; Gowen, R.J.; Harrison, P.J.; Fleming, L.E.; Hoagland, P.; Moschonas, G. Anthropogenic nutrients and harmful algae in coastal waters. J. Environ. Manag. 2014, 146, 206–216. [Google Scholar] [CrossRef]
- Kudela, R.; Berdalet, E.; Urban, E. Harmful Algal Blooms: A Scientific Summary for Policy Makers; UNESCO: Paris, France, 2015. [Google Scholar]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Leaw, C.P.; Lim, P.T.; Ng, B.K.; Cheah, M.Y.; Ahmad, A.; Usup, G. Phylogenetic analysis of Alexandrium species and Pyrodinium bahamense (Dinophyceae) based on theca morphology and nuclear ribosomal gene sequence. Phycologia 2005, 44, 550–565. [Google Scholar] [CrossRef]
- Bravo, I.; Figueroa, R.I.; Garces, E.; Fraga, S.; Massanet, A. The intricacies of dinoflagellate pellicle cysts: The example of Alexandrium minutum cysts from a bloom-recurrent area (Bay of Baiona, NW Spain). Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 166–174. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Vázquez, J.A.; Massanet, A.; Murado, M.A.; Bravo, I. Interactove effects of sality and temperature on planozygote and cysts formation of Alexandrium Minutum (Dinophyceae) in culture 1. J. Phycol. 2011, 47, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, B.; Laabir, M.; Masseret, E.; Collos, Y.; Vaquer, A.; Grzebyk, D. Dormancy and germination features in resting cysts of Alexandrium tamarense species complex (Dinophyceae) can facilitate bloom formation in a shallow lagoon (Thau, southern France). J. Plankton Res. 2009, 31, 1209–1224. [Google Scholar] [CrossRef]
- Kwambai, C.S.; Ennaceri, H.; Lymbery, A.J.; Laird, D.W.; Cosgrove, J.; Moheimani, N.R. Effectiveness of Kaolinite with and without Polyaluminum Chloride (PAC) in Removing Toxic Alexandrium minutum. Toxins 2025, 17, 395. [Google Scholar] [CrossRef]
- McQuoid, M.R.; Godhe, A.; Nordberg, K. Viability of phytoplankton resting stages in the sediments of a coastal Swedish fjord. Eur. J. Phycol. 2002, 37, 191–201. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Schantz, E.J.; Mold, J.D.; Stanger, D.W.; Shavel, J.; Riel, F.J.; Bowden, J.P.; Lynch, J.M.; Wyler, R.S.; Riegel, B.; Sommer, H. Paralytic Shellfish Poison. VI. A Procedure for the Isolation and Purification of the Poison from Toxic Clam and Mussel Tissues. J. Am. Chem. Soc. 1957, 79, 5230–5235. [Google Scholar] [CrossRef]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Terrazas, J.O.; Contreras, H.R.; García, C. Prevalence, variability and bioconcentration of saxitoxin-group in different marine species present in the food chain. Toxins 2017, 9, 190. [Google Scholar] [CrossRef]
- Anderson, D.M.; Kulis, D.M.; Qi, Y.-Z.; Zheng, L.; Lu, S.; Lin, Y.-T. Paralytic shellfish poisoning in Southern China. Toxicon 1996, 34, 579–590. [Google Scholar] [CrossRef] [PubMed]
- García, C.; del Carmen Bravo, M.A.; Lagos, M.; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L.E.; et al. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006, 23, 200–222. [Google Scholar] [CrossRef]
- Ajani, P.; Harwood, D.T.; Murray, A. Recent trends in marine phycotoxins from Australian coastal waters. Mar. Drugs 2017, 15, 33. [Google Scholar] [CrossRef]
- Barua, A.; Ajani, P.A.; Ruvindy, R.; Farrell, H.; Zammit, A.; Brett, S.; Hill, D.; Sarowar, C.; Hoppenrath, M.; Murray, S.A. First detection of paralytic shellfish toxins from Alexandrium pacificum above the regulatory limit in blue mussels (Mytilus galloprovincialis) in New South Wales, Australia. Microorganisms 2020, 8, 905. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Schweibold, L.; Jaffrezic, E.; Rhodes, L.; MacKenzie, L.; Hay, B.; Farrell, H. Overview of Australian and New Zealand harmful algal species occurrences and their societal impacts in the period 1985 to 2018, including a compilation of historic records. Harmful Algae 2021, 102, 101848. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Bolch, C.J. Transport of toxic dinoflagellate cysts via ships’ ballast water. Mar. Pollut. Bull. 1991, 22, 27–30. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Steffensen, D.; Wetherbee, R. Three estuarine Australian dinoflagellates that can produce paralytic shellfish toxins. J. Plankton Res. 1988, 10, 533–541. [Google Scholar] [CrossRef]
- Hallegraeff, G. Harmful algal blooms: A global overview. In Manual on Harmful Marine Microalgae; UNESCO: Paris, France, 2003; Volume 33, pp. 25–49. [Google Scholar]
- Bolch, C.J.S.; de Salas, M.F. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia. Harmful Algae 2007, 6, 465–485. [Google Scholar] [CrossRef]
- Hosja, W.; Hallegraeff, G.M.; Deeley, D. Harmful Phytoplankton Surveillance in Western Australia; Waterways Commission Report; 1994; Volume 43. Available online: https://library.dbca.wa.gov.au/static/Journals/080147/080147-43.pdf (accessed on 25 January 2025).
- Hutchins, J.; Pearce, A.; Walker, D. Dispersal of tropical fishes to temperate seas in the southern hemisphere. J. R. Soc. West. Aust. 1991, 74, 79–84. [Google Scholar]
- Dias, J.; Muñoz, J.; Huisman, J.; McDonald, J. Biosecurity monitoring of harmful algal bloom (HAB) species in Western Australian waters: First confirmed record of Alexandrium catenella (Dinophyceae). BioInvasions Rec. 2015, 4, 233–241. [Google Scholar] [CrossRef]
- Government of Western Australia Department of Health. Environmental Health Directorate Year Book 2017–2018; Government of Western Australia Department of Health: East Perth, WA, USA, 2018. [Google Scholar]
- Trayler, K.; Cosgrove, J. Blooming surprise: Toxic algal blooms in Perth rivers. Landscope 2021, 36, 50–53. [Google Scholar]
- Department of Fisheries. Aquaculture in Western Australia: Industry Overview; Department of Fisheries: Orange, Australia, 2015. [Google Scholar]
- Zhang, Y.; Yu, Z.; Song, X.; Yuan, Y.; Cao, X. Effects of modified clay used for the control of harmful algal blooms on Alexandrium pacificum cysts. Harmful Algae 2018, 72, 36–45. [Google Scholar] [CrossRef]
- Burson, A.; Matthijs, H.C.P.; de Bruijne, W.; Talens, R.; Hoogenboom, R.; Gerssen, A.; Visser, P.M.; Stomp, M.; Steur, K.; van Scheppingen, Y.; et al. Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae 2014, 31, 125–135. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Wang, Y.; Sun, P.; Ma, S.; Chen, T. Algicidal effects of a high-efficiency algicidal bacterium shewanella Y1 on the toxic bloom-causing dinoflagellate Alexandrium pacificum. Mar. Drugs 2022, 20, 239. [Google Scholar] [CrossRef]
- Sun, X.-X.; Choi, J.-K.; Kim, E.-K. A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J. Exp. Mar. Biol. Ecol. 2004, 304, 35–49. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Zhou, Y.; Zheng, W.; Yu, C.; Zheng, T. Novel insights into the algicidal bacterium DH77-1 killing the toxic dinoflagellate Alexandrium tamarense. Sci. Total Environ. 2014, 482–483, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-J.; Li, Y.-H.; Liu, F.; Li, H.-Y.; Liu, J.-S.; Yang, W.-D. Experimental removal of Alexandrium tamarense cells using sulfobetaines and their modified clays. J. Appl. Phycol. 2015, 27, 2313–2319. [Google Scholar] [CrossRef]
- Igwaran, A.; Kayode, A.J.; Moloantoa, K.M.; Khetsha, Z.P.; Unuofin, J.O. Cyanobacteria harmful algae blooms: Causes, impacts, and risk management. Water Air Soil Pollut. 2024, 235, 71. [Google Scholar] [CrossRef]
- Suddleson, M.; Hoagland, P. Proceedings of the Workshop on the Socio-Economic Effects of Harmful Algal Blooms in the United States; U.S. National Office for Harmful Algal Blooms: Falmouth, MA, USA, 2021. [Google Scholar]
- Jin, D.; Thunberg, E.; Hoagland, P. Economic impact of the 2005 red tide event on commercial shellfish fisheries in New England. Ocean Coast. Manag. 2008, 51, 420–429. [Google Scholar] [CrossRef]
- Hoagland, P.; Scatasta, S. The economic effects of harmful algal blooms. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; Volume 189, pp. 391–402. [Google Scholar]
- Van Dolah, E.R.; Paolisso, M.; Sellner, K.; Place, A. Employing a socio-ecological systems approach to engage harmful algal bloom stakeholders. Aquat. Ecol. 2016, 50, 577–594. [Google Scholar] [CrossRef]
- Hoagland, P.; Anderson, D.M.; Kaoru, Y.; White, A.W. The economic effects of harmful algal blooms in the United States: Estimates, assessment issues, and information needs. Estuaries 2002, 25, 819–837. [Google Scholar] [CrossRef]
- Willis, C.; Papathanasopoulou, E.; Russel, D.; Artioli, Y. Harmful algal blooms: The impacts on cultural ecosystem services and human well-being in a case study setting, Cornwall, UK. Mar. Policy 2018, 97, 232–238. [Google Scholar] [CrossRef]
- MacKenzie, A.L. The risk to New Zealand shellfish aquaculture from paralytic shellfish poisoning (PSP) toxins. N. Z. J. Mar. Freshw. Res. 2014, 48, 430–465. [Google Scholar] [CrossRef]
- Santos, M.; Costa, P.R.; Porteiro, F.M.; Moita, M.T. First report of a massive bloom of Alexandrium minutum (Dinophyceae) in middle North Atlantic: A coastal lagoon in S. Jorge Island, Azores. Toxicon 2014, 90, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Fire, S.E.; Pruden, J.; Couture, D.; Wang, Z.; Bottein, M.-Y.D.; Haynes, B.L.; Knott, T.; Bouchard, D.; Lichtenwalner, A.; Wippelhauser, G. Saxitoxin exposure in an endangered fish: Association of a shortnose sturgeon mortality event with a harmful algal bloom. Mar. Ecol. Prog. Ser. 2012, 460, 145–153. [Google Scholar] [CrossRef][Green Version]
- Hattenrath-Lehmann, T.K.; Ossiboff, R.J.; Burnell, C.A.; Rauschenberg, C.D.; Hynes, K.; Burke, R.L.; Bunting, E.M.; Durham, K.; Gobler, C.J. The role of a PSP-producing Alexandrium bloom in an unprecedented diamondback terrapin (Malaclemys terrapin) mortality event in Flanders Bay, New York, USA. Toxicon 2017, 129, 36–43. [Google Scholar] [CrossRef]
- Beckler, J.S.; Arutunian, E.; Moore, T.; Currier, B.; Milbrandt, E.; Duncan, S. Coastal harmful algae bloom monitoring via a sustainable, sail-powered mobile platform. Front. Mar. Sci. 2019, 6, 587. [Google Scholar] [CrossRef]
- Stauffer, B.A.; Bowers, H.A.; Buckley, E.; Davis, T.W.; Johengen, T.H.; Kudela, R.; McManus, M.A.; Purcell, H.; Smith, G.J.; Vander Woude, A. Considerations in harmful algal bloom research and monitoring: Perspectives from a consensus-building workshop and technology testing. Front. Mar. Sci. 2019, 6, 399. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Sengco, M.R.; Anderson, D.M. Using clay to control harmful algal blooms: Deposition and resuspension of clay/algal flocs. Harmful Algae 2005, 4, 123–138. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Bouwman, A.; Brown, C.W.; Flynn, K.J.; Lewitus, A.J.; Madden, C.J. Modeling of HABs and eutrophication: Status, advances, challenges. J. Mar. Syst. 2010, 83, 262–275. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global harmful algal bloom status reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef]
- Anderson, D.M.; Andersen, P.; Bricelj, V.M.; Cullen, J.J.; Rensel, J.J. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters; UNESCO: Paris, France, 2001. [Google Scholar]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef]
- Gobler, C.J.; Lonsdale, D.J.; Boyer, G.L. A review of the causes, effects, and potential management of harmful brown tide blooms caused by Aureococcus anophagefferens (Hargraves et Sieburth). Estuaries 2005, 28, 726–749. [Google Scholar] [CrossRef]
- Kim, H. Mitigation and controls of HABs. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 327–338. [Google Scholar]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar] [CrossRef]
- Anderson, C.R.; Moore, S.K.; Tomlinson, M.C.; Silke, J.; Cusack, C.K. Living with harmful algal blooms in a changing world: Strategies for modeling and mitigating their effects in coastal marine ecosystems. In Coastal and Marine Hazards, Risks, and Disasters; Elsevier: Amsterdam, The Netherlands, 2015; pp. 495–561. [Google Scholar]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef]
- Morón-López, J. A holistic water monitoring approach for an effective ecosystem management. Ecohydrol. Hydrobiol. 2021, 21, 549–554. [Google Scholar] [CrossRef]
- Hardy, F.J.; Bouchard, D.; Burghdoff, M.; Hanowell, R.; LeDoux, B.; Preece, E.; Tuttle, L.; Williams, G. Education and notification approaches for harmful algal blooms (HABs), Washington State, USA. Harmful Algae 2016, 60, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Anderson, C.R.; Berdalet, E.; Kudela, R.M.; Cusack, C.K.; Silke, J.; O’Rourke, E.; Dugan, D.; McCammon, M.; Newton, J.A.; Moore, S.K. Scaling up from regional case studies to a global harmful algal bloom observing system. Front. Mar. Sci. 2019, 6, 250. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, W.; Guo, X.; Liu, L. Inactivation of Scrippsiella trochoidea cysts by different physical and chemical methods: Application to the treatment of ballast water. Mar. Pollut. Bull. 2018, 126, 150–158. [Google Scholar] [CrossRef]
- Stumpf, R.; Culver, M.; Tester, P.; Tomlinson, M.; Kirkpatrick, G.; Pederson, B.; Truby, E.; Ransibrahmanakul, V.; Soracco, M. Monitoring Karenia brevis blooms in the Gulf of Mexico using satellite ocean color imagery and other data. Harmful Algae 2003, 2, 147–160. [Google Scholar] [CrossRef]
- Ryan, J.; Kudela, R.; Birch, J.; Blum, M.; Bowers, H.; Chavez, F.; Doucette, G.; Hayashi, K.; Marin, R., III; Mikulski, C. Causality of an extreme harmful algal bloom in Monterey Bay, California, during the 2014–2016 northeast Pacific warm anomaly. Geophys. Res. Lett. 2017, 44, 5571–5579. [Google Scholar] [CrossRef]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Formal Revision of the Alexandrium tamarense Species Complex (Dinophyceae) Taxonomy: The Introduction of Five Species with Emphasis on Molecular-based (rDNA) Classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef]
- Lilly, E.L.; Halanych, K.M.; Anderson, D.M. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) 1. J. Phycol. 2007, 43, 1329–1338. [Google Scholar] [CrossRef]
- Park, T.G.; Lim, W.A.; Park, Y.T.; Lee, C.K.; Jeong, H.J. Economic impact, management and mitigation of red tides in Korea. Harmful Algae 2013, 30, S131–S143. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Mubashar, M.; Chen, L.; Cheng, S.; Zhang, X. Parallel filtration for solid-liquid separation: A case study of highly-efficient algal removal under parallel configuration driven by magnetic force. Sep. Purif. Technol. 2023, 310, 123098. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Abd Al-Halim, M.A.; Mohammed, S.A. Algae processing by plasma discharge technology: A review. Algal Res. 2023, 70, 102983. [Google Scholar] [CrossRef]
- Turner, J.T.; Tester, P.A. Zooplankton Feeding Ecology: Copepod Grazing During an Expatriate Red Tide; Cosper, E.M., Bricelj, V.M., Carpenter, E.J., Eds.; Novel Phytoplankton Blooms; Springer: Berlin/Heidelberg, Germany, 1989; pp. 359–374. [Google Scholar]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 2017, 69, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Kim, J.S.; Du Yoo, Y.; Kim, S.T.; Song, J.Y.; Kim, T.H.; Seong, K.A.; Kang, N.S.; Kim, M.S.; Kim, J.H. Control of the harmful alga Cochlodinium polykrikoides by the naked ciliate Strombidinopsis jeokjo in mesocosm enclosures. Harmful Algae 2008, 7, 368–377. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, Y.; Guan, C.; Lei, X.; Zheng, W.; Wang, H.; Zheng, T. First evidence of Altererythrobacter sp. LY02 with indirect algicidal activity on the toxic dinoflagellate, Alexandrium tamarense. Curr. Microbiol. 2016, 73, 550–560. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation and Control of Harmful Algal Blooms; Ecological Studies; Springer International Publishing: Cham, Switzerland, 2018; pp. 403–423. [Google Scholar]
- Baek, S.-H.; Sun, X.-X.; Lee, Y.-J.; Wang, S.-Y.; Han, K.-N.; Choi, J.-K.; Noh, J.-H.; Kim, E.-K. Mitigation of harmful algal blooms by sophorolipid. J. Microbiol. Biotechnol. 2003, 13, 651–659. [Google Scholar]
- Ichikawa, S.; Wakao, Y.; Fukuyo, Y. Hydrogen peroxide as an extermination agent against cysts of red tide and toxic dinoflagellates. In Toxic Phytoplankton Blooms in the Sea; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
- Rounsefell, G.A.; Evans, J.E. Large-Scale Experimental Test of Copper Sulfate as a Control for the Florida Red Tide; US Department of the Interior, Fish and Wildlife Service: Bailey’s Crossroads, VA, USA, 1958. [Google Scholar]
- Dorantes-Aranda, J.J.; Seger, A.; Mardones, J.I.; Nichols, P.D.; Hallegraeff, G.M. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE 2015, 10, e0133549. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, Q.; Chen, Q.; Yang, K.; Zhang, D.; Chen, Z.; Lu, S.; Shao, X.; Fan, Y.; Yao, L.; et al. Algicidal Activity of Novel Marine Bacterium Paracoccus sp. Strain Y42 against a Harmful Algal-Bloom-Causing Dinoflagellate, Prorocentrum donghaiense. Appl. Environ. Microbiol. 2018, 84, e01015-18. [Google Scholar] [CrossRef]
- Simberloff, D. How common are invasion-induced ecosystem impacts? Biol. Invasions 2011, 13, 1255–1268. [Google Scholar] [CrossRef]
- Xiao, X.; Li, C.; Huang, H.; Lee, Y.P. Inhibition effect of natural flavonoids on red tide alga Phaeocystis globosa and its quantitative structure-activity relationship. Environ. Sci. Pollut. Res. 2019, 26, 23763–23776. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, J.; Liu, S.; Fan, P.; Wang, W.; Xia, C. Allelochemical induces growth and photosynthesis inhibition, oxidative damage in marine diatom Phaeodactylum tricornutum. J. Exp. Mar. Biol. Ecol. 2013, 444, 16–23. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Cheng, S.-P.; Zhang, S.-H.; He, F.; Liang, W.; Zhang, L.-P.; Hu, C.-Y.; Ge, F.-J.; Wu, Z.-B. Chemical Composition in Aqueous Extracts of Potamogeton malaianus and Potamogeton maackianus and their Allelopathic Effects on Microcystis aeruginosa. Pol. J. Environ. Stud. 2010, 19, 213–218. [Google Scholar]
- Zhan, M.-M.; Liu, P.-R.; Liu, X.-Y.; Hong, Y.; Xie, X. Inactivation and removal technologies for algal-bloom control: Advances and challenges. Curr. Pollut. Rep. 2021, 7, 392–406. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Liu, Y.; Chuang, C.Y.; Kong, F.; Harrison, P.J.; Zhu, X.; Jiang, Y. Exposure of engineered nanoparticles to Alexandrium tamarense (Dinophyceae): Healthy impacts of nanoparticles via toxin-producing dinoflagellate. Sci. Total Environ. 2018, 610, 356–366. [Google Scholar] [CrossRef]
- Zhao, F.; Chu, H.; Tan, X.; Zhang, Y.; Yang, L.; Zhou, X.; Zhao, J. Comparison of axial vibration membrane and submerged aeration membrane in microalgae harvesting. Bioresour. Technol. 2016, 208, 178–183. [Google Scholar] [CrossRef]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Beating the blues: Is there any music in fighting cyanobacteria with ultrasound? Water Res. 2014, 66, 361–373. [Google Scholar] [CrossRef]
- Park, J.; Church, J.; Son, Y.; Kim, K.-T.; Lee, W.H. Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason. Sonochem. 2017, 38, 326–334. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yu, Z. An eco-environmental assessment of harmful algal bloom mitigation using modified clay. Harmful Algae 2021, 107, 102067. [Google Scholar] [CrossRef]
- Lu, G.; Song, X.; Yu, Z.; Cao, X.; Yuan, Y. Effects of modified clay flocculation on major nutrients and diatom aggregation during Skeletonema costatum blooms in the laboratory. Chin. J. Oceanol. Limnol. 2015, 33, 1007–1019. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wu, T.; Yang, W.-D.; Li, H.-Y.; Liu, J.-S. The effectiveness of five natural products against three species of harmful algae: Effectiveness of natural products against harmful algae. Water Environ. J. WEJ 2014, 28, 270–276. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Yu, Z.; Song, X.; Qiu, L. Flocculation of harmful algal cells using modified clay: Effects of the properties of the clay suspension. J. Appl. Phycol. 2016, 28, 1623–1633. [Google Scholar] [CrossRef]
- Zhang, P.; Song, X.; Zhang, Y.; Zhu, J.; Shen, H.; Yu, Z. Assessing the effect of modified clay on the toxicity of Karenia mikimotoi using marine medaka (Oryzias melastigma) as a model organism. Toxics 2022, 10, 105. [Google Scholar] [CrossRef]
- Shirota, A. Red tide problem and countermeasures. II. Int. J. Aqua. Fish. Technol. 1989, 1, 195–223. [Google Scholar]
- Sengco, M.R.; Li, A.; Tugend, K.; Kulis, D.; Anderson, D.M. Removal of red-and brown-tide cells using clay flocculation. I. Laboratory culture experiments with Gymnodinium breve and Aureococcus anophagefferens. Mar. Ecol. Prog. Ser. 2001, 210, 41–53. [Google Scholar] [CrossRef]
- Kim, S.-J. Removal of Red Tide Organisms-2. Flocculation of Red Tide Organisms by Using Loess. Korean J. Fish. Aquat. Sci. 2000, 33, 455–462. [Google Scholar]
- Yu, Z.-M.; Zou, J.-Z.; Ma, X.-N. Application of clays to removal of red tide organisms III. The coagulation of kaolin on red tide organisms. Chin. J. Oceanol. Limnol. 1995, 13, 62–70. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, J.; Ma, X. A new method to improve the capability of clays for removing red tide organisms. Oceanol. Limnol. Sin. 1994, 25, 226–232. [Google Scholar]
- Sengco, M.R. The Aggregation of Clay Minerals and Marine Microalgal Cells: Physicochemical Theory and Implications for Controlling Harmful Algal Blooms; Massachusetts Institute of Technology: Cambridge, MA, USA, 2001. [Google Scholar]
- Lu, G.; Song, X.; Yu, Z.; Cao, X.; Yuan, Y. Environmental effects of modified clay flocculation on Alexandrium tamarense and paralytic shellfish poisoning toxins (PSTs). Chemosphere 2015, 127, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Yu, Z.; Jiang, W.; Song, X.; Cao, X. Dosage-effectiveness of modified clay flocculating red tide organisms: Mechanical mechanism and mathematical model. Sep. Purif. Technol. 2023, 305, 122–422. [Google Scholar] [CrossRef]
- Bae, H. Control of the red tide by yellow clay dispersion. In Harmful Algal Blooms in Korea and China, Proceedings of Korea-China Joint Symposium on Harmful Algal Blooms; Gooduck Press: Delhi, India, 1998. [Google Scholar]
- Choi, H.G.; Kim, P.S.; Lee, W.C.; Yun, S.J.; Kim, H.G.; Lee, H.J. Removal efficiency of cochiodinium polykrikoides by Yellow Loess. Korean J. Fish. Aquat. Sci. 1998, 31, 109–113. [Google Scholar]
- Atkins, R.; Rose, T.; Brown, R.; Robb, M. The microcystis cyanobacteria bloom in the Swan River-February 2000. Water Sci. Technol. 2001, 43, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Archambault, M.-C.; Grant, J.; Bricelj, V.M. Removal efficiency of the dinoflagellate Heterocapsa triquetra by phosphatic clay, and implications for the mitigation of harmful algal blooms. Mar. Ecol. Prog. Ser. 2003, 253, 97–109. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Cao, X.; Yu, Z.; Song, X.; Qiu, L. Controlling harmful algae blooms using aluminum-modified clay. Mar. Pollut. Bull. 2016, 103, 211–219. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Song, X.; Cao, X.; Han, X. Effects of modified clay on cysts of Scrippsiella trochoidea for harmful algal bloom control. Chin. J. Oceanol. Limnol. 2014, 32, 1373–1382. [Google Scholar] [CrossRef]
- Sengco, M.R.; Hagström, J.A.; Granéli, E.; Anderson, D.M. Removal of Prymnesium parvum (Haptophyceae) and its toxins using clay minerals. Harmful Algae 2005, 4, 261–274. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Choi, J.-K.; Kim, E.-K.; Youn, S.-H.; Yang, E.-J. Field experiments on mitigation of harmful algal blooms using a Sophorolipid—Yellow clay mixture and effects on marine plankton. Harmful Algae 2008, 7, 154–162. [Google Scholar] [CrossRef]
- Wang, W.W.; Yan, X.Y.; Li, Y.H.; Yu, D.R.; Li, H.Y.; Yang, W.D.; Liu, J.S. Removal efficiency of different gemini surfactants and related modified clay to Chattonella marina. Water Environ. Res. 2017, 89, 1981–1987. [Google Scholar] [CrossRef]
- Liu, G.; Fan, C.; Zhong, J.; Zhang, L.; Ding, S.; Yan, S.; Han, S. Using hexadecyl trimethyl ammonium bromide (CTAB) modified clays to clean the Microcystis aeruginosa blooms in Lake Taihu, China. Harmful Algae 2010, 9, 413–418. [Google Scholar] [CrossRef]
- Padilla, L.V.; San Diego-McGlone, M.L.; Azanza, R.V. Exploring the potential of clay in mitigating Pyrodinium bahamense var. compressum and other harmful algal species in the Philippines. J. Appl. Phycol. 2010, 22, 761–768. [Google Scholar] [CrossRef]
- Yu, Z.; Zou, J.; Ma, X. A more effective clay for removing red tide organisms. J. Nat. Disasters 1994, 3, 105–109. [Google Scholar]
- Yu, Z.; Ma, X.; Xie, Y. Study of main nutrients adsorption on clays in seawater. Oceanol. Limnol. Sin. 1995, 26, 208–214. [Google Scholar]
- Thomas, D.; Judd, S.; Fawcett, N. Flocculation modelling: A review. Water Res. 1999, 33, 1579–1592. [Google Scholar] [CrossRef]
- Song, W.; Song, X.; Li, J.; Zhang, Y.; Shen, H.; Zhang, P.; Yu, Z. Toxin remained in residual Alexandrium pacificum after flocculation with modified clay. Oceanol. Limnol. Sin 2021, 52, 917–924. [Google Scholar] [CrossRef]
- Song, W.; Song, X.; Shen, H.; Ding, Y.; Cheng, R.; Yu, Z. Degradation of paralytic shellfish toxins during flocculation of Alexandrium pacificum by an oxidized modified clay: A laboratory experiment. Ecotoxicol. Environ. Saf. 2023, 253, 114667. [Google Scholar] [CrossRef]
- Han, M.Y.; Kim, W. A theoretical consideration of algae removal with clays. Microchem. J. 2001, 68, 157–161. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Wang, S.; Wang, C.; Zhou, H.; Kapur, S.; Zhang, J.; Song, Y. Electrostatic charges on microalgae surface: Mechanism and applications. J. Environ. Chem. Eng. 2022, 10, 107516. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Ishika, T.; Moheimani, N.R.; Ennaceri, H. The potential of coupling wastewater treatment with hydrocarbon production using Botryococcus braunii. Algal Res. 2023, 74, 103214. [Google Scholar] [CrossRef]
- Nazloo, E.K.; Danesh, M.; Sarrafzadeh, M.-H.; Moheimani, N.R.; Ennaceri, H. Biomass and hydrocarbon production from Botryococcus braunii: A review focusing on cultivation methods. Sci. Total Environ. 2024, 926, 171734. [Google Scholar] [CrossRef] [PubMed]
- Ennaceri, H.; Nwoba, E.G.; Ogbonna, C.N.; Bahri, P.A.; Moheimani, N.R. Progress of non-destructive hydrocarbon extraction technology of Botryococcus braunii. Algal Res. 2023, 73, 103156. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Moheimani, N.R.; Ennaceri, H. Biofilm and suspension-based cultivation of microalgae to treat anaerobic digestate food effluent (ADFE). Sci. Total Environ. 2024, 924, 171320. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Moheimani, N.R.; Ennaceri, H. Biofilm cultivation of chlorella species. MUR 269 to treat anaerobic digestate food effluent (ADFE): Total ammonia nitrogen (TAN) concentrations effect. Chemosphere 2024, 354, 141688. [Google Scholar] [CrossRef]
- Ennaceri, H.; Benyoussef, A.; Ennaoui, A.; Khaldoun, A. Optical Properties of Front and Second Surface Silver-Based and Molybdenum-Based Mirrors. Int. J. Eng. Technol. 2016, 8, 410–413. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Moheimani, N.R.; Ennaceri, H. Effect of light intensity on Chlorella sp. biofilm growth on anaerobically digested food effluents (ADFE). J. Environ. Manag. 2024, 371, 123015. [Google Scholar] [CrossRef]
- Shammas, N.K. Coagulation and flocculation. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 103–139. [Google Scholar]
- Sengco, M.R.; Anderson, D.M. Controlling harmful algal blooms through clay flocculation. J. Phycol. 2003, 39, 51. [Google Scholar] [CrossRef]
- Vanoss, C.J.; Giese, R.F. The hydrophilicity and hydrophobicity of clay-minerals. Clay Clay Min. 1995, 43, 474–477. [Google Scholar] [CrossRef]
- Bos, R.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions—Its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, N. Surface chemistry of solid and liquid interfaces. By H. Y. Erbil. ChemPhysChem 2008, 9, 646–647. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Syngouna, V.I. Attachment of bacteriophages MS2 and ΦX174 onto kaolinite and montmorillonite: Extended-DLVO interactions. Colloids Surf. B Biointerfaces 2012, 92, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Baraka, A.E.; Ennaceri, H.; Ennaoui, A.; Ghennioui, A.; Jorio, A.; Khaldoun, A. A novel approach to evaluate soiling adhesion on the surface of CSP reflectors via extended DLVO theory. Appl. Phys. A 2019, 125, 515. [Google Scholar] [CrossRef]
- Ennaceri, H.; Alami, H.E.; Brik, H.; Mokssit, O.; Khaldoun, A. Lotus effect and super-hydrophobic coatings for concentrated solar power systems (CSP). In Proceedings of the 2014 International Conference on Composite Materials & Renewable Energy Applications (ICCMREA), Sousse, Tunisia, 22–24 January 2014; pp. 1–4. [Google Scholar]
- Ennaceri, H.; Mkpuma, V.O.; Moheimani, N.R. Nano-clay modified membranes: A promising green strategy for microalgal antifouling filtration. Sci. Total Environ. 2023, 902, 166479. [Google Scholar] [CrossRef]
- Nabweteme, R.; Yoo, M.; Kwon, H.S.; Kim, Y.J.; Hwang, G.; Lee, C.H.; Ahn, I.S. Application of the extended DLVO approach to mechanistically study the algal flocculation. J. Ind. Eng. Chem. 2015, 30, 289–294. [Google Scholar] [CrossRef]
- Dai, D.; Qv, M.; Liu, D.; Tang, C.; Wang, W.; Wu, Q.; Yin, Z.; Zhu, L. Structural insights into mechanisms of rapid harvesting of microalgae with pH regulation by magnetic chitosan composites: A study based on E-DLVO model and component fluorescence analysis. Chem. Eng. J. 2023, 456, 141071. [Google Scholar] [CrossRef]
- Sharma, P.K.; Rao, K.H. Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: Surface thermodynamics and extended DLVO theory. Colloids Surf. B Biointerfaces 2003, 29, 21–38. [Google Scholar] [CrossRef]
- Yu, Z.; Sengco, M.R.; Anderson, D.M. Flocculation and removal of the brown tide organism, Aureococcus anophagefferens (Chrysophyceae), using clays. J. Appl. Phycol. 2004, 16, 101–110. [Google Scholar] [CrossRef]
- Qi, J.; Yu, J.; Shah, K.J.; Shah, D.D.; You, Z. Applicability of clay/organic clay to environmental pollutants: Green way—An overview. Appl. Sci. 2023, 13, 9395. [Google Scholar] [CrossRef]
- Li, J.; Song, X.; Zhang, Y.; Xu, X.; Yu, Z. Effect of modified clay on the transition of paralytic shellfish toxins within the bay scallop Argopecten irradians and sediments in laboratory trials. Aquaculture 2019, 505, 112–117. [Google Scholar] [CrossRef]
- Lewis, M.A.; Dantin, D.D.; Walker, C.C.; Kurtz, J.C.; Greene, R.M. Toxicity of clay flocculation of the toxic dinoflagellate, Karenia brevis, to estuarine invertebrates and fish. Harmful Algae 2003, 2, 235–246. [Google Scholar] [CrossRef]
- Cao, S.; Liu, Z.; Zhou, B.; Jiang, Y.; Xu, M.; Wang, Y. Post-ecological effect and risk assessment of using modified clay in harmful algal bloom mitigation: An attempt based on the responses of zooplankton Brachionus plicatilis and bivalve Mytilus edulis. Ecotoxicol. Environ. Saf. 2022, 230, 113134. [Google Scholar] [CrossRef]
- Devillier, V.M.; Hall, E.R.; Anderson, D.M.; Lewis, K.A. Exposure of blue crab (Callinectes sapidus) to modified clay treatment of Karenia brevis as a bloom control strategy. Harmful Algae 2023, 128, 102492. [Google Scholar] [CrossRef] [PubMed]
- Devillier, V.M.; Hall, E.R.; Lovko, V.; Pierce, R.; Anderson, D.M.; Lewis, K.A. Mesocosm study of PAC-modified clay effects on Karenia brevis cells and toxins, chemical dynamics, and benthic invertebrate physiology. Harmful Algae 2024, 134, 102609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, X.; Shen, H.; Cao, X.; Yuan, Y.; Wu, Z.; Yu, Z. The effects of modified clay on abalone (Haliotis discus hannai) based on laboratory and field experiments. Environ. Toxicol. Chem. 2020, 39, 2065–2075. [Google Scholar] [CrossRef]
- Archambault, M.-C.; Bricelj, V.M.; Grant, J.; Anderson, D.M. Effects of suspended and sedimented clays on juvenile hard clams, Mercenaria mercenaria, within the context of harmful algal bloom mitigation. Mar. Biol. 2004, 144, 553–565. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Yu, Z.; Zhang, P.; Cao, X.; Yuan, Y. Impact assessment of modified clay on embryo-larval stages of turbot Scophthalmus maximus L. J. Oceanol. Limnol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Lin, M.-Z.; Li, W.-X.; Hu, T.; Bu, H.; Li, Z.-L.; Wu, T.; Wu, X.-X.; Sun, C.; Li, Y.; Jiang, G.-B. One-step removal of harmful algal blooms by dual-functional flocculant based on self-branched chitosan integrated with flotation function. Carbohydr. Polym. 2021, 259, 117710. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.H.; Henry, M.S.; Higham, C.J.; Blum, P.; Sengco, M.R.; Anderson, D.M. Removal of harmful algal cells (Karenia brevis) and toxins from seawater culture by clay flocculation. Harmful Algae 2004, 3, 141–148. [Google Scholar] [CrossRef]
- Yu, Z.; Rao, S. Impact of halloysite on growth of Psuedonitzschia pungens F. multiseries and production of algal toxin. Oceanol. Limnol. Sin./Haiyang Yu Huzhao 1998, 29, 47–52. [Google Scholar]
- Haubois, A.-G.; Bricelj, V.M.; Naar, J. Transfer of brevetoxins to a tellinid bivalve by suspension-and deposit-feeding and its implications for clay mitigation of Karenia brevis blooms. Mar. Biol. 2007, 151, 2003–2012. [Google Scholar] [CrossRef]
- Awasthi, A.; Jadhao, P.; Kumari, K. Clay nano-adsorbent: Structures, applications and mechanism for water treatment. SN Appl. Sci. 2019, 1, 1076. [Google Scholar] [CrossRef]
- Huang, Y.; Pang, Y.; Wang, G.; Han, R.; Wang, J.; Zhang, P.; Xu, L. Using PAC-modified clays to control black-bloom-induced black suspended matter in Lake Taihu: Deposition and resuspension of black matter/clay flocs. Water Sci. Technol. Water Supply 2016, 16, 180–185. [Google Scholar] [CrossRef]
- Yukselen, M.A.; Gregory, J. Properties of flocs formed using different coagulants. Water Sci. Technol. Water Supply 2002, 2, 95–101. [Google Scholar] [CrossRef]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Larkin, S.L.; Adams, C.M. Harmful algal blooms and coastal business: Economic consequences in Florida. Soc. Nat. Resour. 2007, 20, 849–859. [Google Scholar] [CrossRef]
- Wang, M.; Cao, X.; Zhang, B.; Mu, Q.; Song, X.; Yu, Z. Single source with series modifications: New method for preparing modified clay to control harmful algae blooms. Mater. Des. 2023, 232, 112077. [Google Scholar] [CrossRef]
- Llamas-Orozco, J.A.; Meng, F.; Walker, G.S.; Abdul-Manan, A.F.N.; MacLean, H.L.; Posen, I.D.; McKechnie, J. Estimating the environmental impacts of global lithium-ion battery supply chain: A temporal, geographical, and technological perspective. PNAS Nexus 2023, 2, pgad361. [Google Scholar] [CrossRef] [PubMed]
- Reutter, F.; Lehmann, P. Environmental trade-offs of (de) centralized renewable electricity systems. Energy Sustain. Soc. 2024, 14, 37. [Google Scholar] [CrossRef]
- Choi, D.Y.; Wittig, T.W.; Kluever, B.M. An evaluation of bird and bat mortality at wind turbines in the Northeastern United States. PLoS ONE 2020, 15, e0238034. [Google Scholar] [CrossRef]

| HAB Species | Modified Clay Treatment | Conc Clay (g L−1) | Environmental Conditions Tested | Removal Efficiency (RE) (%) | Findings | Ref. |
|---|---|---|---|---|---|---|
| Aureococcus anophagefferens | Aluminium sulfate-Modified clay (AS-MC) | 0.1–2.0 | pH: 3–11; DW vs. SW; pH: 7–11; SW; Temp: 15–25 °C | 95—5% (pH 3–7, DW) ~70% (pH 7–11, SW) | DW: 95%–5%; SW: 95%–30% (pH 3–7); Improves to ~70% at pH 11 | [118] |
| SW: 30% at pH 7 increases to ~70% at pH 11 | ||||||
| Aluminium chloride (AC-MC) | 0.1–2.0 | pH: 3–11; DW vs. SW; pH: 7–11; SW; Temp: 15–25 °C | 95—22% (pH 3–7, DW) ~70% (pH 7–11, SW) | DW: 95%–22%; SW: 95%–35%; up to ~70% at pH 11; | ||
| SW: 30% at pH 7 increases to ~70% at pH 11 | ||||||
| Poly aluminium chloride (PAC-MC) | 0.1–2.0 | pH: 3–11; DW vs. SW; pH: 5–11; SW; Temp: 15–25 °C | 95—20% (pH 3–7, DW) ~75% (pH 7–11, SW) | DW: ~90% at pH 3; SW: 20–60% (pH 3–5); ~75% at pH > 7 | ||
| SW: 20% at pH 5 increases ~75% at pH 11 | ||||||
| Phaeocystis globosa | PAC-MC | 0.5–1.0 | pH: 3 to 11; temp: 10–40 °C | 26–44.6% (sulphate removed) 24.5% (sulphate restored) ~75% at pH 11 | Salinity improves when sulfate is removed; increase in pH increases RE. | [104] |
| Skelotonema costatum | PAC-MC | 0.025–2.00 | Turbidity, pH stability, | 98.79% at 1 g L−1; >97% at 0.25 g L−1; 98.98% with sediment; 97.81% without sediment | 0.25 g L−1 preferred due to high RE minimal turbidity; sediment enhances removal. pH > 7.77 supported effective coagulation prevented re-blooming | [102] |
| Scrippsiella trochoidea cysts | Kaolin, and polymeric aluminium chloride PACl | 0.1, 0.5 & 1.0 | Removal efficiency. final cyst formation; germination rate. | 69.1%—0.1 g L−1; 94.3%—0.5 g L−1; 97.7%—1.0 g L−1 | Cyst formation increased with clay concentration (17.9%, 22% & 24.6%); germination rate decreased with increase in clay concentration (71.3%, 47.5% & 53.3%). | [119] |
| Prymnesium parvum | Wet Bentonite + PAC | 0.05 & 0.5 | pH: 8; temp: 20 °C; cell densities: (1 × 106 & 1 × 108 cells L−1). | 64%—0.05 g L−1; 77%—0.5 g L−1 | Highest removal 0.5 g L−1 & at lower cell density temperature, pH and salinity kept constant. | [120] |
| Gymnodinium breve | Florida hosphatic clay (IMC-P2 + PAC) | 0.25 | pH: 6.98 Temp: 20 °C | ~90% | PAC enhanced removal | [107] |
| Aureococcus anophagefferens | Kaolinite acid treated clay (H-DP) | 4.0 | pH: 4.86 Temp: 20 °C | ~85% | Required mixing for effective removal | |
| Margalefidinium polykrikoides | Sophorolipid-yellow-modified clay (SMC) | 0.01 & 0.02 | Temp: 20 °C | 0.01 g L−1—80% 0.02 g L−1—90% | [56] | |
| Margalefidinium polykrikoides | SMC | 0.005 (Sophorolipid) + 1.0 (yellow clay) | pH: 3.5 Temp: 25 °C | 95% | 95% removal efficiency within 30 min, outperforming yellow clay alone (10 g L−1) | [121] |
| Chattonella marina | Ethylene bis (dodecyl dimethyl ammonium bromide) (EDAB) | 0.003 | Temp: 22 °C | 3.0 mg/L 100% | Doses depend at higher removal efficiency and higher dose | [122] |
| Microcystis aeruginosa | Hexadecyltrimethyl ammonium bromide (CTAB) | 0.02 (clay-lake sediment) + 0.8 CTAB | Temp: 22 °C | 98.92% | CTAB concentration 0.1 to 1 g L−1 had RE of >90% | [123] |
| HAB Species | Modified Clay Treatment | Conc Clay (g L−1) | Environmental Conditions Tested | Removal Efficiencies (RE) (%) | Findings | Ref. |
|---|---|---|---|---|---|---|
| Alexandrium tamrense | Kaolinite clay + Polyaluminum chloride (K-PAC) | 0.25 g L−1 | Temp: 20 ± 1 °C Cell densities: (3.1 × 107 cells L−1) | >90% | RE increased when concentration of K-PAC increased (2.0 g L−1—99.35%); detoxification of PSTs Nutrient removal | [112] |
| Montmorillonite intercalated with palmityl sulfobetaine | 0.02 g L−1 | pH: 8 Temp: 22 ± 1 °C Cell densities: (3.1 × 106 cells L−1) | ~70% | Removal increased with sulfo betaine content in clay. | [42] | |
| Alexandrium pacificum | (K-PAC) | 0.2, 0.4, 0.6 & 0.8 g L−1 | Temp: 20 ± 1 °C pH: 8.9 Cell densities: (1.0 × 104 cells L−1) | 1 g L−1—~99% 0.6 g L−1—~90% 0.2 g L−1—~75% | RE increased with increase in concentration of K-PAC | [37] |
| Kaolin + potassium peroxymonosulfate (PMPS-MC) | 0.005 & 0.01 g L−1 | Temp: 20 ± 1 °C Cell densities: (2.3 × 106 cells L−1) | >95% | RE achieved within 3 h; increased when pH was >8 | [128,129] | |
| (PMPS-MC) (Toxin removal experiment) | 0.005 & 0.01 g L−1 | Temp: 20 ± 1 °C Cell densities: (2.3 × 106 cells L−1) | >93% | 29–46% toxin reduction via transformation (i.e., GTX1&4 to GTX2&3 and C1&C2) in 15 min; 46–50% toxicity reduction compared to control | ||
| Alexandrium minutum | Kaolinite + Polyaluminum chloride (KPAC) | 0.05, 0.1, 025 & 0.3 g L−1 | Salinity: ~32 psu Temp: 20 ± 1 °C pH: 7 & 8 Cell densities: (1.0 × 107 & 2.0 × 107 cells L−1) | 100% | Best RE 0.1 g L−1 with no significant difference in RE compared to other concentrations; pH had no significant effect. KPAC prepared with seawater | [13] |
| HAB species | Modified clay treatment | Conc clay (g L−1) | Environmental conditions tested | Resting cyst formation and germination rate | Findings | Ref. |
| Alexandrium pacificum cysts | Kaolinite clay + Polyaluminum chloride (K-PAC) | 0.2, 0.4, 0.6 & 0.8 g L−1 | Temp: 20 ± 1 °C pH: 8.9 Cell densites: (1.0 × 104 cells L−1) | formation rate Control—29.7% 1 g L−1—~12% 0.6 g L−1—~15.5% 0.2 g L−1—~30% | Higher MC reduced resting cyst formation and germination; MC concentrations > 0.4 g/L reduced “seed” cysts for future blooms | [37] |
| germination rate Control—68.0% 1 g L−1—~26.5% 0.6 g L−1—~171.4% 0.2 g L−1—~68.6% |
| Species Tested | Clay Type & Concentration | Study | Exposure/Conditions | Findings | Ref. |
|---|---|---|---|---|---|
| Argopecten irradians (Bay scallop) | Modified clay 0.1 & 0.5 g L−1 | Laboratory | 16-day exposure to Alexandrium tamarense bloom | MC treatment: toxin accumulation in scallop tissues was greatly reduced: ~13.5% of initial toxin incorporated at 0.1 g L−1, and almost none at 0.5 g L−1. Toxins in sediments were rapidly detoxified, falling below detection within 4 days, whereas toxins persisted in scallop tissues for ~16 day | [154] |
| Ampelisca abdita, Leptocheirus plumulosus (Infaunal amphipods) | Phosphatic clay + polyaluminum hydroxy chloride (PAHC) 0.0005, 0.005 & 0.05 g L−1 | Laboratory | Acute and chronic toxicity tests with Karenia brevis and phosphatic clay + PAC | Phosphatic clay + PAC; mostly non-toxic; K. brevis moderately toxic to L. plumulosus | |
| Cyprinodon variegatus (Larval sheepshead minnows) | K. brevis alone: highly toxic; Phosphatic clay + PAHC; did not consistently reduce toxicity; Phosphatic clay alone; mostly non-toxic | [155] | |||
| Palaemonetes pugio (Embryos of grass shrimp) | Phosphatic clay + PAC mostly non-toxic | ||||
| Brachionus plicatilis (Rotifer) | PAC-modified clay 0.1–1.0 g L−1 | Simulated laboratory bloom | 2 h and 2 d exposures during K. mikimotoi bloom | 0.1 g L−1: minimal effect on population and reproduction. higher concentrations (0.5–1.0 g L−1) not tested on rotifers specifically | [156] |
| Mytilus edulis (Blue mussels) | Sedimentation to benthos during K. mikimotoi bloom | 0.1 g L−1: minimal impact; 0.5–1.0 g L−1: gill/digestive gland damage, reduced filtering rate, enzymes, condition index, and increased mortality | |||
| Callinectes sapidus (Adult blue crabs) | PAC-modified clay (Modified Clay II) 0.5 g L−1 | Laboratory | 8-day exposure to clay alone, K. brevis, or clay + K. brevis | PAC-modified clay alone: no effect; PAC-modified clay reduced K. brevis concentration by 95%; no significant impact on mortality or behavioral reflexes | [157] |
| Callinectes sapidus (Adult blue crabs) | PAC-modified clay (Modified Clay II) 0.2 g L−1 | Simulated bloom in 1400 L mesocosm | 72-h exposure with K. brevis bloom-level density (1 × 106 cells/L) | RE of 57% of K. brevis cells after 8 h and 95% after 48 h; No significant lethal or sublethal effects on blue crabs, sea urchins, or hard clams at 72 h; Suggests MC II at 0.2 g/L is environmentally safe for these benthic species under short-term exposure | [158] |
| Lytechinus variegatus (Sea urchin) | |||||
| Mercenaria campechiensis (Hard clam) | |||||
| Haliotis discus hannai (Abalone) | Modified clay | Laboratory and field experiments | Short-term (1–15 days) and recovery period (16–30 days) in lab; typical aquaculture field conditions | Survival: No significant effect at tested concentrations Decrease in shell length and weight day 1–15 rapid recovery day 16–30 | [159] |
| Mercenaria mercenaria (Juvenile hard clams) | Phosphatic clay | Large flume (simulated field) | 2-week exposure, low-flow (sedimentation) vs. high-flow (suspension) with nontoxic algae | Low-flow: minor or no growth inhibition; High-flow: strong growth reduction (~90%); no mortality in either case | [160] |
| Scophthalmus maximus (Turbot embryos) | PAC-modified clay 0–1.7 g L−1 | Large flume (simulated field) | 24–48 h exposure | LC50: 1.70 g L−1 (24 h), 1.65 g L−1 (48 h); Safe concentration: 0.47 g L−1; Hatchability not affected at ≤0.5 g L−1; deformities increased above 0.5 g L−1; growth and yolk absorption peaked at g L−1, then decreased | [161] |
| Modified Clay Process | Procedure | Relative Carbon Impact |
|---|---|---|
| 1. Calcination | 750 °C for 2 h | High (most energy intensive) |
| 2. Acid etching | Sitrred at stirred for 40 minuted at a fixed stirring rate of 400 r/min at 93 °C. | Medium |
| 3. Alkali neutralization | Addition of Sodium hydroxide (NaOH) to adjust pH | Low |
| 4. Raw clay supplementation | Addition of raw clay to ratio 2:1(addition of weight) | Low |
| 5. Aging | at 500 rpm at 70 °C for 3 h | Low-medium |
| 6. Drying | 70 °C | Low-medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwambai, C.S.; Ennaceri, H.; Lymbery, A.J.; Laird, D.W.; Cosgrove, J.; Moheimani, N.R. Toxic Alexandrium Treatment in Western Australia: Investigating the Efficacy of Modified Nano Clay. Toxins 2025, 17, 495. https://doi.org/10.3390/toxins17100495
Kwambai CS, Ennaceri H, Lymbery AJ, Laird DW, Cosgrove J, Moheimani NR. Toxic Alexandrium Treatment in Western Australia: Investigating the Efficacy of Modified Nano Clay. Toxins. 2025; 17(10):495. https://doi.org/10.3390/toxins17100495
Chicago/Turabian StyleKwambai, Cherono Sheilah, Houda Ennaceri, Alan J. Lymbery, Damian W. Laird, Jeff Cosgrove, and Navid Reza Moheimani. 2025. "Toxic Alexandrium Treatment in Western Australia: Investigating the Efficacy of Modified Nano Clay" Toxins 17, no. 10: 495. https://doi.org/10.3390/toxins17100495
APA StyleKwambai, C. S., Ennaceri, H., Lymbery, A. J., Laird, D. W., Cosgrove, J., & Moheimani, N. R. (2025). Toxic Alexandrium Treatment in Western Australia: Investigating the Efficacy of Modified Nano Clay. Toxins, 17(10), 495. https://doi.org/10.3390/toxins17100495






