A Multi-Year Study of Mycotoxin Co-Occurrence in Wheat and Corn Grown in Ontario, Canada
Abstract
1. Introduction
2. Results
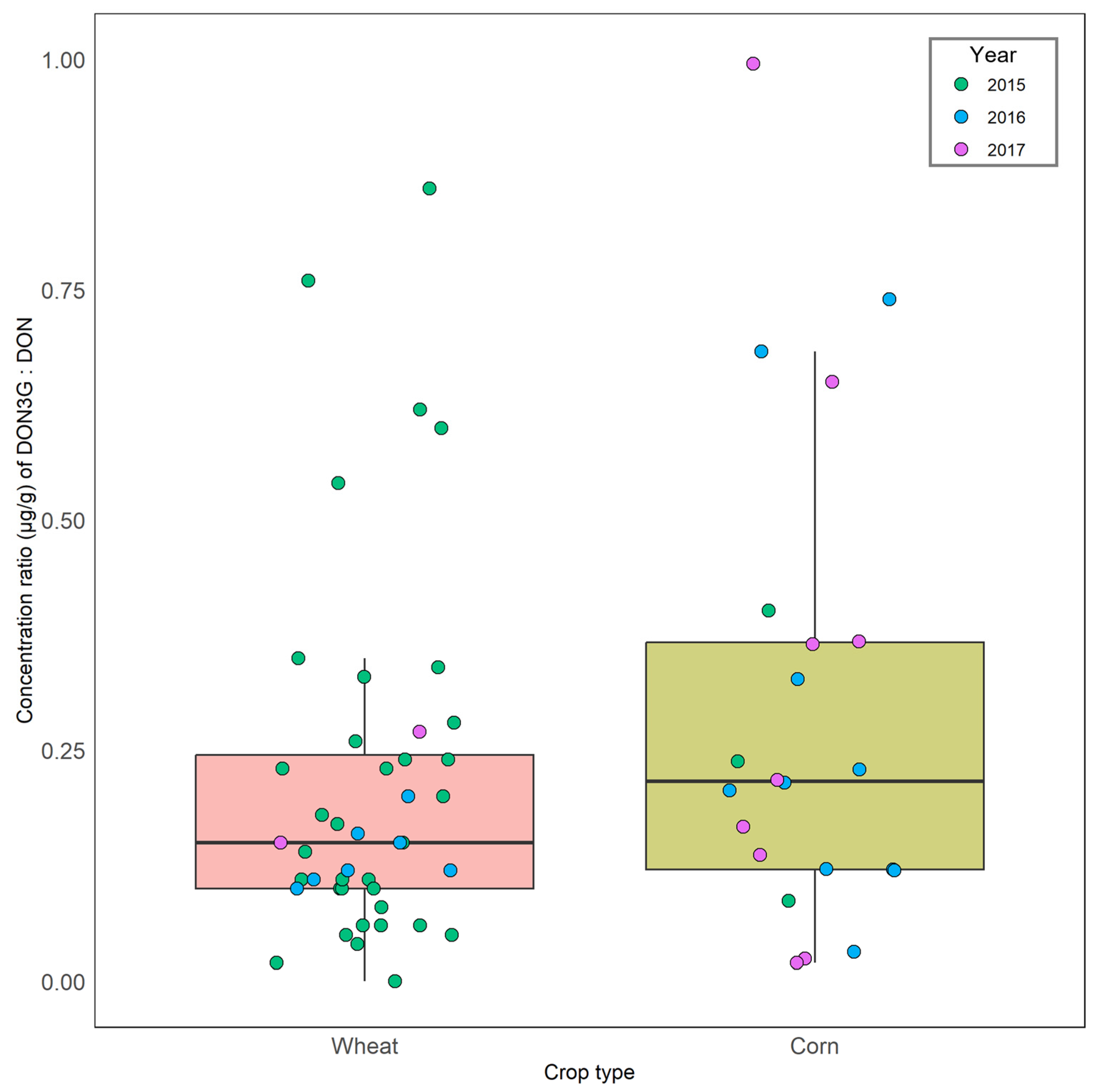
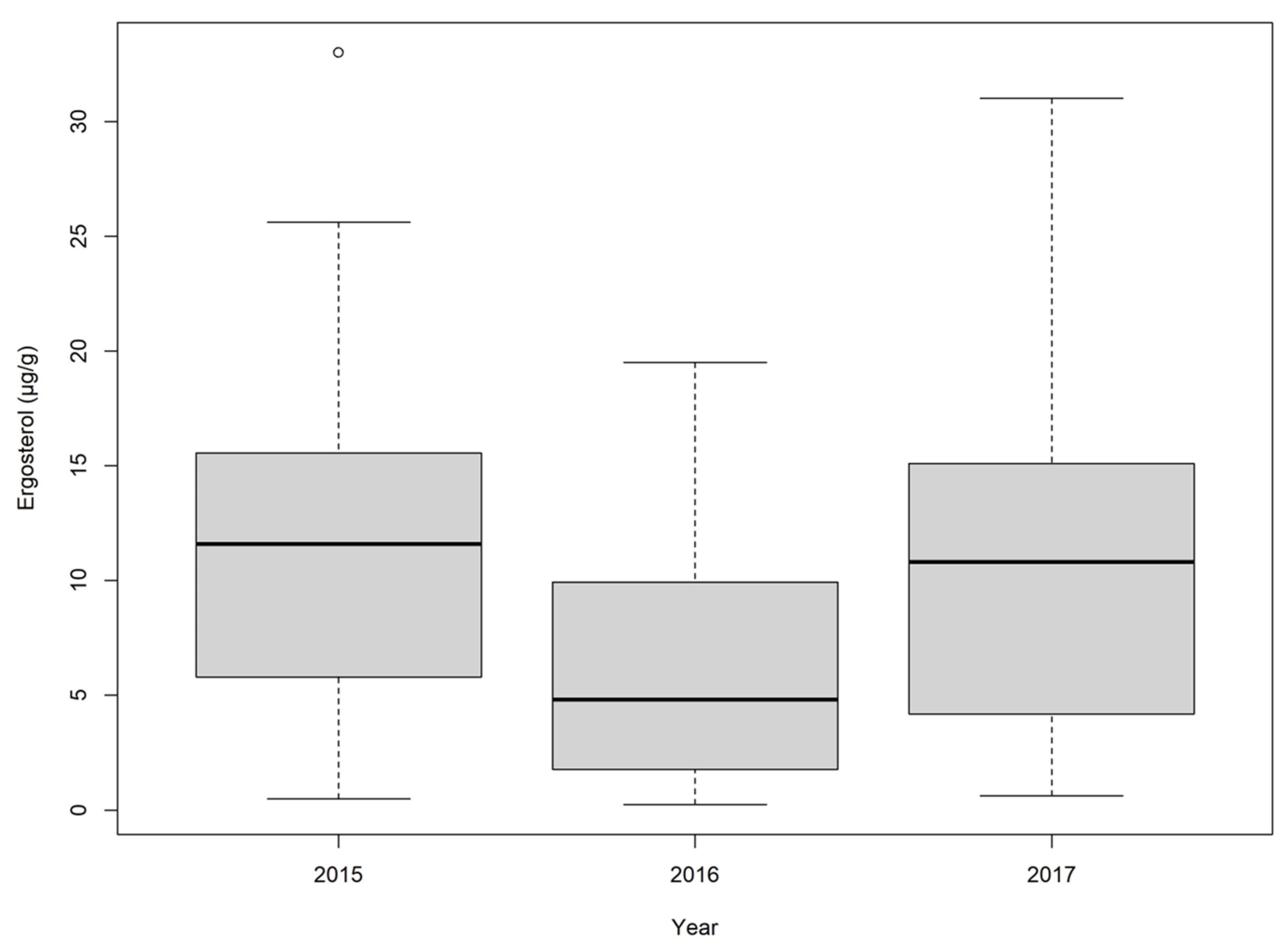
2.1. Wheat
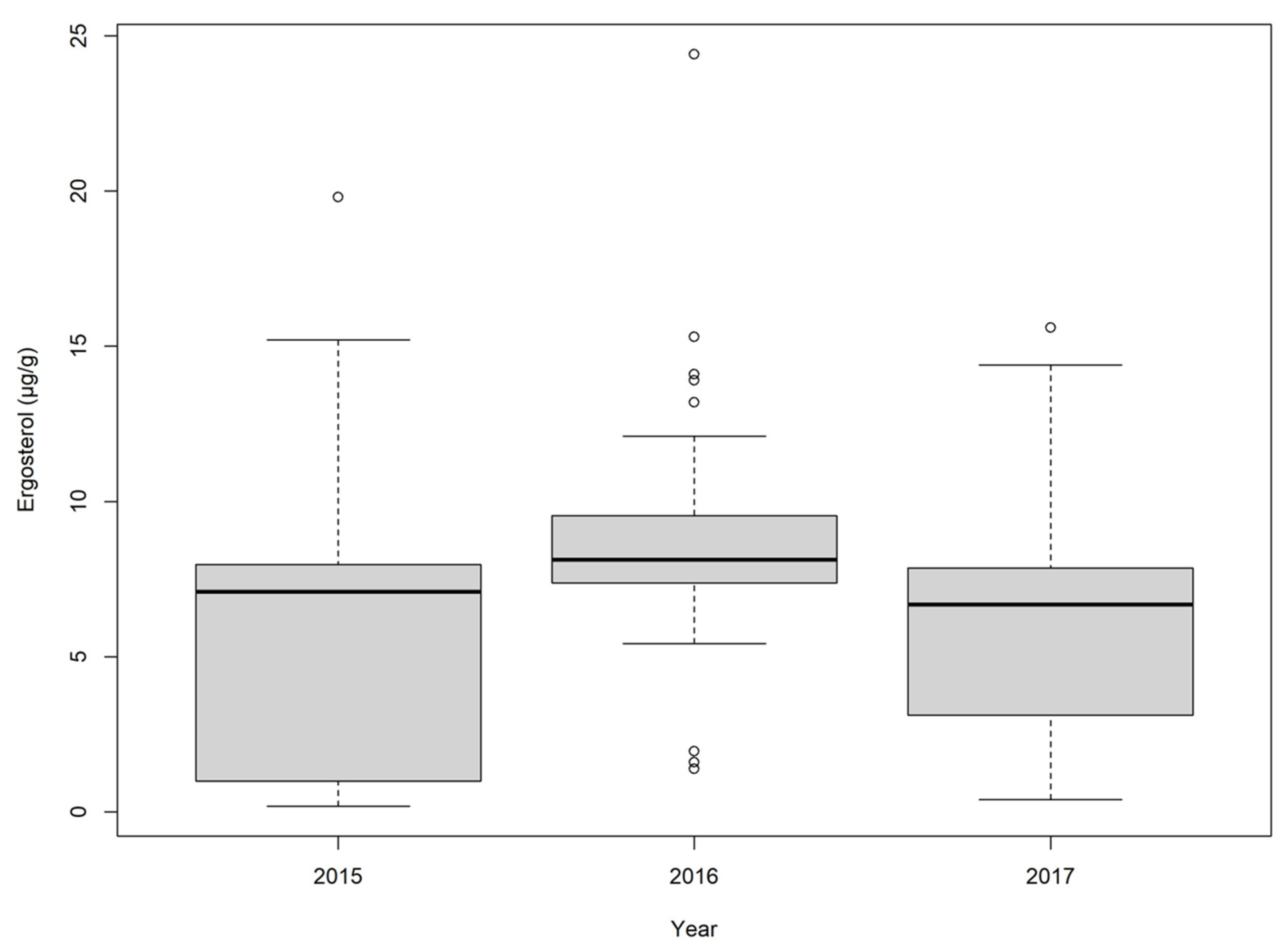
2.2. Corn
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Corn and Wheat Sample Collection
5.2. Mycotoxin Extraction
5.3. LC-MS/MS Analysis and Quantification of Mycotoxins
5.4. Ergosterol Extraction and Quantification by LC-MS/MS
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hesseltine, C. Natural occurrence of mycotoxins in cereals. Mycopathol. Mycol. Appl. 1974, 53, 141–153. [Google Scholar] [CrossRef]
- Pitt, J.I.; Miller, J.D. A concise history of mycotoxin research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. 2008, 25, 219–230. [Google Scholar] [CrossRef]
- Sutton, J. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Andrews, R.; Thompson, B.; Trenholm, H.L. A national survey of mycotoxins in Canada. J. Am. Oil Chem. Soc. 1981, 58, A989–A991. [Google Scholar] [CrossRef]
- Greenway, J.; Puls, R. Fusariotoxicosis from barley in British Columbia. I. Natural occurrence and diagnosis. Can. J. Comp. Med. 1976, 40, 12. [Google Scholar]
- Prior, M. Mycotoxin determinations on animal feedstuffs and tissues in Western Canada. Can. J. Comp. Med. 1976, 40, 75. [Google Scholar]
- Scott, P.; Walbeek, W.v.; Harwig, J.; Fennell, D. Occurrence of a mycotoxin, ochratoxin A, in wheat and isolation of ochratoxin A and citrinin producing strains of Penicillium viridicatum. Can. J. Plant Sci. 1970, 50, 583–585. [Google Scholar] [CrossRef]
- Scott, P.M.; Van Walbeek, W.; Kennedy, B.; Anyeti, D. Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and toxigenic fungi in grains and other agricultural products. J. Agric. Food Chem. 1972, 20, 1103–1109. [Google Scholar] [CrossRef]
- Fitzpatrick, D.W. Mycotoxins in the food chain: Nutritional and toxicological considerations. Can. J. Physiol. Pharmacol. 1990, 68, 979–981. [Google Scholar] [CrossRef]
- Hooker, D.; Schaafsma, A.; Tamburic-Ilincic, L. Using weather variables pre-and post-heading to predict deoxynivalenol content in winter wheat. Plant Dis. 2002, 86, 611–619. [Google Scholar] [CrossRef]
- Funnell, H. Mycotoxins in animal feedstuffs in Ontario: 1972 to 1977. Can. J. Comp. Med. 1979, 43, 243. [Google Scholar]
- Sutton, J.; Baliko, W.; Funnell, H. Relation of weather variables to incidence of zearalenone in corn in southern Ontario. Can. J. Plant Sci. 1980, 60, 149–155. [Google Scholar] [CrossRef]
- Miller, J.D. Factors that affect the occurrence of fumonisin. Environ. Health Perspect. 2001, 109, 321–324. [Google Scholar]
- Miller, J.D. Changing patterns of fungal toxins in crops: Challenges for analysts. J. AOAC Int. 2016, 99, 837–841. [Google Scholar] [CrossRef]
- Campbell, H.; Choo, T.M.; Vigier, B.; Underhill, L. Comparison of mycotoxin profiles among cereal samples from eastern Canada. Can. J. Bot. 2002, 80, 526–532. [Google Scholar] [CrossRef]
- Scott, P. Multi-year monitoring of Canadian grains and grain-based foods for trichothecenes and zearalenone. Food Addit. Contam. 1997, 14, 333–339. [Google Scholar] [CrossRef]
- Crippin, T.; Renaud, J.B.; Sumarah, M.W.; Miller, J.D. Comparing genotype and chemotype of Fusarium graminearum from cereals in Ontario, Canada. PLoS ONE 2019, 14, e0216735. [Google Scholar] [CrossRef]
- Sumarah, M.W. The deoxynivalenol challenge. J. Agric. Food Chem. 2022, 70, 9619–9624. [Google Scholar] [CrossRef]
- Miller, J.D. Epidemiology of Fusarium Ear Diseases of Cereals. In Mycotoxins in Grain; Compounds Other than Aflatoxin; Eagen Press: St. Paul, MN, USA, 1994; pp. 19–36. [Google Scholar]
- Xue, A.G.; Chen, Y.; Seifert, K.; Guo, W.; Blackwell, B.A.; Harris, L.J.; Overy, D.P. Prevalence of Fusarium species causing head blight of spring wheat, barley and oat in Ontario during 2001–2017. Can. J. Plant Pathol. 2019, 41, 392–402. [Google Scholar] [CrossRef]
- Balbus, J.M.; Boxall, A.B.; Fenske, R.A.; McKone, T.E.; Zeise, L. Implications of global climate change for the assessment and management of human health risks of chemicals in the natural environment. Environ. Toxicol. Chem. 2013, 32, 62–78. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins, climate change, and food system management. J. Food Diagn. 2022, 4, 26–35. [Google Scholar]
- Bianchini, A.; Horsley, R.; Jack, M.M.; Kobielush, B.; Ryu, D.; Tittlemier, S.; Wilson, W.W.; Abbas, H.K.; Abel, S.; Harrison, G. DON occurrence in grains: A North American perspective. Cereal Foods World 2015, 60, 32–56. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; Patrick, S.K.; Sulyok, M.; Krska, R.; McKendry, T.; Gräfenhan, T. Fusarium damage in small cereal grains from Western Canada. 2. Occurrence of Fusarium toxins and their source organisms in durum wheat harvested in 2010. J. Agric. Food Chem. 2013, 61, 5438–5448. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Gaba, D.; Chan, J.M. Monitoring of Fusarium trichothecenes in Canadian cereal grain shipments from 2010 to 2012. J. Agric. Food Chem. 2013, 61, 7412–7418. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Roscoe, M.; Blagden, R.; Kobialka, C. Occurrence of ochratoxin A in Canadian wheat shipments, 2010–12. Food Addit. Contam. Part A 2014, 31, 910–916. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Gaba, D.; Mckendry, T.; Pleskach, K.; Roscoe, M. Fusarium and Alternaria mycotoxins present in Canadian wheat and durum harvest samples. Can. J. Plant Pathol. 2019, 41, 403–414. [Google Scholar] [CrossRef]
- JECFA (World Health Organization Joint FAO/WHO Expert Committee on Food Additives). Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. Contaminants, 3.2: 4,15-Diacetoxyscirpenol; World Health Organization: Geneva, The Netherlands, 2017; pp. 40–54. [Google Scholar]
- JECFA (World Health Organization Joint FAO/WHO Expert Committee on Food Additives). Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. Contaminants, 3.6-Sterigmatocystin; World Health Organization: Geneva, The Netherlands, 2017; pp. 106–122. [Google Scholar]
- CMOS (Canadian Meterological and Oceanographic Society). Canada’s Top Ten Weather Stories for 2015. Available online: https://cmos.in1touch.org/uploaded/web/website/top_ten/EN_Final%20Top%20Ten%20Weather%20Stories%20in%202015.pdf (accessed on 12 July 2024).
- ECCC (Environment Climate Change Canada). Climate Trends and Variations Bulletin–Summer 2015. Available online: https://publications.gc.ca/collections/collection_2016/eccc/En81-23-2015-3-eng.pdf (accessed on 28 June 2024).
- ECCC (Environment Climate Change Canada). Climate Trends and Variations Bulletin–Summer 2016. Available online: https://www.canada.ca/content/dam/eccc/migration/main/sc-cs/cabbe0ee-bb3d-4e6c-96dc-6f83a9beb427/4325-20summer-20eng04.pdf (accessed on 28 June 2024).
- ECCC (Environment Climate Change Canada). Climate Trends and Variations Bulletin–Summer 2017. Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/climate-change/trends-variations/summer-2017-en.pdf (accessed on 28 June 2024).
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin production in Fusarium according to contemporary species concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef]
- Reid, L.; Nicol, R.; Ouellet, T.; Savard, M.; Miller, J.; Young, J.; Stewart, D.; Schaafsma, A. Interaction of Fusarium graminearum and F. moniliforme in maize ears: Disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 1999, 89, 1028–1037. [Google Scholar] [CrossRef]
- Crippin, T.; Limay-Rios, V.; Renaud, J.; Schaafsma, A.; Sumarah, M.; Miller, J. Fusarium graminearum populations from maize and wheat in Ontario, Canada. World Mycotoxin J. 2020, 13, 355–366. [Google Scholar] [CrossRef]
- Limay-Rios, V.; Schaafsma, A.W. Effect of prothioconazole application timing on Fusarium mycotoxin content in maize grain. J. Agric. Food Chem. 2018, 66, 4809–4819. [Google Scholar] [CrossRef]
- Limay-Rios, V.; Schaafsma, A.W. Relationship between Mycotoxin Content in Winter Wheat Grain and Aspirated Dust Collected during Harvest and after Storage. ACS Omega 2021, 6, 1857–1871. [Google Scholar] [CrossRef]
- Miller, J.; Savard, M.; Schaafsma, A.; Seifert, K.; Reid, L. Mycotoxin production by Fusarium moniliforme and Fusarium proliferatum from Ontario and occurrence of fumonisin in the 1993 corn crop. Can. J. Plant Pathol. 1995, 17, 233–239. [Google Scholar]
- Fusilier, K.; Chilvers, M.I.; Limay-Rios, V.; Singh, M.P. Mycotoxin co-occurrence in Michigan harvested maize grain. Toxins 2022, 14, 431. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-occurrence of 35 mycotoxins: A seven-year survey of corn grain and corn silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Tothill, I.E.; Harris, D.; Magan, N. The relationship between fungal growth and ergosterol content of wheat grain. Mycol. Res. 1992, 96, 965–970. [Google Scholar] [CrossRef]
- Abramson, D.; Hulasare, R.; York, R.K.; White, N.D.G.; Jayas, D.S. Mycotoxins, ergosterol, and odor volatiles in durum wheat during granary storage at 16% and 20% moisture content. J. Stored Prod. Res. 2005, 41, 67–76. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Roscoe, M.; Pleskach, K. A multi-year survey of mycotoxins and ergosterol in Canadian oats. Mycotoxin Res. 2020, 36, 103–114. [Google Scholar] [CrossRef]
- Perkowski, J.; Buśko, M.; Stuper, K.; Kostecki, M.; Matysiak, A.; Szwajkowska-Michałek, L. Concentration of ergosterol in small-grained naturally contaminated and inoculated cereals. Biologia 2008, 63, 542–547. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Pallaroni, L.; Piva, G. Occurrence of mycotoxins and ergosterol in maize harvested over 5 years in Northern Italy. Food Addit. Contam. 2004, 21, 479–487. [Google Scholar] [CrossRef]
- David Miller, J.; Arnison, P.G. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins: Still with us after all these years. In Present Knowledge in Food Safety; Elsevier: Amsterdam, The Netherlands, 2023; pp. 62–78. [Google Scholar]
- Zhang, Z.; Nie, D.; Fan, K.; Yang, J.; Guo, W.; Meng, J.; Zhao, Z.; Han, Z. A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit. Rev. Food Sci. Nutr. 2020, 60, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Duncan, G.; Richardson, A.J. The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary deepoxy-deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine small and large intestinal microbiota rapidly hydrolyze the masked mycotoxin deoxynivalenol-3-glucoside and release deoxynivalenol in spiked batch cultures in vitro. Appl. Environ. Microbiol. 2018, 84, e02106–e02117. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar]
- Blackwell, B.A.; Schneiderman, D.; Thapa, I.; Bosnich, W.; Pimentel, K.; Kebede, A.Z.; Reid, L.M.; Harris, L.J. Assessment of deoxynivalenol and deoxynivalenol derivatives in Fusarium graminearum-inoculated Canadian maize inbreds. Can. J. Plant Pathol. 2022, 44, 504–517. [Google Scholar] [CrossRef]
- Stoev, S.D. Food security, underestimated hazard of joint mycotoxin exposure and management of the risk of mycotoxin contamination. Food Control 2023, 159, 110235. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Chen, C.; Riley, R.T.; Wu, F. Dietary fumonisin and growth impairment in children and animals: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1448–1464. [Google Scholar] [CrossRef]
- Rotter, R.; Trenholm, H.; Prelusky, D.; Hartin, K.; Thompson, B.; Miller, J. A preliminary examination of potential interactions between deoxynivalenol (DON) and other selected Fusarium metabolites in growing pigs. Can. J. Anim. Sci. 1992, 72, 107–116. [Google Scholar] [CrossRef]
- Trenholm, H.; Friend, D.; Thompson, B.; Prelusky, D. Effects of zearalenone and deoxynivalenol combinations fed to pigs. JSM Mycotoxins 1988, 1988, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Blaney, B.; Magee, M. Responses of pigs fed wheat naturally infected with Fusarium graminearum and containing the mycotoxins 4-deoxynivalenol and zearalenone. Aust. J. Agric. Res. 1988, 39, 1095–1105. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Andersen, B.; Thrane, U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol. Res. 2008, 112, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Larsen, T.O.; De Vries, R.; Meijer, M.; Houbraken, J.; Cabañes, F.; Ehrlich, K.; Samson, R. Secondary metabolite profiling, growth profiles and other tools for species recognition and important Aspergillus mycotoxins. Stud. Mycol. 2007, 59, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef]
- Andersen, B.; Dongo, A.; Pryor, B.M. Secondary metabolite profiling of Alternaria dauci, A. porri, A. solani, and A. tomatophila. Mycol. Res. 2008, 112, 241–250. [Google Scholar] [CrossRef]
- Kelman, M.; Renaud, J.; Baines, D.; Yeung, K.-C.; Miller, J.; Sumarah, M. Mycotoxin determination in fungal contaminated Canadian silage toxic to dairy cows and goats. World Mycotoxin J. 2022, 15, 429–438. [Google Scholar] [CrossRef]
- Teeter-Wood, K.R.; Kelman, M.J.; Teeter, D.P.; Sumarah, M.W. Prevalence of mycotoxins from silage in a small beef cattle feedlot over a storage season: A case study. Can. J. Plant Pathol. 2024, 46, 319–328. [Google Scholar] [CrossRef]
- Borman, P.; Elder, D. Chapter 5, validation of analytical procedures Q2 (R1). In ICH Quality Guidelines: An Implementation Guide; Teasdale, A.E.D., Nims, R., Eds.; John Wiley and Sons: Geneva, Switzerland, 2017; pp. 127–166. [Google Scholar]
- Dong, Y.; Steffenson, B.J.; Mirocha, C.J. Analysis of ergosterol in single kernel and ground grain by gas chromatography−mass spectrometry. J. Agric. Food Chem. 2006, 54, 4121–4125. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Harrell, M.F.E., Jr. Package ‘hmisc’. CRAN2018 2019, 2019, 235–236. [Google Scholar]


| 2015, n = 59 | 2016, n = 50 | 2017, n = 50 | ||||
|---|---|---|---|---|---|---|
| Median (# 2 Hits) µg/kg | Range (Min–Max) µg/kg | Median (# 2 Hits) µg/kg | Range (Min–Max) µg/kg | Median (# 2 Hits) µg/kg | Range (Min–Max) µg/kg | |
| Deoxynivalenol (DON) | 948 (53) | 20.1–14,600 | 537 (16) | 8.96–8070 | 109 (11) | 18.2–563 |
| 15-Acetyldeoxynivalenol (15-ADON) | 82.3 (45) | 38.7–165 | 77.0 (4) | 70.0–165 | ||
| 3-Acetyldeoxynivalenol (3-ADON) | 113 (6) | 81.6–148 | 118 (1) | |||
| Deoxynivalenol-3-glucoside (DON-3G) | 271 (33) | 35.5–67 | 315 (7) | 114–993 | 107 (2) | 85.1–128 |
| Zearalenone (ZEN) | 28.4 (45) | 16.5–1640 | 19.3 (13) | 16.2–59.5 | 18.5 (17) | 16.1–4670 |
| HT-2 toxin (HT-2) | 237 (1) | |||||
| Fumonisin FB1 (FB1) | 42.8 (3) | 41.8–47.1 | ||||
| Fumonisin FB2 (FB2) | 33.6 (3) | 33.6–34.2 | 34.0 (1) | |||
| Fumonisin FB3 (FB3) | 46.3 (3) | 45.9–47.4 | 48.3 (1) | |||
| Sterigmatocystin (STE) | 3630 (4) | 135–9420 | 1420 (2) | 322–2510 | ||
| α-cyclopiazonic acid (αCPA) | 121 (7) | 118–163 | 118 (1) | 117 (2) | 117–117 | |
| Mycophenolic acid (MPA) | 494 (2) | 132–855 | ||||
| Penitrem A (PENTA) | 51.3 (3) | 51.0–53.9 | 51.6 (1) | |||
| Roquefortine C (ROC) | 26.2 (5) | 25.1–42.4 | 28.2 (2) | 26.9–29.4 | ||
| Viridicatin (VIRI) | 54.9 (4) | 52.3–63.7 | 60.6 (2) | 58.2–63.0 | ||
| Altenuene (ALT) | 13.6 (12) | 11.6–18.6 | 14.2 (3) | 12.9–14.4 | 11.9 (1) | |
| Tentoxin (TEN) | 41.9 (40) | 4.73–120 | 45.6 (9) | 2.70–110 | 16.7 (11) | 3.05–96.5 |
| Alternariol (AOH) | 23.5 (42) | 15.7–412 | 23.2 (12) | 17.2–85.7 | 19.3 (8) | 18.3–36.1 |
| Alternariol monomethyl ether (AME) | 20.4 (42) | 16.0–84.2 | 20.6 (12) | 17.5–39.2 | 19.8 (8) | 17.5–27.0 |
| Beauvericin (BEA) | 118 (21) | 9.27–748 | 324 (5) | 52.4–448 | 80.4 (4) | 50.0–554 |
| Enniatin B (ENNB) | 65.6 (59) | 5.29–6500 | 8.84 (41) | 5.30–1580 | 6.59 (38) | 3.08–50.4 |
| Enniatin B1 (ENNB1) | 66.8 (59) | 22.6–4100 | 26.2 (41) | 22.5–677 | 23.0 (39) | 22.0–50.0 |
| Fusaric acid (FUSA) | 116 (34) | 112–230 | 115 (12) | 112–133 | 114 (12) | 111–216 |
| 2015, n = 44 | 2016, n = 51 | 2017, n = 65 | ||||
|---|---|---|---|---|---|---|
| Median (# 2 Hits) µg/kg | Range (Min–Max) µg/kg | Median (#2 Hits) µg/kg | Range (Min–Max) µg/kg | Median (# 2 Hits) µg/kg | Range (Min–Max) µg/kg | |
| Deoxynivalenol (DON) | 125 (16) | 15.5–3760 | 393 (20) | 17.9–17,600 | 319 (15) | 63.8–18,500 |
| 15-Acetyldeoxynivalenol (15-ADON) | 126 (4) | 63.9–178 | 195 (13) | 62.2–335 | 128 (10) | 57.3–1080 |
| 3-Acetyldeoxynivalenol (3-ADON) | 136 (1) | 302 (6) | 92.7–1184 | 502 (5) | 218–981 | |
| Deoxynivalenol-3-glucoside (DON-3G) | 331 (4) | 107–1300 | 447 (9) | 75.6–6650 | 176 (9) | 89.0–4930 |
| Zearalenone (ZEN) | 16.5 (20) | 12.2–1790 | 13.1 (30) | 12.0–1620 | 14.1 (31) | 12.0–1390 |
| HT-2 Toxin (HT-2) | 1260 (2) | 901–1630 | 1240 (1) | 883 (1) | ||
| Fumonisin FB1 (FB1) | 47.4 (4) | 46.0–52.1 | 51.7 (10) | 45.2–1600 | 55.0 (4) | 45.4–76.6 |
| Fumonisin FB2 (FB2) | 35.8 (4) | 35.6–38.2 | 36.8 (7) | 35.5–118 | 36.3 (2) | 35.4–37.3 |
| Fumonisin FB3 (FB3) | 54.0 (2) | 53.4–54.7 | 55.6 (7) | 53.3–101 | 55.0 (1) | |
| Sterigmatocystin (STE) | 750 (1) | |||||
| α-cyclopiazonic acid (αCPA) | 100.0 (1) | |||||
| Penitrem A (PENTA) | 54.4 (1) | |||||
| Roquefortine C (ROC) | 79.7 (6) | 36.8–371 | 28.3 (1) | |||
| Viridicatin (VIRI) | 139 (3) | 71.4–250 | ||||
| Altenuene (ALT) | 20.1 (8) | 13.8–28.3 | 17.5 (4) | 12.1–41.1 | 17.1 (3) | 12.5–26.3 |
| Tentoxin (TEN) | 4.12 (2) | 3.81–4.42 | ||||
| Alternariol (AOH) | 69.4 (16) | 24.1–214 | 27.4 (19) | 21.3–218 | 28.0 (16) | 19.0–177 |
| Alternariol monomethyl ether (AME) | 16.1 (2) | 14.6–17.6 | 74.5 (3) | 23.3–332 | ||
| Beauvericin (BEA) | 168 (8) | 34.5–5670 | 180 (8) | 34.0–6430 | 605 (5) | 77.0–13,000 |
| Enniatin B (ENNB) | 5.27 (26) | 4.81–6.78 | 5.03 (10) | 4.83–5.54 | 5.33 (24) | 4.81–8.40 |
| Enniatin B1 (ENNB1) | 32.8 (1) | 25.3 (1) | 35.1 (1) | |||
| Fusaric acid (FUSA) | 318 (1) | 411 (8) | 107–1180 | 982 (6) | 199–2060 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelman, M.J.; Miller, J.D.; Renaud, J.B.; Baskova, D.; Sumarah, M.W. A Multi-Year Study of Mycotoxin Co-Occurrence in Wheat and Corn Grown in Ontario, Canada. Toxins 2024, 16, 372. https://doi.org/10.3390/toxins16080372
Kelman MJ, Miller JD, Renaud JB, Baskova D, Sumarah MW. A Multi-Year Study of Mycotoxin Co-Occurrence in Wheat and Corn Grown in Ontario, Canada. Toxins. 2024; 16(8):372. https://doi.org/10.3390/toxins16080372
Chicago/Turabian StyleKelman, Megan J., J. David Miller, Justin B. Renaud, Daria Baskova, and Mark W. Sumarah. 2024. "A Multi-Year Study of Mycotoxin Co-Occurrence in Wheat and Corn Grown in Ontario, Canada" Toxins 16, no. 8: 372. https://doi.org/10.3390/toxins16080372
APA StyleKelman, M. J., Miller, J. D., Renaud, J. B., Baskova, D., & Sumarah, M. W. (2024). A Multi-Year Study of Mycotoxin Co-Occurrence in Wheat and Corn Grown in Ontario, Canada. Toxins, 16(8), 372. https://doi.org/10.3390/toxins16080372





