Influence of Apis mellifera syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia
Abstract
1. Introduction
2. Results
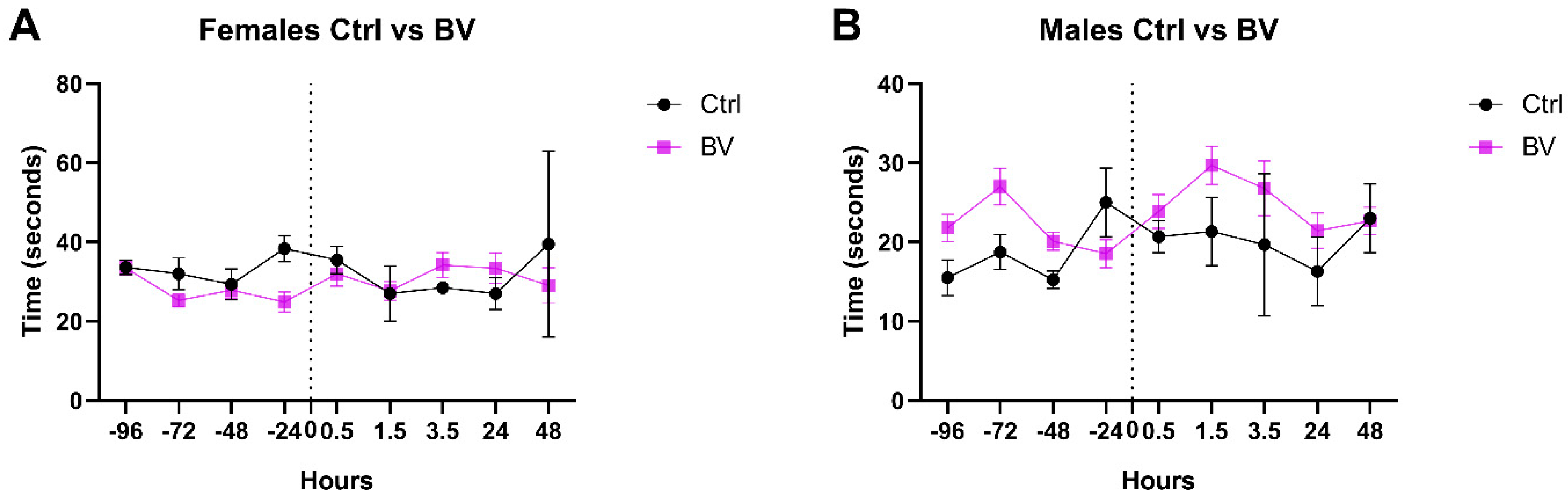
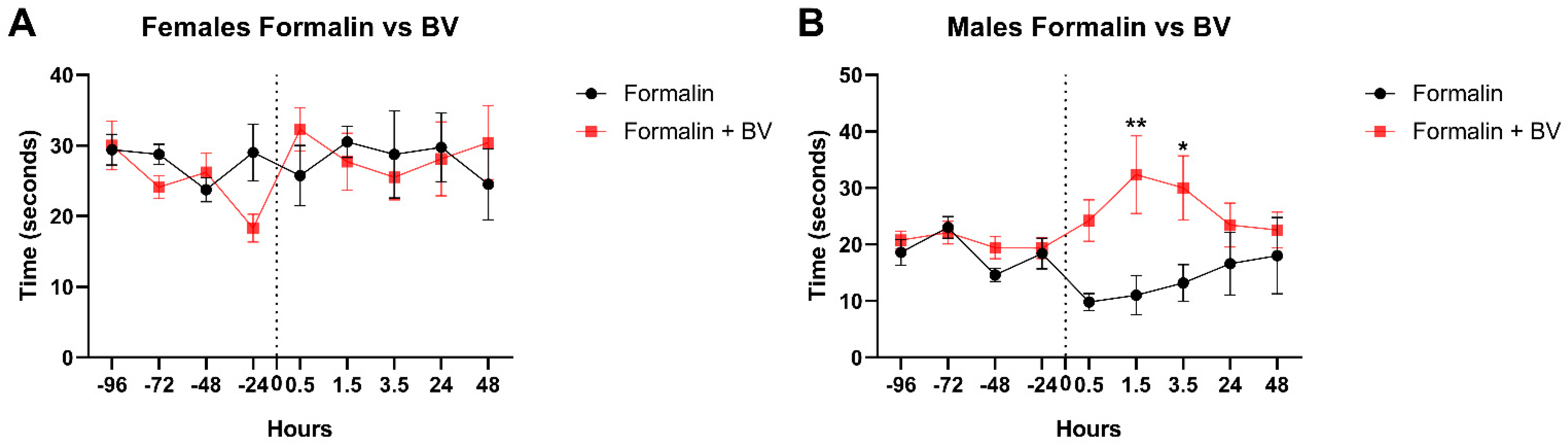
2.1. The Effect of Apis mellifera syriaca Bee Venom (AmsBV) on Pain Sensitivity in Hyperalgesia
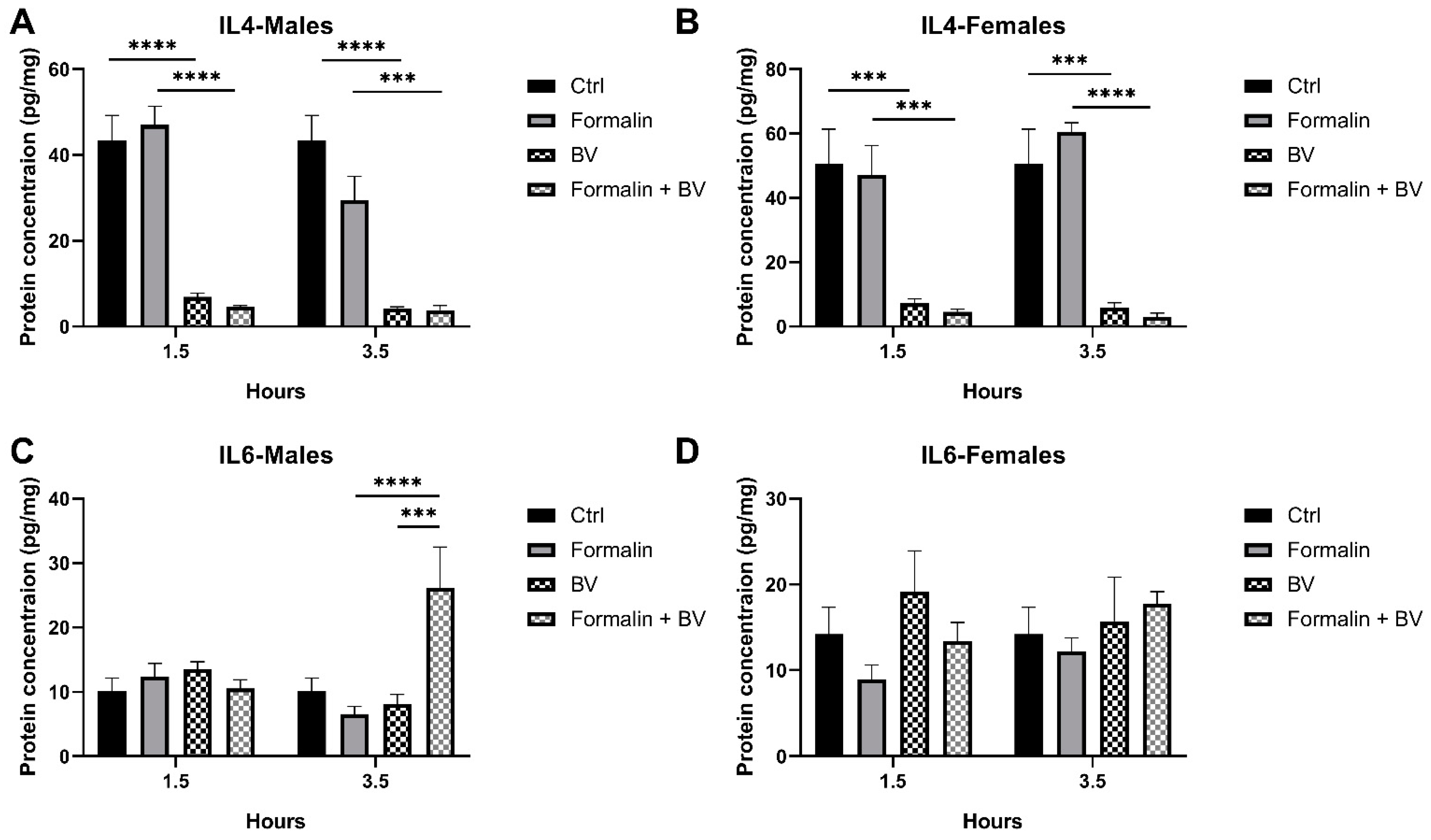
2.2. The Effect of AmsBV on IL-4 and IL-6 Levels in the Spleens of Formalin-Induced Hyperalgesia Mice Model
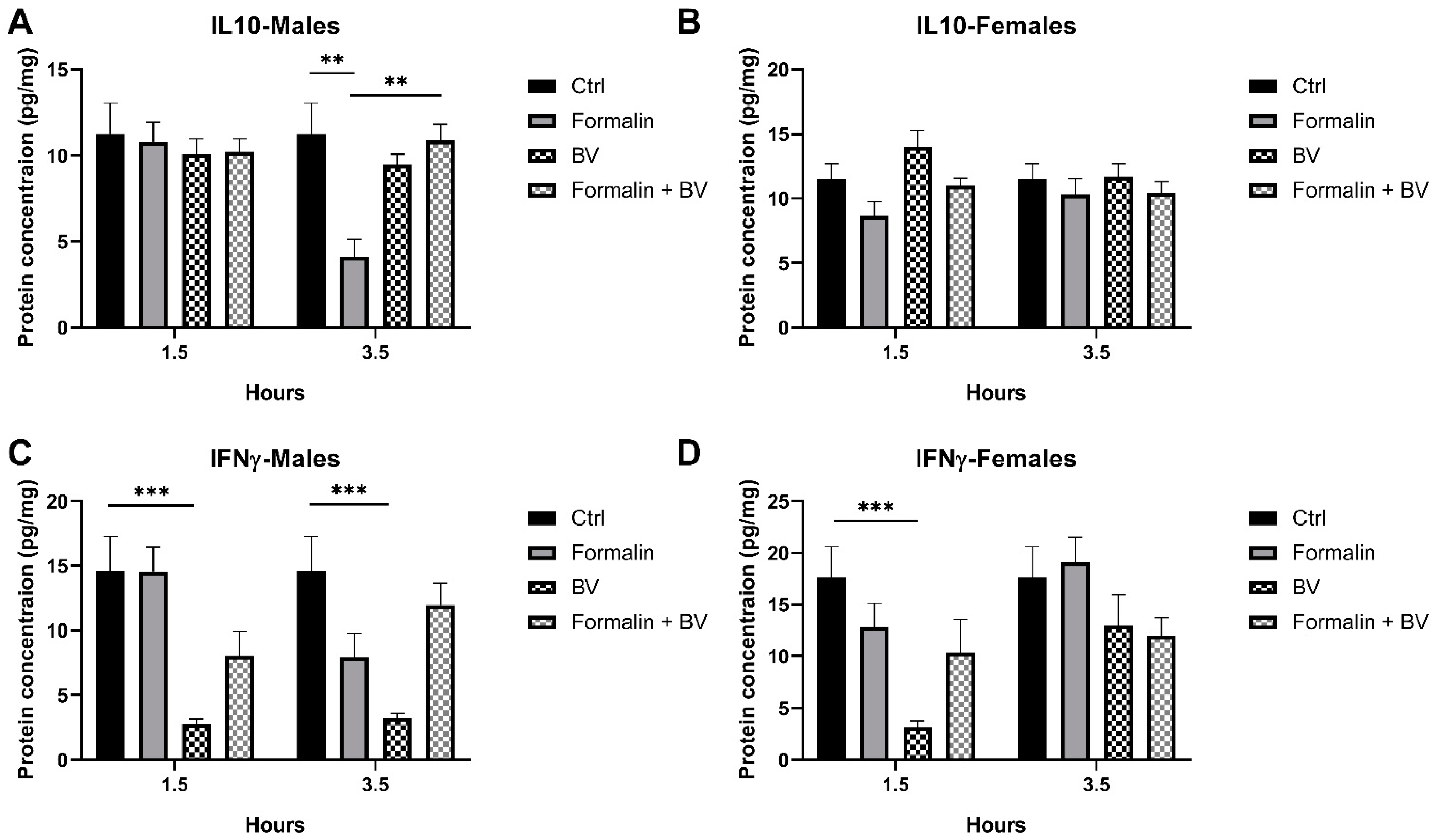
2.3. The Effect of AmsBV on the Levels of IL-10 and IFN-γ in the Spleens of Formalin-Induced Hyperalgesia Mice Model
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venom and Formalin Solutions
5.2. Mice Handling and Treatment
5.3. Hotplate Test
5.4. Dissection
5.5. Tissue Homogenization
5.6. Cytokine Measurement
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Chiu, Y.; Wu, W.; Hsu, P.; Özçakar, L. Botulinum toxin injections for shoulder and upper limb pain: A narrative review. Pain Manag. 2020, 10, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef]
- Fujii, T.; Yamasaki, R.; Kira, J.-I. Novel neuropathic pain mechanisms associated with allergic inflammation. Front. Neurol. 2019, 10, 1337. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef]
- Poole, S.; Cunha, F.Q.; Selkirk, S.; Lorenzetti, B.B.; Ferreira, S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br. J. Pharmacol. 1995, 115, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, S.; Sun, W.; Fu, H. Melittin, the major pain-producing substance of bee venom. Neurosci. Bull. 2016, 32, 265–272. [Google Scholar] [CrossRef]
- Hossen, M.S.; Khalil, M.I.; Gan, S.H. Melittin, a potential natural toxin of crude bee venom: Probable future arsenal in the treatment of diabetes mellitus. J. Chem. 2017, 1, 4035626. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on freund’s complete adjuvant-induced arthritis model in rats. Toxicon 2019, 161, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Oh, M.J.; Lee, D.H.; Lee, Y.S.; Lee, J.; Kim, D.; Choi, C.; Song, M.J.; Song, H.S.; Hong, J.T. Anti-inflammatory effect of bee venom in phthalic anhydride-induced atopic dermatitis animal model. Inflammopharmacology 2020, 28, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.; Jiang, J.; Chen, Z.J. Signal-induced ubiquitination of I kappa B alpha by the F-box protein slimb/ beta -TrCP. Genes Dev. 1999, 13, 284–294. [Google Scholar] [CrossRef]
- Abboud, A. Effect of Bee Venom on Hyperalgesia Hot-Plate Latency. 2023. Available online: https://scholarhub.balamand.edu.lb/handle/uob/6926 (accessed on 15 April 2024).
- Yaacoub, C.; Wehbe, R.; Salma, Y.; El-Obeid, D.; El Bersaoui, R.; Coutard, B.; Fajloun, Z. Apis mellifera syriaca venom: Evaluation of its anticoagulant effect, proteolytic activity, and cytotoxicity along with its two main Compounds—MEL and PLA2—On HeLa cancer cells. Molecules 2022, 27, 1653. [Google Scholar] [CrossRef]
- KBakhiet, E.; AMHussi, H.; Elshehaby, M. Apis mellifera venom inhibits bacterial and fungal pathogens in vitro. Pak. J. Biol. Sci. 2022, 25, 875–884. [Google Scholar] [CrossRef]
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.; Coutard, B.; Fajloun, Z. The cytotoxic effect of Apis mellifera venom with a synergistic potential of its two main Components—Melittin and PLA2—On colon cancer HCT116 cell lines. Molecules 2021, 26, 2264. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First characterization of the venom from Apis mellifera syriaca, A honeybee from the middle east region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Lee, Y.M.; Cho, S.; Son, E.; Song, C.; Kim, D. Apamin from bee venom suppresses inflammation in a murine model of gouty arthritis. J. Ethnopharmacol. 2020, 257, 112860. [Google Scholar] [CrossRef] [PubMed]
- Poudineh, M.; Parvin, S.; Omidali, M.; Nikzad, F.; Mohammadyari, F.; Sadeghi Poor Ranjbar, F.; Rasouli, F.; Nanbakhsh, S.; Olangian-Tehrani, S. The effects of vitamin therapy on ASD and ADHD: A narrative review. CNS Neurol. Disord.-Drug Targets 2023, 22, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, W.; Xu, Q.; Wu, H.; Jiao, C.; Chen, X. Estrogen modulation of visceral pain. J. Zhejiang Univ. Sci. B 2019, 20, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, C.; Khoury, M.; Mouawad, C.; Darazy, D.; Roufayel, R.; Mattei, C.; Fajloun, Z.; Legros, C.; Karam, M. Apis mellifera syriaca venom modulates splenic cytokines levels in BALB/c mice. Infect. Disord.-Drug Targets 2024, 24, e230623218222. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, V.; Toti, A.; Ghelardini, C.; Mannelli, L. Interferon-gamma and neuropathy: Balance between pain and neuroprotection. Neural Regen. Res. 2022, 17, 2700–2701. [Google Scholar] [CrossRef]
- Karam, M.C.; Merckbawi, R.; Salman, S.; Mobasheri, A. Atenolol reduces leishmania major-induced hyperalgesia and TNF-α without affecting IL-1β or keratinocyte derived chemokines (KC). Front. Pharmacol. 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, L.; Roche, M.; Finn, D.P. The role of the brain’s endocannabinoid system in pain and its modulation by stress. Int. Rev. Neurobiol. 2015, 125, 203–255. [Google Scholar] [CrossRef]
- Fu, K.; Light, A.R.; Maixner, W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J. Pain 2001, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Janjigian, M.; Myers, R.R. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain 1998, 74, 35–42. [Google Scholar] [CrossRef]
- Yanik, B.M.; Dauch, J.R.; Cheng, H.T. Interleukin-10 reduces neurogenic inflammation and pain behavior in a mouse model of type 2 diabetes. J. Pain Res. 2020, 13, 3499–3512. [Google Scholar] [CrossRef]
- Jafari, Z.; Sadeghi, S.; Dehaghi, M.M.; Bigham, A.; Honarmand, S.; Tavasoli, A.; Hoseini MH, M.; Varma, R.S. Immunomodulatory activities and biomedical applications of melittin and its recent advances. Arch. Der Pharm. 2024, 357, e2300569. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayoub, M.; Fayjaloun, S.; Roufayel, R.; El Obeid, D.; Fajloun, Z.; Rima, M.; Karam, M. Influence of Apis mellifera syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia. Toxins 2025, 17, 18. https://doi.org/10.3390/toxins17010018
Ayoub M, Fayjaloun S, Roufayel R, El Obeid D, Fajloun Z, Rima M, Karam M. Influence of Apis mellifera syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia. Toxins. 2025; 17(1):18. https://doi.org/10.3390/toxins17010018
Chicago/Turabian StyleAyoub, Mohamad, Salma Fayjaloun, Rabih Roufayel, Dany El Obeid, Ziad Fajloun, Mohamad Rima, and Marc Karam. 2025. "Influence of Apis mellifera syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia" Toxins 17, no. 1: 18. https://doi.org/10.3390/toxins17010018
APA StyleAyoub, M., Fayjaloun, S., Roufayel, R., El Obeid, D., Fajloun, Z., Rima, M., & Karam, M. (2025). Influence of Apis mellifera syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia. Toxins, 17(1), 18. https://doi.org/10.3390/toxins17010018





