Exploring the Venom Diversity of Australian Taipans: Comparative Characterization of Oxyuranus microlepidotus and Oxyuranus scutellatus
Abstract
1. Introduction
2. Results and Discussion
2.1. Venom Profile
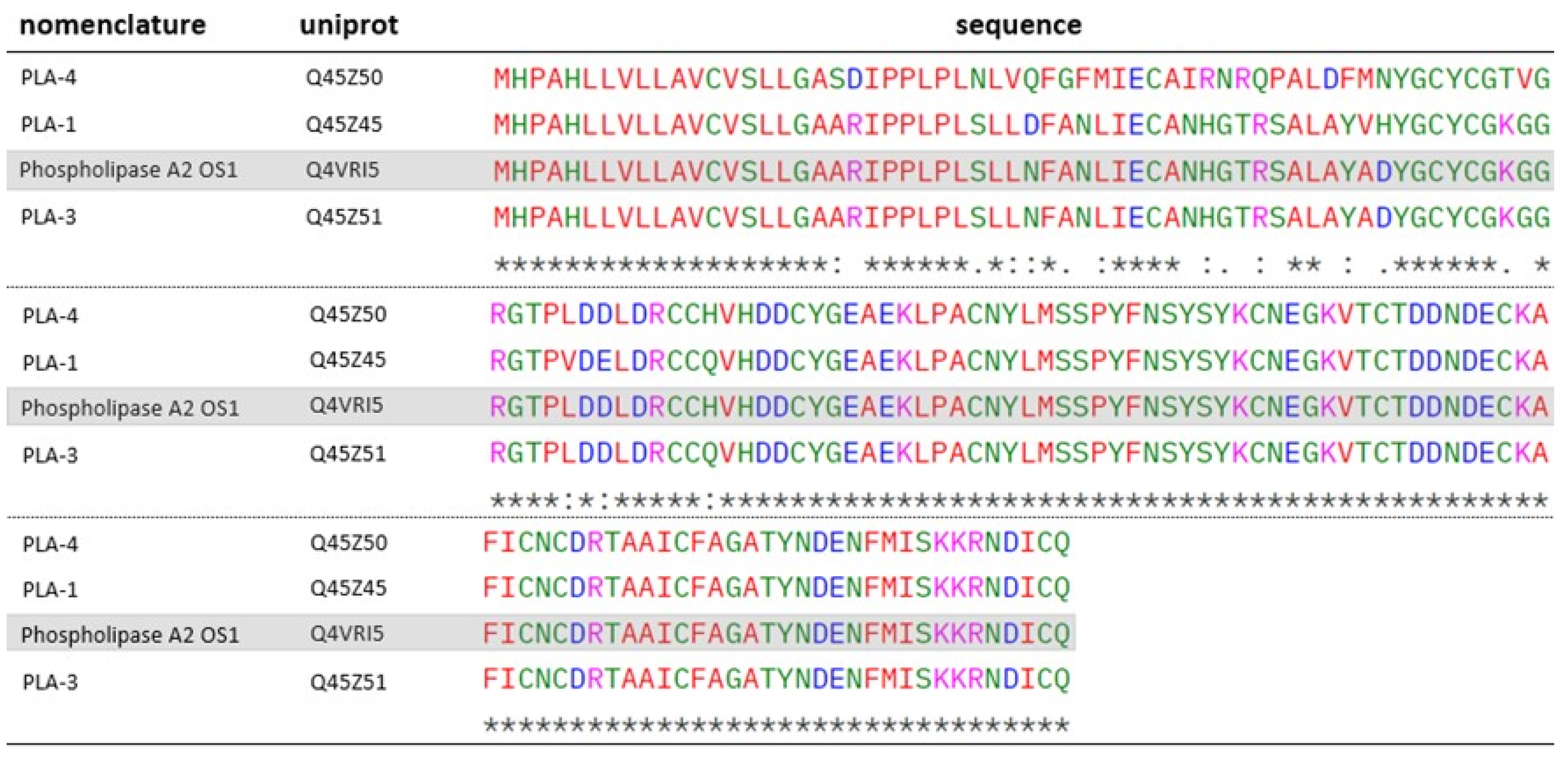
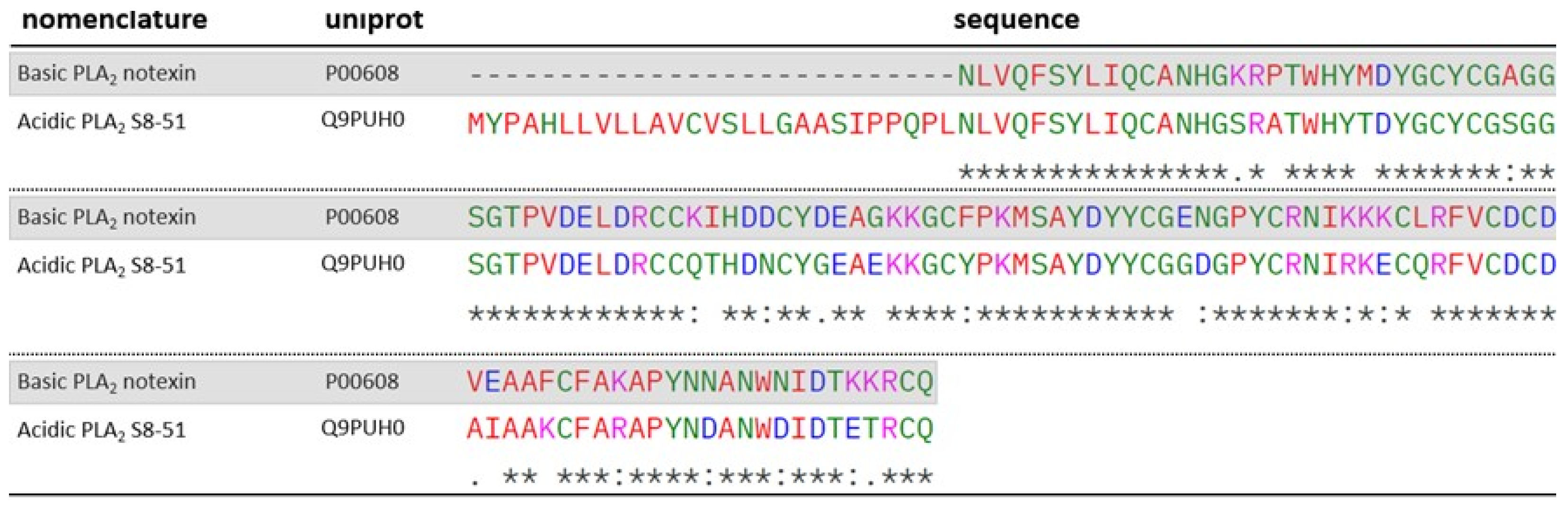
2.2. Phospholipases A2 (PLA2)
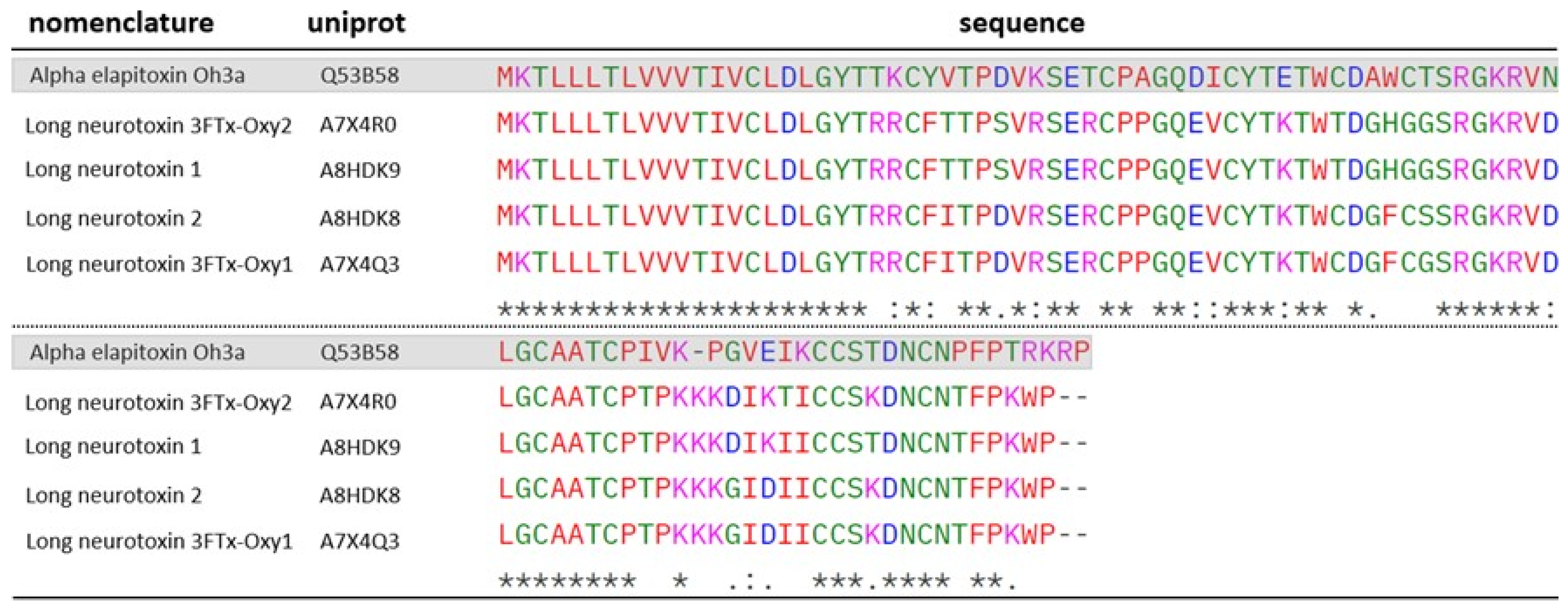
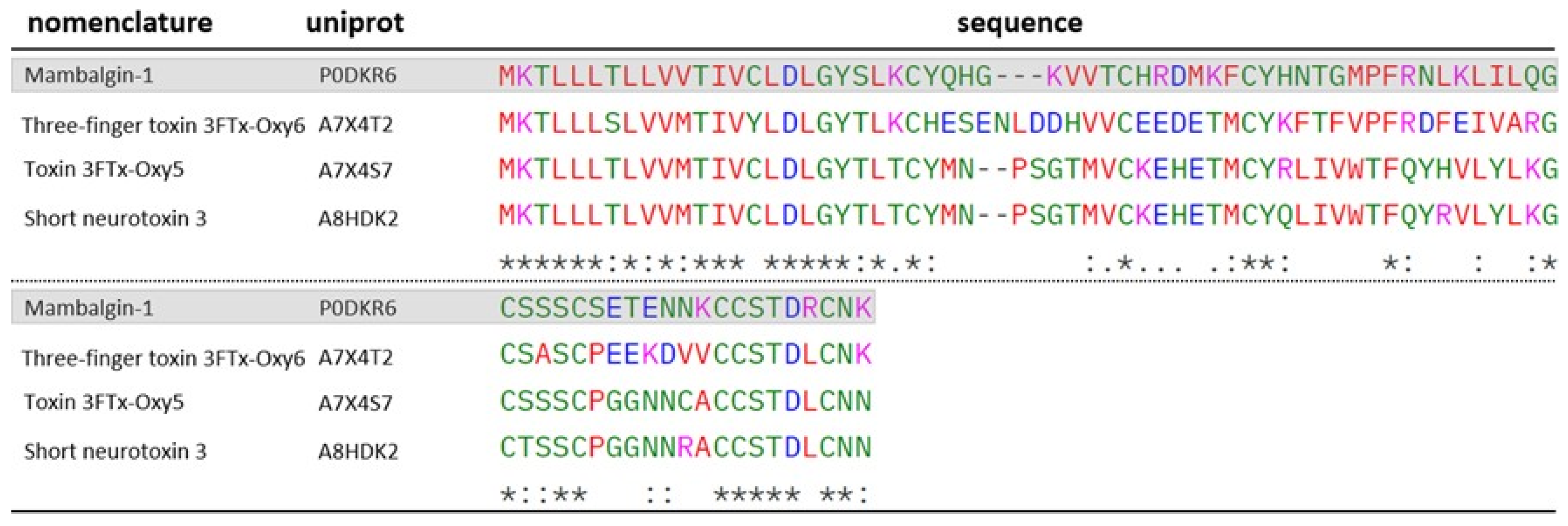
2.3. Three-Fingers Toxins (3FTx)
2.4. Other Proteins
3. Conclusions
4. Materials and Methods
4.1. Venom Fractioning
4.2. Fractions Characterization
4.2.1. Protein Digestion
4.2.2. Analysis by LC-ESI-IT-TOF/MS
4.2.3. Analysis by MALDI-TOF/MS
4.2.4. Proteomic Data Processing and Data Analysis
4.2.5. Quantitative Analysis of Venom Proteins
4.3. Computational Pocket Mapping
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardy, M.C.; Cochrane, J.; Allavena, R.E. Venomous and poisonous Australian animals of veterinary importance: A rich source of novel therapeutics. BioMed Res. Int. 2014, 2014, 671041. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Wüster, W.; Fry, B.G. The good, the bad and the ugly: Australian snake taxonomists and a history of the taxonomy of Australia’s venomous snakes. Toxicon 2006, 48, 919–930. [Google Scholar] [CrossRef]
- Jackson, T.N.W.; Sunagar, K.; Undheim, E.A.B.; Koludarov, I.; Chan, A.H.C.; Sanders, K.; Ali, S.A.; Hendrikx, I.; Dunstan, N.; Fry, B.G. Venom down under: Dynamic evolution of Australian elapid snake toxins. Toxins 2013, 5, 2621–2655. [Google Scholar] [CrossRef]
- Kocholaty, W.F.; Ledford, E.B.; Daly, J.G.; Billings, T.A. Toxicity and some enzymatic properties and activities in the venoms of Crotalidae, Elapidae and Viperidae. Toxicon 1971, 9, 131–138. [Google Scholar] [CrossRef]
- Doughty, P.; Maryan, B.; Donnellan, S.C.; Hutchinson, M.N. A new species of taipan (Elapidae: Oxyuranus) from central Australia. Zootaxa 2007, 1422, 45–58. [Google Scholar] [CrossRef]
- Kornhauser, R.; Hart, A.J.; Reeve, S.; Smith, A.I.; Fry, B.G.; Hodgson, W.C. Variations in the pharmacological profile of post-synaptic neurotoxins isolated from the venoms of the Papuan (Oxyuranus scutellatus canni) and coastal (Oxyuranus scutellatus scutellatus) taipans. Neurotoxicology 2010, 31, 239–243. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Trevett, A.J.; Korinhona, A.; Nwokolo, N.; Laurenson, I.F.; Paul, M.; Black, J.; Naraqi, S.; Mavo, B.; Saweri, A. Snakebites by the Papuan taipan (Oxyuranus scutellatus canni): Paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am. J. Trop. Med. Hyg. 1995, 52, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.M.; Madaras, F.; Turnbull, R.K.; Morley, T.; Dunstan, N.; Allen, L.; Kuchel, T.; Mirtschin, P.; Hodgson, W.C. Comparative studies of the venom of a new Taipan species, Oxyuranus temporalis, with other members of its genus. Toxins 2014, 6, 1979–1995. [Google Scholar] [CrossRef] [PubMed]
- Crachi, M.T.; Hammer, L.W.; Hodgson, W.C. The effects of antivenom on the in vitro neurotoxicity of venoms from the taipans Oxyuranus scutellatus, Oxyuranus microlepidotus and Oxyuranus scutellatus canni. Toxicon 1999, 37, 1771–1778. [Google Scholar] [CrossRef]
- Brennan, K.E.C.; Morley, T.; Hutchinson, M.; Donnellan, S. Redescription of the western desert taipan, Oxyuranus temporalis (Serpentes: Elapidae), with notes on its distribution, diet and genetic variation. Aust. J. Zool. 2011, 59, 227. [Google Scholar] [CrossRef]
- Johnston, C.I.; Ryan, N.M.; O’Leary, M.A.; Brown, S.G.A.; Isbister, G.K. Australian taipan (Oxyuranus spp.) envenoming: Clinical effects and potential benefits of early antivenom therapy—Australian Snakebite Project (ASP-25). Clin. Toxicol. 2017, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Bal, B. Papuan Taipan (‘Oxyuranus scutellatus canni’) envenomation in rural Papua New Guinea. Ann. ACTM Int. J. Trop. Travel Med. 2003, 4, 6–9. [Google Scholar]
- Su, M.J.; Chang, C.C. Presynaptic effects of snake venom toxins which have phospholipase A2 activity (beta-bungarotoxin, taipoxin, crotoxin). Toxicon 1984, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, M.C.; Yen, C.H.; Hseu, M.J.; Tseng, C.C.; Tsai, M.D.; Dupureur, C.M. Binding proteins on synaptic membranes for crotoxin and taipoxin, two phospholipases A2 with neurotoxicity. Toxicon 1995, 33, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fathi, B.; Harvey, A.L.; Rowan, E.G. The effect of temperature on the effects of the phospholipase A neurotoxins beta-bungarotoxin and taipoxin at the neuromuscular junction. Toxicon 2013, 70, 86–89. [Google Scholar] [CrossRef]
- Treppmann, P.; Brunk, I.; Afube, T.; Richter, K.; Ahnert-Hilger, G. Neurotoxic phospholipases directly affect synaptic vesicle function. J. Neurochem. 2011, 117, 757–764. [Google Scholar] [CrossRef]
- Harris, J.B.; Maltin, C.A. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br. J. Pharmacol. 1982, 76, 61–75. [Google Scholar] [CrossRef]
- Possani, L.D.; Martin, B.M.; Yatani, A.; Mochca-Morales, J.; Zamudio, F.Z.; Gurrola, G.B.; Brown, A.M. Isolation and physiological characterization of taicatoxin, a complex toxin with specific effects on calcium channels. Toxicon 1992, 30, 1343–1364. [Google Scholar] [CrossRef]
- Earl, S.T.H.; Richards, R.; Johnson, L.A.; Flight, S.; Anderson, S.; Liao, A.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Identification and characterisation of Kunitz-type plasma kallikrein inhibitors unique to Oxyuranus sp. snake venoms. Biochimie 2012, 94, 365–373. [Google Scholar] [CrossRef]
- Su, M.C.; Lee, S.Y.; Tan, C.T.; Su, C.C.; Li, S.Y.; Lin, R.H.; Hung, C.C.; Lin, M.J. Taicatoxin inhibits the calcium-dependent slow motility of mammalian outer hair cells. Hear. Res. 2005, 203, 172–179. [Google Scholar] [CrossRef]
- Rouault, M.; Rash, L.D.; Escoubas, P.; Boilard, E.; Bollinger, J.; Lomonte, B.; Maurin, T.; Guillaume, C.; Canaan, S.; Deregnaucourt, C.; et al. Neurotoxicity and other pharmacological activities of the snake venom phospholipase A2 OS2: The N-terminal region is more important than enzymatic activity. Biochemistry 2006, 45, 5800–5816. [Google Scholar] [CrossRef]
- Herrera, M.; Fernández, J.; Vargas, M.; Villalta, M.; Segura, Á.; León, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef]
- St Pierre, L.; Fischer, H.; Adams, D.J.; Schenning, M.; Lavidis, N.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Distinct activities of novel neurotoxins from Australian venomous snakes for nicotinic acetylcholine receptors. Cell. Mol. Life Sci. 2007, 64, 2829–2840. [Google Scholar] [CrossRef]
- Fry, B.G.; Wickramaratana, J.C.; Lemme, S.; Beuve, A.; Garbers, D.; Hodgson, W.C.; Alewood, P. Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): Isolation, chemical and biological characterization. Biochem. Biophys. Res. Commun. 2005, 327, 1011–1015. [Google Scholar] [CrossRef]
- Morrison, J.; Pearn, J.; Covacevich, J.; Tanner, C.; Coulter, A. Studies on the venom of Oxyuranus microlepidotus. J. Toxicol. Clin. Toxicol. 1983, 21, 373–385. [Google Scholar] [CrossRef]
- Broad, A.J.; Sutherland, S.K.; Coulter, A.R. The lethality in mice of dangerous Australian and other snake venom. Toxicon 1979, 17, 661–664. [Google Scholar] [CrossRef]
- Tasoulis, T.; Silva, A.; Veerati, P.C.; Baker, M.; Hodgson, W.C.; Dunstan, N.; Isbister, G.K. Intra-Specific Venom Variation in the Australian Coastal Taipan Oxyuranus scutellatus. Toxins 2020, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- van Thiel, J.; Alonso, L.L.; Slagboom, J.; Dunstan, N.; Wouters, R.M.; Modahl, C.M.; Vonk, F.J.; Jackson, T.N.W.; Kool, J. Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.). Toxins 2023, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Wong, H.Y.; Desai, M.; Moochhala, S.; Kuchel, P.W.; Kini, R.M. Identification of a novel family of proteins in snake venoms. J. Biol. Chem. 2003, 278, 40097–40104. [Google Scholar] [CrossRef]
- Wilkinson, T.S.; Roghanian, A.; Simpson, A.J.; Sallenave, J.M. WAP domain proteins as modulators of mucosal immunity. Biochem. Soc. Trans. 2011, 39, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.G.; Fry, B.G.; Alewood, P.; Kumar, P.P.; Kini, R.M. Antimicrobial activity of omwaprin, a new member of the Waprin family of snake venom proteins. Biochem. J. 2007, 402, 93–104. [Google Scholar] [CrossRef]
- Thankappan, B.; Angayarkanni, J. Biological characterization of omw1 and omw2: Antimicrobial peptides derived from omwaprin. 3 Biotech 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Dhananjaya, B.L.; D’Souza, C.J.M. The pharmacological role of nucleotidases in snake venoms. Cell Biochem. Funct. 2010, 28, 171–177. [Google Scholar] [CrossRef]
- Samel, M.; Vija, H.; Kurvet, I.; Künnis-Beres, K.; Trummal, K.; Subbi, J.; Kahru, A.; Siigur, J. Interactions of PLA2-s from Vipera lebetina, Vipera berus berus and Naja naja oxiana venom with platelets, bacterial and cancer cells. Toxins 2013, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Zieler, H.; Keister, D.B.; Dvorak, J.A.; Ribeiro, J.M. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J. Exp. Biol. 2001, 204, 4157–4167. [Google Scholar] [CrossRef] [PubMed]
- Gebrim, L.C.; Marcussi, S.; Menaldo, D.L.; de Menezes, C.S.R.; Nomizo, A.; Hamaguchi, A.; Silveira-Lacerda, E.P.; Homsi Brandeburgo, M.I.; Sampaio, S.V.; Soares, A.M.; et al. Antitumor effects of snake venom chemically modified Lys49 phospholipase A2-like BthTX-I and a synthetic peptide derived from its C-terminal region. Biologicals 2009, 37, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Fohlman, J.; Eaker, D.; Karlsoon, E.; Thesleff, S. Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties. Eur. J. Biochem. 1976, 68, 457–469. [Google Scholar] [CrossRef]
- Cendron, L.; Mičetić, I.; Polverino de Laureto, P.; Paoli, M. Structural analysis of trimeric phospholipase A2 neurotoxin from the Australian taipan snake venom. FEBS J. 2012, 279, 3121–3135. [Google Scholar] [CrossRef]
- Harrison, J.A.; Aquilina, J.A. Insights into the subunit arrangement and diversity of paradoxin and taipoxin. Toxicon 2016, 112, 45–50. [Google Scholar] [CrossRef]
- Hodgson, W.C.; Dal Belo, C.A.; Rowan, E.G. The neuromuscular activity of paradoxin: A presynaptic neurotoxin from the venom of the inland taipan (Oxyuranus microlepidotus). Neuropharmacology 2007, 52, 1229–1236. [Google Scholar] [CrossRef]
- Apel, A.R.; Hoban, K.; Chuartzman, S.; Tonikian, R.; Sidhu, S.; Schuldiner, M.; Wendland, B.; Prosser, D. Syp1 regulates the clathrin-mediated and clathrin-independent endocytosis of multiple cargo proteins through a novel sorting motif. Mol. Biol. Cell 2017, 28, 2434–2448. [Google Scholar] [CrossRef]
- Oishi, Y.; Arakawa, T.; Tanimura, A.; Itakura, M.; Takahashi, M.; Tajima, Y.; Mizoguchi, I.; Takuma, T. Role of VAMP-2, VAMP-7, and VAMP-8 in constitutive exocytosis from HSY cells. Histochem. Cell Biol. 2006, 125, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, D.; Pennuto, M.; Rigoni, M.; Rossetto, O.; Montecucco, C.; Valtorta, F. Taipoxin induces synaptic vesicle exocytosis and disrupts the interaction of synaptophysin I with VAMP2. Mol. Pharmacol. 2005, 67, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Schmid-Alliana, A.; Lazdunski, M.; Barhanin, J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J. Biol. Chem. 1990, 265, 9526–9532. [Google Scholar] [CrossRef]
- Kolko, M.; Bruhn, T.; Christensen, T.; Lazdunski, M.; Lambeau, G.; Bazan, N.G.; Diemer, N.H. Secretory phospholipase A2 potentiates glutamate-induced rat striatal neuronal cell death in vivo. Neurosci. Lett. 1999, 274, 167–170. [Google Scholar] [CrossRef]
- Jabeen, T.; Sharma, S.; Singh, N.; Singh, R.K.; Kaur, P.; Perbandt, M.; Betzel, C.; Srinivasan, A.; Singh, T.P. Crystal structure of a calcium-induced dimer of two isoforms of cobra phospholipase A2 at 1.6 Å resolution. Proteins 2005, 59, 856–863. [Google Scholar] [CrossRef]
- Takasaki, C.; Kimura, S.; Kokubun, Y.; Tamiya, N. Isolation, properties and amino acid sequences of a phospholipase A2 and its homologue without activity from the venom of a sea snake, Laticauda colubrina, from the Solomon Islands. Biochem. J. 1988, 253, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Halpert, J.; Eaker, D.; Karlsson, E. The role of phospholipase activity in the action of a presynaptic neurotoxin from the venom of Notechis scutatus scutatus (Australian tiger snake). FEBS Lett. 1976, 61, 72–76. [Google Scholar] [CrossRef]
- Zimmerman, S.E.; Yong, L.C. Nephrotoxicity of notexin in experimental mice. Exp. Toxicol. Pathol. 1995, 47, 149–155. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Hiremath, K.; Dodakallanavar, J.; Sampat, G.H.; Patil, V.S.; Harish, D.R.; Chavan, R.; Hegde, H.V.; Roy, S. Three finger toxins of elapids: Structure, function, clinical applications and its inhibitors. Mol. Divers. 2024, 28, 3409–3426. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, F.; Wolf, K.M.; Martin, B.M.; Possani, L.D.; Chiappinelli, V.A. Two novel alpha-neurotoxins isolated from the taipan snake, Oxyuranus scutellatus, exhibit reduced affinity for nicotinic acetylcholine receptors in brain and skeletal muscle. Biochemistry 1996, 35, 7910–7916. [Google Scholar] [CrossRef]
- Huynh, T.M.; Silva, A.; Isbister, G.K.; Hodgson, W.C. Isolation and pharmacological characterization of α-elapitoxin-Oh3a, a long-chain post-synaptic neurotoxin from King Cobra (Ophiophagus hannah) venom. Front. Pharmacol. 2022, 13, 815069. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555. [Google Scholar] [CrossRef]
- Trummal, K.; Tõnismägi, K.; Paalme, V.; Järvekülg, L.; Siigur, J.; Siigur, E. Molecular diversity of snake venom nerve growth factors. Toxicon 2011, 58, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.J.; Suh, B.C. Acid-sensing ion channels (ASICs): Therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013, 46, 295–304. [Google Scholar] [CrossRef]
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-sensing ion channels: Focus on physiological and some pathological roles in the brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar]
- Levi-Montalcini, R.; Angeletti, P.U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev. Biol. 1963, 7, 653–659. [Google Scholar] [CrossRef]
- Paalme, V.; Trummal, K.; Samel, M.; Tõnismägi, K.; Järvekülg, L.; Vija, H.; Subbi, J.; Siigur, J.; Siigur, E. Nerve growth factor from Vipera lebetina venom. Toxicon 2009, 54, 329–336. [Google Scholar] [CrossRef]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Fry, B.G.; Jackson, T.N.W.; Casewell, N.R.; Undheim, E.A.B.; Vidal, N.; Ali, S.A.; King, G.F.; Vasudevan, K.; Vasconcelos, V.; et al. Molecular evolution of vertebrate neurotrophins: Co-option of the highly conserved nerve growth factor gene into the advanced snake venom arsenal. PLoS ONE 2013, 8, e81827. [Google Scholar] [CrossRef]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve growth factor: Early studies and recent clinical trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef]
- Capsoni, S.; Cattaneo, A. On the molecular basis linking Nerve Growth Factor (NGF) to Alzheimer’s disease. Cell. Mol. Neurobiol. 2006, 26, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Rosing, J.; Tans, G. Structural and functional properties of snake venom prothrombin activators. Toxicon 1992, 30, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Rosing, J.; Tans, G. Snake venom prothrombin activators—The history. In Toxins and Hemostasis; Springer: Dordrecht, The Netherlands, 2010; pp. 485–499. [Google Scholar]
- Kini, R.M. The intriguing world of prothrombin activators from snake venom. Toxicon 2005, 45, 1133–1145. [Google Scholar] [CrossRef]
- St Pierre, L.; Masci, P.P.; Filippovich, I.; Sorokina, N.; Marsh, N.; Miller, D.J.; Lavin, M.F. Comparative analysis of prothrombin activators from the venom of Australian elapids. Mol. Biol. Evol. 2005, 22, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Tacon, C.L.; Munas, A.; Little, M. Case report: Rotational thromboelastometry in taipan envenomation. Am. J. Trop. Med. Hyg. 2021, 106, 746–749. [Google Scholar] [CrossRef]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake venom: From deadly toxins to life-saving therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef]
- Daniels, L.B.; Maisel, A.S. Natriuretic peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef]
- Nakao, K.; Ogawa, Y.; Suga, S.I.; Imura, H. Molecular biology and biochemistry of the natriuretic peptide system. In Molecular Reviews in Cardiovascular Medicine; Springer: Dordrecht, The Netherlands, 1996; pp. 74–82. [Google Scholar]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Vink, S.; Jin, A.H.; Poth, K.J.; Head, G.A.; Alewood, P.F. Natriuretic peptide drug leads from snake venom. Toxicon 2012, 59, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Yu, G.; Chen, Z.; Zhou, X.; Zhu, S.; Li, R.; Zhang, Y.; Lu, Q. A novel natriuretic peptide from the cobra venom. Toxicon 2011, 57, 134–140. [Google Scholar] [CrossRef]
- Chen, H.H.; Burnett Jr, J.C. Clinical application of the natriuretic peptides in heart failure. Eur. Heart J. Suppl. 2006, 8, E18–E25. [Google Scholar] [CrossRef]
- Ang, W.F.; Koh, C.Y.; Kini, R.M. From snake venoms to therapeutics: A focus on natriuretic peptides. Pharmaceuticals 2022, 15, 1153. [Google Scholar] [CrossRef]
- Krätzschmar, J.; Haendler, B.; Eberspaecher, U.; Roosterman, D.; Donner, P.; Schleuning, W.D. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur. J. Biochem. 1996, 236, 827–836. [Google Scholar] [CrossRef]
- Cohen, D.J.; Maldera, J.A.; Weigel Muñoz, M.; Ernesto, J.I.; Vasen, G.; Cuasnicu, P.S. Cysteine-Rich Secretory Proteins (CRISP) and their role in mammalian fertilization. Biol. Res. 2011, 44, 135–138. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Modahl, C.M.; Maenaka, K.; Aoki-Shioi, N. Cysteine-rich secretory proteins (CRISPs) from venomous snakes: An overview of the functional diversity in a large and underappreciated superfamily. Toxins 2020, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Snutch, T.P. Voltage-Gated Calcium Channels. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 427–441. [Google Scholar]
- Yamazaki, Y.; Brown, R.L.; Morita, T. Purification and cloning of toxins from elapid venoms that target cyclic nucleotide-gated ion channels. Biochemistry 2002, 41, 11331–11337. [Google Scholar] [CrossRef]
- Lecht, S.; Chiaverelli, R.A.; Gerstenhaber, J.; Calvete, J.J.; Lazarovici, P.; Casewell, N.R.; Harrison, R.; Lelkes, P.I.; Marcinkiewicz, C. Anti-angiogenic activities of snake venom CRISP isolated from Echis carinatus sochureki. Biochim. Biophys. Acta 2015, 1850, 1169–1179. [Google Scholar] [CrossRef]
- Adade, C.M.; Carvalho, A.L.O.; Tomaz, M.A.; Costa, T.F.R.; Godinho, J.L.; Melo, P.A.; Lima, A.P.C.A.; Rodrigues, J.C.F.; Zingali, R.B.; Souto-Padrón, T. Crovirin, a snake venom cysteine-rich secretory protein (CRISP) with promising activity against Trypanosomes and Leishmania. PLoS Negl. Trop. Dis. 2014, 8, e3252. [Google Scholar] [CrossRef]
- Zupunski, V.; Kordis, D.; Gubensek, F. Adaptive evolution in the snake venom Kunitz/BPTI protein family. FEBS Lett. 2003, 547, 131–136. [Google Scholar] [CrossRef]
- Ranasinghe, S.; McManus, D.P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 2013, 39, 219–227. [Google Scholar] [CrossRef]
- Kunitz, M.; Northrop, J.H. Isolation from beef pancreas of crystalline trypsinogen, trypsin, a trypsin inhibitor, and an inhibitor trypsin compound. J. Gen. Physiol. 1936, 19, 991–1007. [Google Scholar] [CrossRef]
- Huber, R.; Kukla, D.; Rhlmann, A.; Epp, O.; Formanek, H. The basic trypsin inhibitor of bovine pancreas. Sci. Nat. 1970, 57, 389–392. [Google Scholar] [CrossRef]
- Millers, E.K.I.; Trabi, M.; Masci, P.P.; Lavin, M.F.; de Jersey, J.; Guddat, L.W. Crystal structure of textilinin-1, a Kunitz-type serine protease inhibitor from the venom of the Australian common brown snake (Pseudonaja textilis). FEBS J. 2009, 276, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hsu, C.H.; Su, N.Y.; Lin, Y.C.; Chiou, S.H.; Wu, S.H. Solution structure of a Kunitz-type chymotrypsin inhibitor isolated from the elapid snake Bungarus fasciatus. J. Biol. Chem. 2001, 276, 45079–45087. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Twenty years of dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- Schweitz, H.; Heurteaux, C.; Bois, P.; Moinier, D.; Romey, G.; Lazdunski, M. Calcicludine, a venom peptide of the Kunitz-type protease inhibitor family, is a potent blocker of high-threshold Ca2+ channels with a high affinity for L-type channels in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Droctové, L.; Ciolek, J.; Mendre, C.; Chorfa, A.; Huerta, P.; Carvalho, C.; Gouin, C.; Lancien, M.; Stanajic-Petrovic, G.; Braco, L.; et al. A new Kunitz-type snake toxin family associated with an original mode of interaction with the vasopressin 2 receptor. Br. J. Pharmacol. 2022, 179, 3470–3481. [Google Scholar] [CrossRef]
- Morjen, M.; Honoré, S.; Bazaa, A.; Abdelkafi-Koubaa, Z.; Ellafi, A.; Mabrouk, K.; Kovacic, H.; El Ayeb, M.; Marrakchi, N.; Luis, J. PIVL, a snake venom Kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014, 95, 149–156. [Google Scholar] [CrossRef]
- Morjen, M.; Kallech-Ziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouari-Kessentini, R.; Sanz, L.; Calvete, J.J.; Srairi-Abid, N.; El Ayeb, M.; et al. PIVL, a new serine protease inhibitor from Macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. 2013, 32, 52–62. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Mackessy, S.P. Pharmacological properties and pathophysiological significance of a Kunitz-type protease inhibitor (Rusvikunin-II) and its protein complex (Rusvikunin complex) purified from Daboia russelii russelii venom. Toxicon 2014, 89, 55–66. [Google Scholar] [CrossRef]
- Jeong, J.K.; Diano, S. Prolyl carboxypeptidase and its inhibitors in metabolism. Trends Endocrinol. Metab. 2013, 24, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.H. Prolylcarboxypeptidase (PrCP) inhibitors and the therapeutic uses thereof: A patent review. Expert. Opin. Ther. Pat. 2017, 27, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Beraldo-Neto, E.; Vigerelli, H.; Coelho, G.R.; da Silva, D.L.; Nencioni, A.L.A.; Pimenta, D.C. Unraveling and profiling Tityus bahiensis venom: Biochemical analyses of the major toxins. J. Proteom. 2023, 274, 104824. [Google Scholar] [CrossRef] [PubMed]
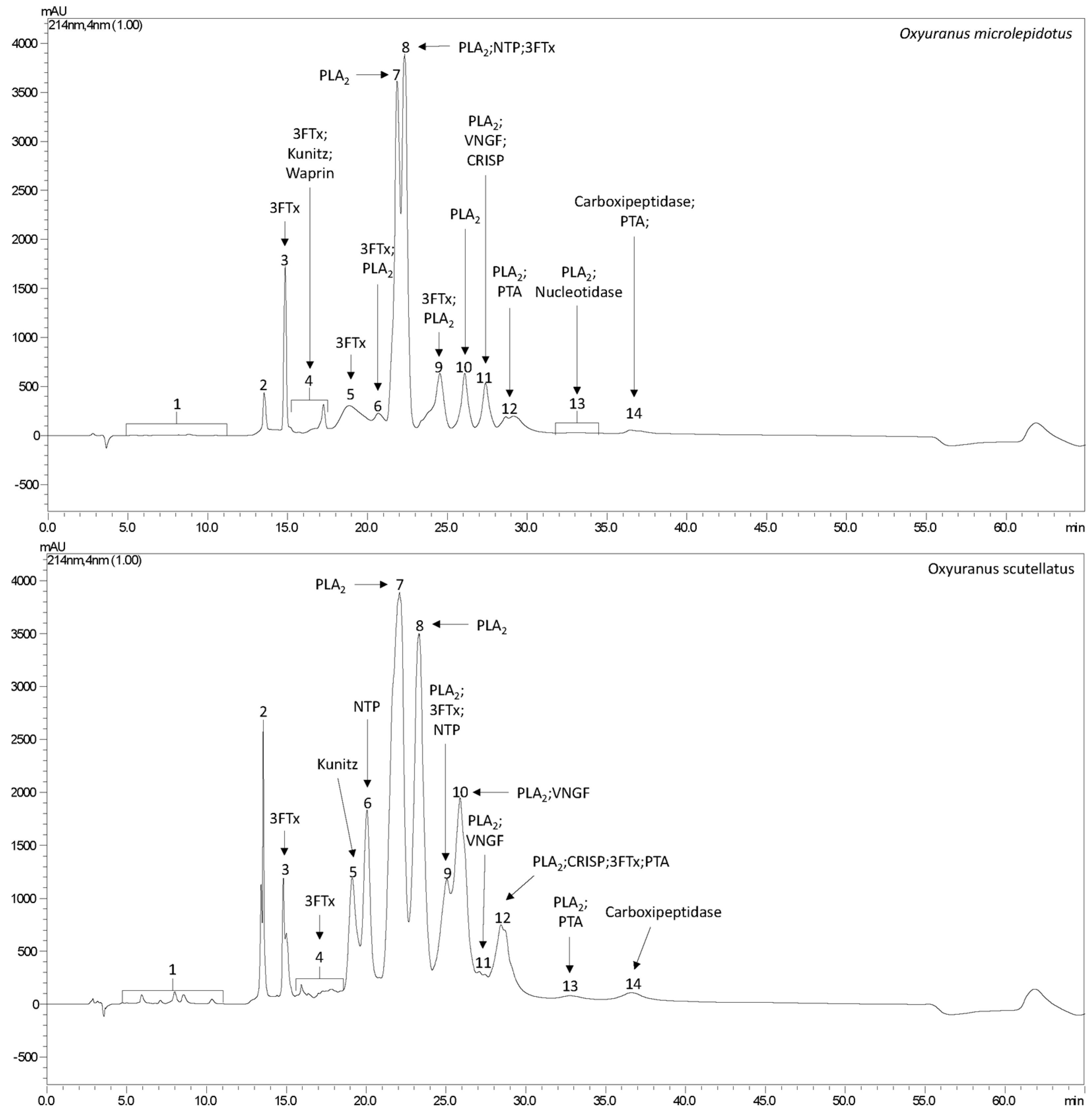
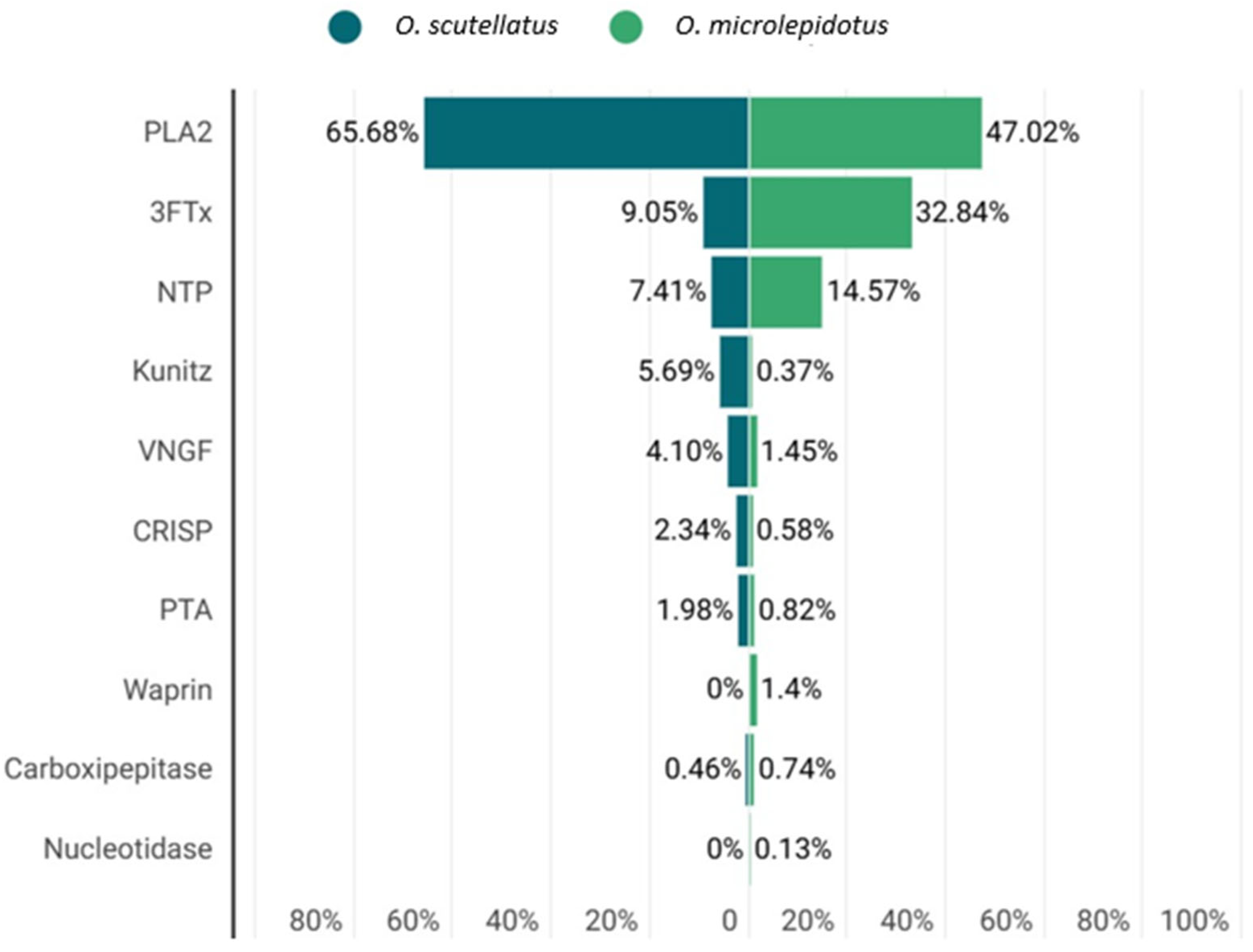
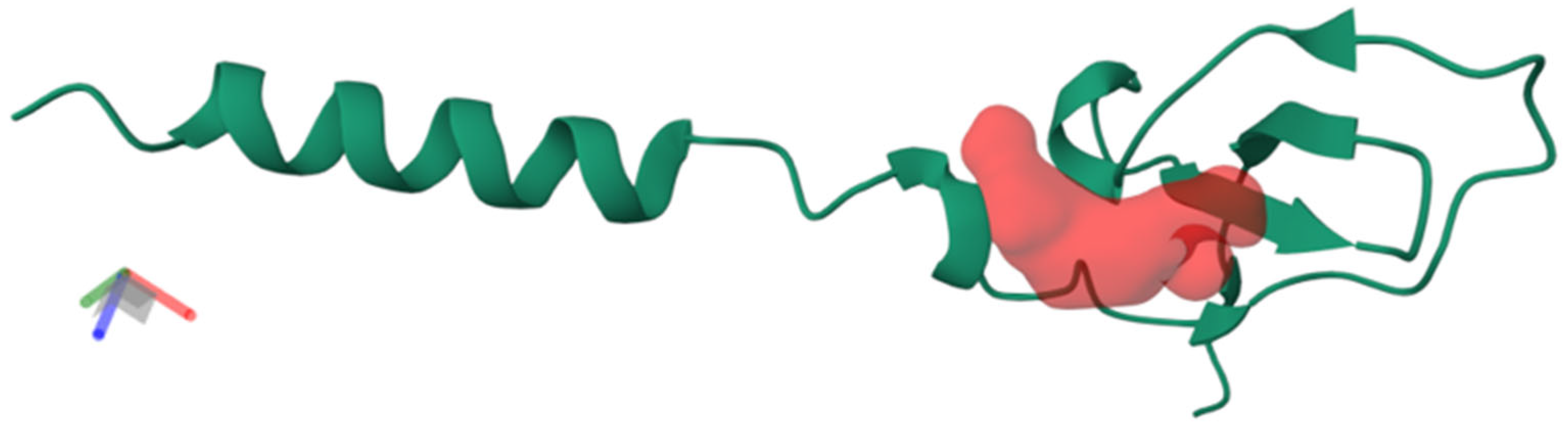

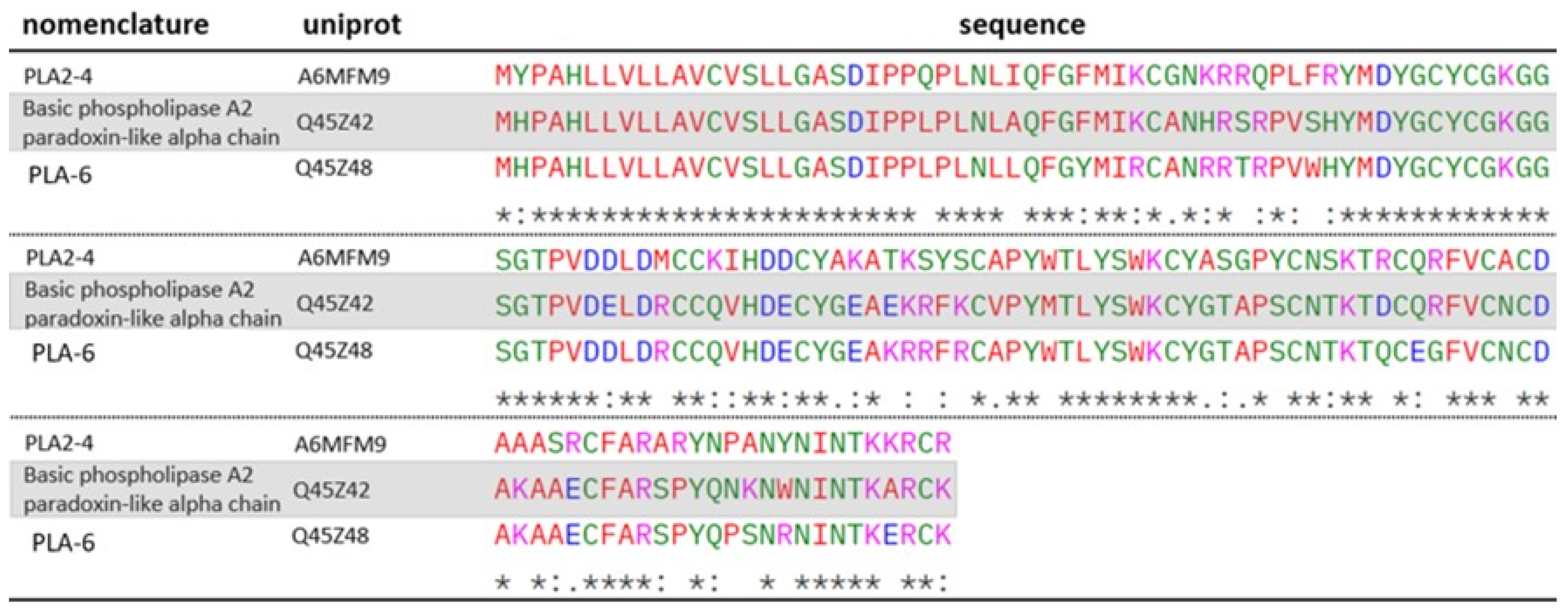


| Fraction | UNIPROT Code | Theoretical Mass (kDa) 1 | −10lgP | Peptides | Unique Peptides | Protein Description | Type |
|---|---|---|---|---|---|---|---|
| 3 | A7X4S0 | 6.79 | 137.57 | 8 | 8 | Short neurotoxin 3FTx-Oxy4 (O. microlepidotus) | 3FTx |
| 4 | P0CB06 F4 | 6.79 | 64.89 | 3 | 1 | Short neurotoxin 2 (O. scutellatus) | 3FTx |
| B7S4N9 F5 | 7 | 61.68 | 3 | 3 | Kunitz-type protease inhibitor taicotoxin (O. scutellatus) | Kunitz | |
| B5G6G7 | 5.72 | 119.78 | 12 | 12 | Omwaprin-b (O. microlepidotus) | Waprin | |
| 5 | A7X4R0 | 7.91 | 137.90 | 8 | 1 | Long neurotoxin 3FTx-Oxy2 (O. microlepidotus) | 3FTx |
| 6 | Q45Z49 | 14.32 | 85.63 | 4 | 1 | PLA-5 (O. scutellatus) | PLA2 |
| A8HDK8 | 7.98 | 191.13 | 16 | 1 | Long neurotoxin 2 (O. microlepidotus) | 3FTx | |
| A7X4Q3 | 7.95 | 188.04 | 16 | 1 | Long neurotoxin 3FTx- Oxy1 (O. microlepidotus) | 3FTx | |
| 7 | Q45Z41 | 14.22 | 152.09 | 12 | 4 | PLA-5 (O. microlepidotus) | PLA2 |
| 8 | Q4VRI7 F8 | 14.35 | 99.72 | 5 | 1 | Alpha taipoxin-2 (O. scutellatus) | PLA2 |
| A7X4S7 | 6.28 | 102.05 | 4 | 4 | Toxin 3FTx- Oxy5 (O. microlepidotus) | 3FTx | |
| Q3SAF8 | 4.11 | 123.71 | 6 | 6 | Natriuretic peptide OmNP-d (fragment) (O. microlepidotus) | NP | |
| 9 | P00614 F9 | 13.83 | 138.13 | 12 | 6 | Basic phospholipase A2 taipoxin alpha chain (O. scutellatus) | PLA2 |
| A7X4T2 F12 | 6.87 | 129.48 | 6 | 6 | Three-finger Toxin 3FTx- Oxy6 (O. microlepidotus) | 3FTx | |
| A8HDK2 F9 | 6.34 | 66.99 | 3 | 3 | Short neurotoxin 3 (O. scutellatus) | 3FTx | |
| 10 | Q45Z45 F9 | 15 | 173.78 | 18 | 6 | PLA-1 (O. microlepidotus) | PLA2 |
| Q45Z51F10 | 14.95 | 159.33 | 12 | 2 | PLA-3 (O. microlepidotus) | PLA2 | |
| P0CG57 | 13.31 | 115.25 | 5 | 1 | Neutral phospholipase A2 homolog taipoxin beta chain 2 (O. microlepidotus) | PLA2 | |
| 11 | Q45Z46 F10 | 13.33 | 184.44 | 22 | 10 | Neutral phospholipase A2 paradoxin-like beta chain (O. microlepidotus) | PLA2 |
| P00615 F11 | 13.23 | 99.21 | 6 | 1 | Neutral phospholipase A2 homolog taipoxin beta chain 1 (O. scutellatus) | PLA2 | |
| Q3HXZ0 F10 | 13.39 | 116.17 | 5 | 5 | Venom nerve growth factor 2 (O. microlepidotus) | NGF | |
| Q3SB07 F12 | 23.55 | 83.52 | 3 | 2 | Cysteine-rich venom protein pseudechetoxin-like (O. scutellatus) | CRISP | |
| 12 | P00616 F12 | 14.60 | 214.03 | 27 | 16 | Acidic phospholipase A2 homolog taipoxin gamma chain (O. scutellatus) | PLA2 |
| Q9PUH0 | 13.44 | 53.51 | 2 | 1 | Acidic phospholipase A2 S8-51 (Austrelaps superbus) | PLA2 | |
| Q58L95 | 15.99 | 85.41 | 3 | 3 | Venom prothrombin activator omicarin-C catalytic subunit (O. microlepidotus) | PTA | |
| P60043 | 13.21 | 63.09 | 3 | 1 | Basic phospholipase A2 1 (Fragment) (Naja. Sagittifera) | PLA2 | |
| 13 | Q45Z50 F13 | 15.21 | 171.55 | 5 | 2 | PLA-4 (O. scutellatus) | PLA2 |
| Q4VRI5 F9 | 14.10 | 123.21 | 4 | 1 | Phospholipase A2 OS1 (O. scutellatus) | PLA2 | |
| A0A670YUG0 | 48.31 | 74.98 | 3 | 1 | 5′ nucleotidase ecto (Pseudonaja textilis) | Nucleotidase | |
| A0A2I4HXH5 | 57.81 | 70.13 | 3 | 1 | Snake venom 5′-nucleotidase (Naja atra) | Nucleotidase | |
| 14 | A0A670Y1M6 F14 | 53.81 | 134.99 | 12 | 6 | Prolylcarboxypeptidase (Pseudonaja textilis) | Peptidase |
| A0A8C6XE75 | 51.58 | 107.01 | 8 | 1 | Prolylcarboxipeptidase (Naja naja) | Peptidase | |
| V8NPH4 F14 | 42.76 | 75.53 | 4 | 2 | Lysosomal Pro-X carboxypeptidase (Ophiophagus hannah) | Peptidase |
| Fraction | UNIPROT Code | Theoretical Mass (kDa) 1 | −10lgP | Peptides | Unique Peptides | Protein Description | Type |
|---|---|---|---|---|---|---|---|
| 3 | Q45Z11 | 6.73 | 122.20 | 6 | 1 | Short neurotoxin 1 (O. scutellatus) | 3FTx |
| 4 | P0CB06 F4 | 6.79 | 94.79 | 4 | 4 | Short neurotoxin 2 (O. scutellatus) | 3FTx |
| A8HDK9 | 7.89 | 67.94 | 5 | 5 | Long neurotoxin 1 (O. scutellatus) | 3FTx | |
| 5 | B7S4N9 F4 | 7 | 142.30 | 8 | 7 | Kunitz-type serine protease inhibitor taicotoxin (O. scutellatus) | Kunitz |
| 6 | P83228 | 3.66 | 82.03 | 3 | 3 | Natriuretic peptide TNP-b (O. scutellatus) | NP |
| 7 | Q45Z47 | 13.33 | 178.33 | 14 | 2 | Phospholipase A2 OS2 (O. scutellatus) | PLA2 |
| Q4VRI4 | 14.2 | 172.76 | 14 | 1 | OS6 (O. scutellatus) | PLA2 | |
| P10116 | 13.35 | 78.09 | 4 | 1 | Basic phospholipase A2 2 (Laticauda colubrina) | PLA2 | |
| A0A898INR6 | 14.71 | 71.72 | 2 | 1 | Phospholipase A (2) (Calliophis bivirgatus) | PLA2 | |
| 8 | Q4VRI7 F8 | 14.35 | 157.86 | 10 | 5 | Alpha taipoxin-2 (O. scutellatus) | PLA2 |
| Q45Z48 | 14.59 | 144.13 | 7 | 2 | PLA-6 (O. scutellatus) | PLA2 | |
| A6MFM9 | 14.52 | 101.38 | 3 | 2 | PLA2-4 (Crytophis nigrescens) | PLA2 | |
| P14615 | 13.42 | 50.97 | 2 | 1 | Neutral phospholipase A2 3 (Bungarus fasciatus) | PLA2 | |
| 9 | Q45Z45 F10 | 15 | 122.02 | 7 | 1 | PLA-1 (O. microlepidotus) | PLA2 |
| P00614 F9 | 13.83 | 179.92 | 13 | 8 | Basic phospholipase A2 taipoxin alpha chain (O. scutellatus) | PLA2 | |
| Q4VRI5 F13 | 14.1 | 172.67 | 11 | 2 | Phospholipase A2 OS1 (O. scutellatus) | PLA2 | |
| A6MFM9 | 14.52 | 65.31 | 3 | 2 | PLA2-4 (Crytophis nigrescens) | PLA2 | |
| A8HDK2 F9 | 6.34 | 102.63 | 4 | 4 | Short neurotoxin 3 (O. scutellatus) | 3FTx | |
| Q3SAX8 | 4.11 | 96.42 | 5 | 4 | Natriuretic peptide OsNP-d (fragment) (O. scutellatus) | NP | |
| 10 | Q45Z46 F11 | 13.33 | 125.32 | 8 | 3 | Neutral phospholipase A2 paradoxin-like beta chain (O. microlepidotus) | PLA2 |
| Q45Z51 F10 | 14.95 | 171.88 | 10 | 5 | PLA-3 (O. scutellatus) | PLA2 | |
| Q3HXZ0 F11 | 13.39 | 64.42 | 3 | 3 | Venom nerve growth factor 2 (O. microlepidotus) | NGF | |
| 11 | P00615 F11 | 13.24 | 170.01 | 16 | 9 | Neutral phospholipase A2 homolog taipoxin beta chain 1 (O. scutellatus) | PLA2 |
| P0CG57 | 13.31 | 132.04 | 9 | 1 | Neutral phospholipase A2 homolog taipoxin beta chain 2 (O. scutellatus) | PLA2 | |
| Q3I5F4 | 13.38 | 99.42 | 4 | 4 | Venom nerve growth factor (O. scutellatus) | NGF | |
| 12 | P00616 F12 | 44.72 | 172.56 | 12 | 9 | Acidic phospholipase A2 homolog taipoxin gamma chain (O. scutellatus) | PLA2 |
| A7X4T2 F9 | 6.87 | 83.17 | 2 | 2 | Three-finger toxin 3FTx-Oxy6 (O. microlepidotus) | 3FTx | |
| Q58L96 | 44.69 | 128.30 | 6 | 6 | Venom prothrombin activator oscutarin-C catalytic subunit (O. scutellatus) | PTA | |
| Q3SB07 F11 | 23.55 | 151.14 | 16 | 3 | Cysteine-rich venom protein pseudechetoxin-like (O. scutellatus) | CRISP | |
| Q8UW11 | 24.54 | 94.05 | 7 | 1 | Cystein-rich venom protein 2 (Hydrophis hardwickii) | CRISP | |
| 13 | Q45Z50 F13 | 15.21 | 135.44 | 5 | 1 | PLA-4 (O. scutellatus) | PLA2 |
| Q58L91 | 157.79 | 200.61 | 34 | 3 | Venom prothrombin activator oscutarin-C non catalytic subunit (O. scutellatus) | PTA | |
| 14 | A0A670Y1M6 F14 | 53.81 | 130.34 | 12 | 6 | Prolylcarboxypeptidase (Pseudonaja textilis) | Peptidase |
| A0A8C6XDV9 | 54.70 | 117.08 | 8 | 1 | Prolylcarboxipeptidase (Naja naja) | Peptidase | |
| V8NPH4 F14 | 42.76 | 78.45 | 4 | 2 | Lysosomal Pro-X carboxypeptidase (Ophiophagus hannah) | Peptidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, G.G.; Spencer, P.J.; Pimenta, D.C.; Beraldo-Neto, E. Exploring the Venom Diversity of Australian Taipans: Comparative Characterization of Oxyuranus microlepidotus and Oxyuranus scutellatus. Toxins 2025, 17, 488. https://doi.org/10.3390/toxins17100488
Paz GG, Spencer PJ, Pimenta DC, Beraldo-Neto E. Exploring the Venom Diversity of Australian Taipans: Comparative Characterization of Oxyuranus microlepidotus and Oxyuranus scutellatus. Toxins. 2025; 17(10):488. https://doi.org/10.3390/toxins17100488
Chicago/Turabian StylePaz, Guilherme Gonelli, Patrick Jack Spencer, Daniel Carvalho Pimenta, and Emidio Beraldo-Neto. 2025. "Exploring the Venom Diversity of Australian Taipans: Comparative Characterization of Oxyuranus microlepidotus and Oxyuranus scutellatus" Toxins 17, no. 10: 488. https://doi.org/10.3390/toxins17100488
APA StylePaz, G. G., Spencer, P. J., Pimenta, D. C., & Beraldo-Neto, E. (2025). Exploring the Venom Diversity of Australian Taipans: Comparative Characterization of Oxyuranus microlepidotus and Oxyuranus scutellatus. Toxins, 17(10), 488. https://doi.org/10.3390/toxins17100488






