Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama
Abstract
1. Introduction
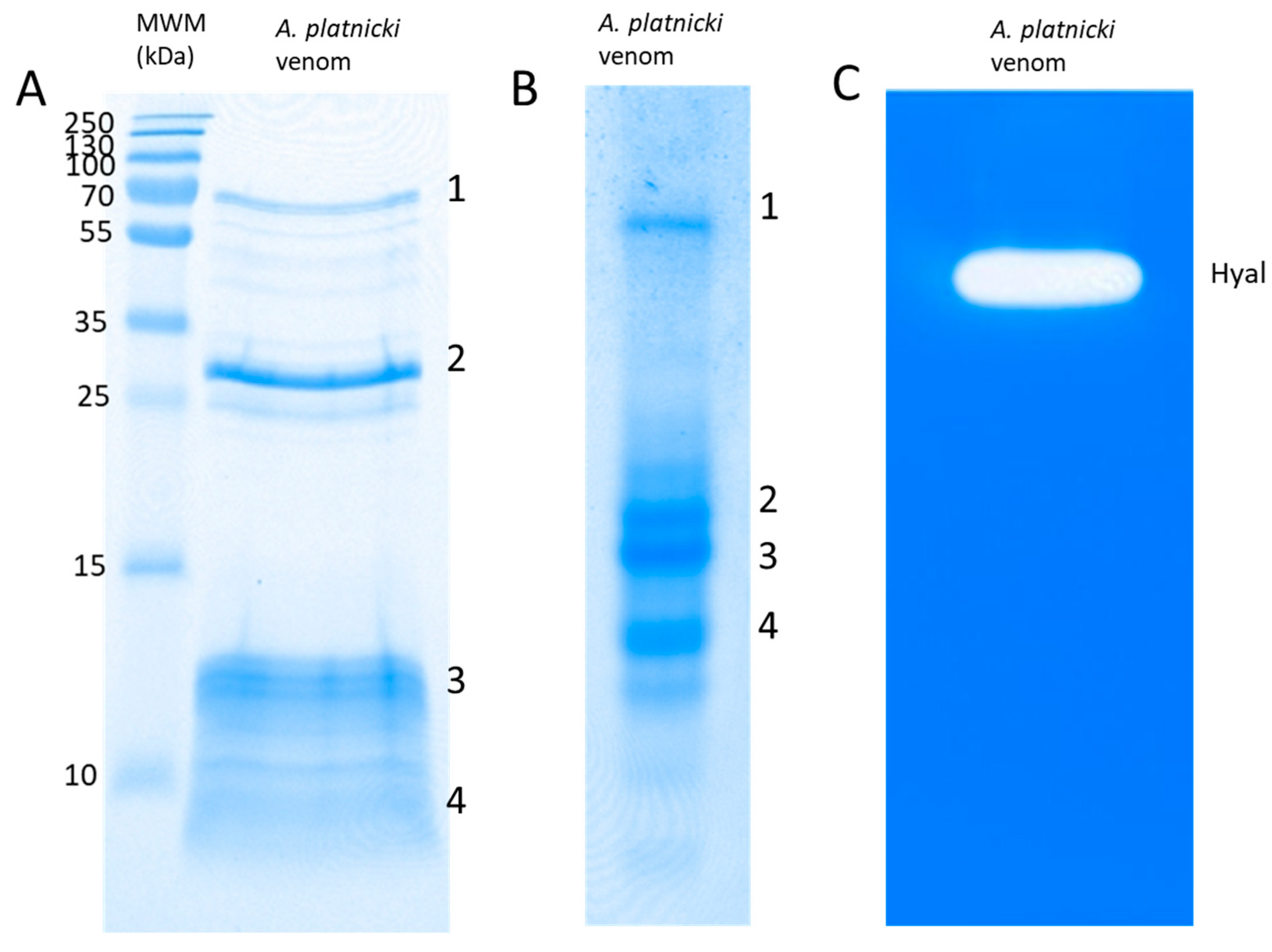
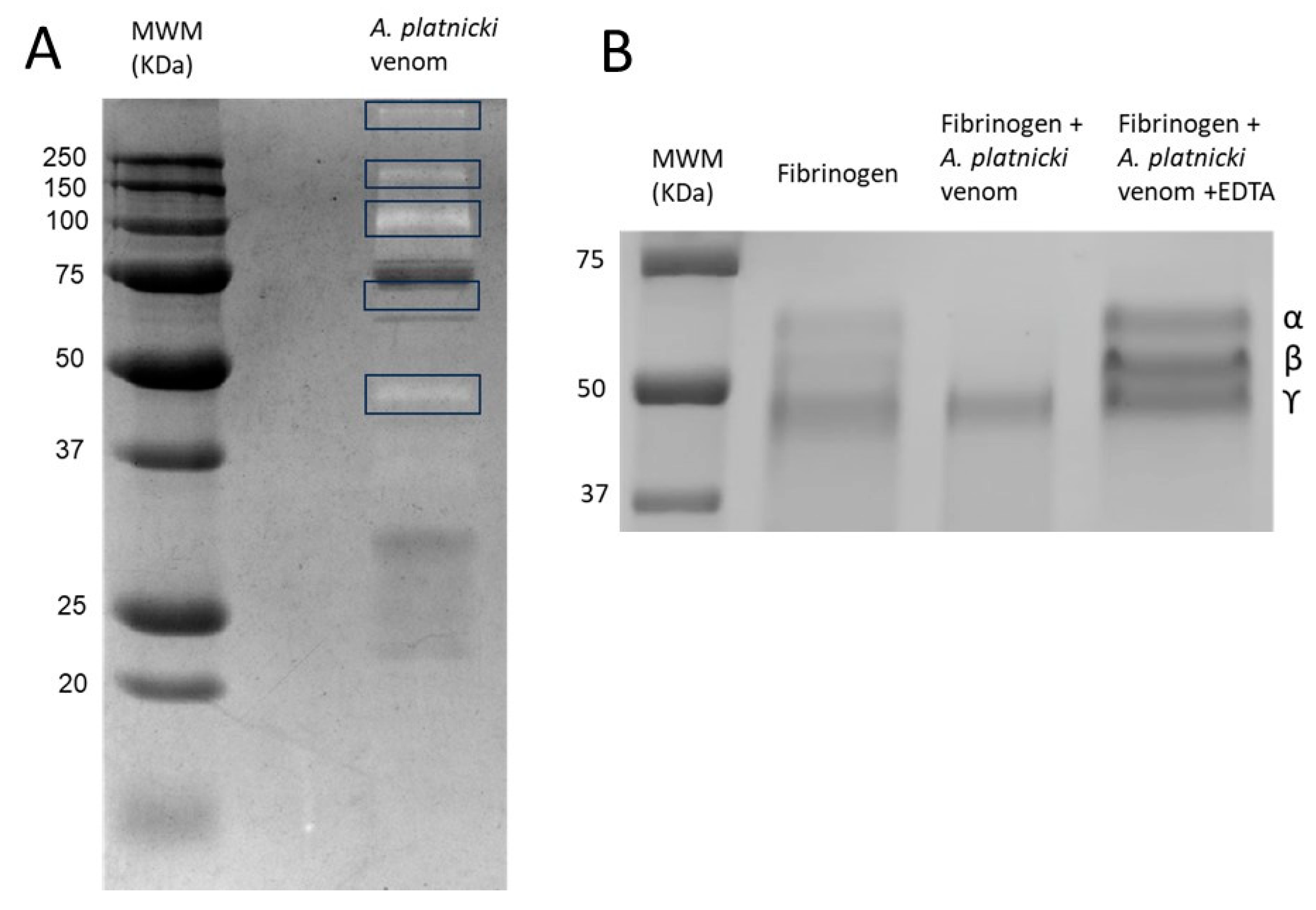
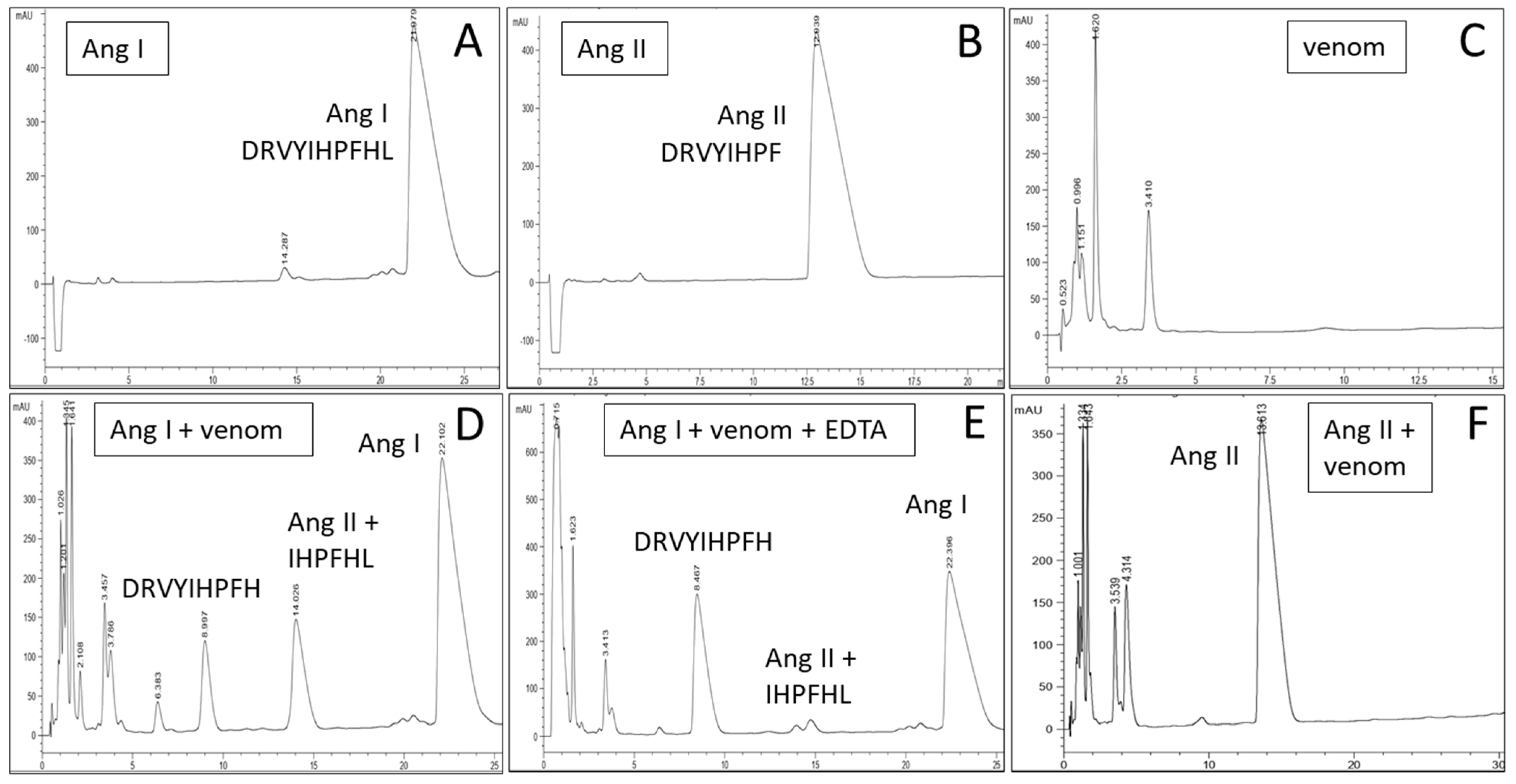
2. Results
3. Discussion
4. Materials and Methods
4.1. Scorpion Specimens and Venom
4.2. Electrophoresis and Determination of Enzymatic Activities
4.3. RP-HPLC for Venom Protein Separation
4.4. Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fet, V.; Lowe, G. Family Buthidae C.L. Koch, 1837. In Catalog of the Scorpions of the World; The New York Entomological Society: New York, NY, USA, 2000; pp. 54–286. [Google Scholar]
- Ward, M.J.; Ellsworth, S.A.; Nystrom, G.S. A global accounting of medically significant scorpions: Epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon 2018, 151, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Fet, V.; Soleglad, M.E.; Lowe, G. A new trichobothrial character for the high-level systematics of Buthoidea (Scorpiones: Buthida). Euscorpious 2005, 23, 1–40. [Google Scholar] [CrossRef]
- Stundlová, J.; Stahlavsky, F.; Opatova, V.; Stundl, J.; Kovarík, K.F.; Dolejs, P.; Smíd, J. Molecular data do not support the traditional morphology-based groupings in the scorpion family Buthidae (Arachnida: Scorpiones). Mol. Phylogenet. Evol. 2022, 173, 107511. [Google Scholar] [CrossRef]
- Santibañez-López, C.E.; Ojanguren-Affilastro, A.A.; Graham, M.R.; Sharma, P.P. Congruence between ultraconserved element-based matrices and phylotranscriptomic datasets in the scorpion Tree of Life. Cladistics 2023, 39, 533–547. [Google Scholar] [CrossRef]
- Pocock, R.I. Arachnida. The Fauna of British India, including Ceylon and Burma; Published under the Authority of the Secretary of State for India in Council; W.T. Blandford: London, UK, 1900; 279p. [Google Scholar]
- Lourenço, W.R. Révision du genre Ananteris Thorell, 1891 (Scorpiones, Buthidae) et déscription de six espèces nouvelles. Bull. Muséum Natl. d’Histoire Nat. Paris (Zool. Biol. Écol. Anim.) 1982, 4, 119–151. [Google Scholar] [CrossRef]
- Lourenço, A. The ‘Ananteris Group’ (Scorpiones: Buthidae), suggested composition and possible links with other Buthids. Bol. Soc. Entom. Arag. 2011, 48, 105–113. [Google Scholar]
- Ythier, E. A new high-altitude scorpion species of the genus Ananteris Thorell, 1891 (Scorpiones: Ananteridae) from the Pico da Neblina, Brazil. Faunitaxys 2024, 12, 1–9. [Google Scholar]
- Botero-Trujillo, R.; Noriega, J.A. On the identity of Microananteris, with a discussion on pectinal morphology, and description of a new Ananteris from Brazil (Scorpiones, Buthidae). Zootaxa 2011, 2747, 37–52. [Google Scholar] [CrossRef]
- Ojanguren-Affilastro, A.A.; Adilardi, R.S.; Mattoni, C.I.; Ramírez, M.J.; Ceccarelli, F.S. Dated phylogenetic studies of the southernmost American buthids (Scorpiones; Buthidae). Mol. Phylogenet. Evol. 2017, 110, 39–49. [Google Scholar] [CrossRef]
- Sharma, P.P.; Fernández, R.; Esposito, L.A.; González-Santillán, A.; Monod, L. Phylogenomic resolution of scorpions reveals multilevel discordance with morphological phylogenetic signal. Proc. R. Soc. B 2015, 282, 20142953. [Google Scholar] [CrossRef]
- Deshpande, S.; Joshi, M.; Kawai, K.; Deb, A.; Lee, J.D.; Bastawade, D.; Gowande, G.; Sulakhe, S. Molecular and morphological confirmation of Isometrus maculatus (DeGeer, 1778) (Scorpiones: Buthidae) from Northeast India and East Asia. Euscorpius 2023, 374, 1–19. [Google Scholar]
- Teruel, R. Morfología, ecología y distribución de Isometrus maculatus (Degeer 1778) en Cuba (Scorpiones: Buthidae). Bol. Soc. Entom. Arag. 2009, 45, 173–179. [Google Scholar]
- Froy, O.; Gurevitz, M. New insight on scorpion divergence inferred from comparative analysis of toxin structure, pharmacology and distribution. Toxicon 2003, 42, 549–555. [Google Scholar] [CrossRef]
- Lourenço, W.R. Scorpions trapped in amber: A remarkable window on their evolution over time from the Mesozoic period to present days. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230040. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Runjaic, F.J.M.; Portillo-Quintero, C.; Borges, A. Un nuevo escorpión del género Ananteris Thorell, 1891 (Scorpiones: Buthidae) para la Sierra Perijá, Venezuela. Mem. Fund. Salle Cienc. Nat. 2008, 169, 5–81. [Google Scholar]
- Botero-Trujillo, R.; Florez, E. A revisionary approach of Colombian Ananteris (Scorpiones, Buthidae): Two new species, a new synonymy, and notes on the value of trichobothria and hemispermatophore for the taxonomy of the group. Zootaxa 2011, 2904, 1–44. [Google Scholar] [CrossRef]
- Pucca, M.B.; Oliveira, F.N.; Schwartz, E.F.; Arantes, E.C.; Lira-da-Silva, R.M. Scorpionism and Dangerous Species of Brazil. In Scorpion Venoms; Gopalakrishnakone, P., Possani, L., Schwartz, E.F., Rodríguez de la Vega, R., Eds.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, Y.; He, Y.; Di, Z.; Wu, Y.; Cao, Z.; Li, W. Comparative venom gland transcriptome analysis of the scorpion Lychas mucronatus reveals intraspecific toxic gene diversity and new venom components. BMC Genom. 2010, 11, 452. [Google Scholar]
- Ma, Y.; He, Y.; Zhao, R.; Wu, Y.; Li, W.; Cao, Z. Extreme diversity of scorpion venom peptides and proteins revealed by transcriptomic analysis: Implication for proteome evolution of scorpion venom arsenal. J. Proteom. 2012, 75, 1563–1576. [Google Scholar] [CrossRef]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Expert Rev. Proteom. 2019, 17, 67–83. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–551. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.; Zhou, X.; Liu, Z. CAP superfamily proteins from venomous animals: Who we are and what to do? Int. J. Biol. Macromol. 2022, 221, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Z.; Ma, Y. Adaptive evolution of insect selective excitatory β-type sodium channel neurotoxins from scorpion venom. Peptides 2017, 92, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, Y.; Yin, S.; Zhao, R.; Fan, S.; Hu, Y.; Wu, Y.; Cao, Z.; Li, W. Molecular cloning and functional identification of a new K+ channel blocker, LmKTx10, from the scorpion Lychas mucronatus. Peptides 2009, 30, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, H.; Yang, F.; Liang, S.; Cao, Z.; Li, W.; Wu, Y. Functional characterization of two novel scorpion sodium channel toxins from Lychas mucronatus. Toxicon 2014, 90, 318–325. [Google Scholar] [CrossRef]
- Triana, F.; Bonilla, F.; Alfaro-Chinchilla, A.; Víquez, C.; Díaz, C.; Sasa, M. Report of thanatosis in the Central American scorpions Tityus ocelote and Ananteris platnicki (Scorpiones: Buthidae). Euscorpius 2022, 359, 1–5. [Google Scholar]
- Lourenço, A. Review of the geographical distribution of the genus Ananteris Thoreli (Scorpiones: Buthidae), with description of a new species. Rev. Biol. Trop. 1993, 41, 697–701. [Google Scholar]
- Lourenço, W.R. Comments on the Ananterinae Pocock, 1900 (Scorpiones: Buthidae) and description of a new remarkable species of Ananteris from Perú. C.R. Biologies 2015, 330, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Mattoni, C.I.; García-Hernández, S.; Botero-Trujillo, R.; Ochoa, J.A.; Ojanguren-Affilastro, A.A.; Pintoda-Rocha, R.; Prendini, L. Scorpion Sheds ‘Tail’ to Escape: Consequences and Implications of Autotomy in Scorpions (Buthidae: Ananteris). PLoS ONE 2015, 10, e0116639. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Harvey, P.J.; Craik, D.J.; Ronjat, M.; de Waard, M.; Zhu, S. Functional evolution of scorpion venom peptides with an inhibitor cystine knot fold. Biosci. Rep. 2013, 33, e00047. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, E.; Pairet, B.; Hartmann, H.; Decker, H. Crystallization and Preliminary Analysis of Crystals of the 24-Meric Hemocyanin of the Emperor Scorpion (Pandinus imperator). PLoS ONE 2012, 7, e32548. [Google Scholar] [CrossRef]
- Cunningham, M.; Laino, A.; Romero, S.; García, F. Arachnid hemocyanins. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins, Subcellular Biochemistry 94; Hoeger, U., Harris, J.R., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 219–231. [Google Scholar]
- Barth, T.; Coelho Mandacaru, S.; Charneau, S.; Valle de Souza, M.; Ornelas Ricart, C.A.; Ferreira Noronha, E.; Araújo Souza, A.; de Freitas, S.M.; Roepstorff, P.; Fontes, W.; et al. Biochemical and structural characterization of a protein complex containing a hyaluronidase and a CRISP-like protein isolated from the venom of the spider Acanthoscurria natalensis. J. Proteom. 2019, 192, 102–113. [Google Scholar] [CrossRef]
- Cajado-Carvalho, D.; Kazuo Kuniyoshi, A.; Duzzi, B.; Kei Iwai, L.; Castro de Oliveira, U.; Junqueira de Azevedo, I.L.M.; Tadashi Kodama, R.; Vieira Portaro, F. Insights into the hypertensive effects of Tityus serrulatus scorpion venom: Purification of an Angiotensin-converting enzyme-like peptide. Toxins 2016, 8, 348. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Malachize Alano-da-Silva, N.; Gobbo Ferreira, I.; Cerni, F.A.; de Almeida Goncalves Sachett, J.; Monteiro, W.M.; Pucca, M.B.; Candiani Arantes, E. Understanding the complexity of Tityus serrulatus venom: A focus on high molecular weight components. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230046. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, S. Mesobuthus venom-derived antimicrobial peptides possess intrinsic multifunctionality and differential potential as drugs. Front. Microbiol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.B.; Chan, A.H.C.; Koludarov, I.; Muñoz-Gómez, S.A.; Antunes, A.; Fry, B.G. Evolution stings: The origin and diversification of scorpion toxin peptide scaffolds. Toxins 2013, 5, 2456–2487. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.J.; de Armas, L.F.; Cambra, R.A. Predation of Ananteris spp. (Scorpions: Buthidae) by ants and social wasps (Hymenoptera, Formicidae, Vespidae) in Panama, Central America. Euscorpius 2021, 329, 1–4. [Google Scholar]
- Botero-Trujillo, R. Anuran predators of Scorpions: Bufo marinus (Linnaeus, 1758) (Anura: Bufonidae), first known natural enemy of Tityus nematochirus Mello-Leitão, 1940 (Scorpiones: Buthidae). Rev. Iber. Aracnol. 2006, 13, 199–202. [Google Scholar]
- Salabi, F.; Vazirianzadeh, B.; Baradaran, M. Identification, classification, and characterization of alpha and beta subunits of LVP1 protein from the venom gland of four Iranian scorpion species. Sci. Rep. 2023, 13, 22277. [Google Scholar] [CrossRef]
- de Oliveira, U.C.; Nishiyama, M.Y., Jr.; dos Santos, M.B.V.; Santos-da-Silva, A.d.P.; Chalkidis, H.d.M.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, A.C.; Junqueira-de-Azevedo, I.d.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and Tserrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef]
- Kalapothakis, Y.; Miranda, K.; Pereira, A.H.; Witt, A.S.A.; Marani, C.; Martins, A.P.; Gomes Leal, H.; Campos-Júnior, E.; Pimenta, A.M.C.; Borges, A.; et al. Novel components of Tityus serrulatus venom: A transcriptomic approach. Toxicon 2021, 189, 91–104. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B. Molecular characterization of a possible progenitor sodium channel toxin from the Old World scorpion Mesobuthus martensii. FEBS Lett. 2006, 580, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, W.; Zeng, X.; Jiang, D.; Mao, X.; Liu, H. Molecular cloning and sequencing of two ‘short chain’ K+ channel blocking peptides from the Chinese scorpion Buthus martensii Karsch. FEBS Lett. 1999, 457, 509–514. [Google Scholar] [CrossRef] [PubMed]
- So, W.L.; Leung, T.C.N.; Nong, W.; Bendena, W.G.; Ngai, S.M.; Hui, J.H.L. Transcriptomic and proteomic analyses of venom glands from scorpions Liocheles australasiae, Mesobuthus martensii, and Scorpio maurus palmatus. Peptides 2021, 146, 170643. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Chen, L.; Wei, J.-F.; Yang, X.; Ma, D.; Xu, X.; Xu, X.; He, S.; Lu, J.; Laiz, R. Purification and characterization of two new allergens from the venom of Vespa magnifica. PLoS ONE 2012, 7, e31920. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Rivera, J.; Lomonte, B.; Bonilla, F.; Diego-García, E.; Camacho, E.; Tytgat, J.; Sasa, M. Venom characterization of the bark scorpion Centruroides edwardsii (Gervais 1843): Composition, biochemical activities and in vivo toxicity for potential prey. Toxicon 2019, 171, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Diego-García, E.; Peigneur, S.; Clynen, E.; Marien, T.; Czech, L.; Schoofs, L.; Tytgat, J. Molecular diversity of the telson and venom components from Pandinus cavimanus (Scorpionidae Latreille 1802): Transcriptome, venomics and function. Proteomics 2012, 12, 313–328. [Google Scholar] [CrossRef]
- Salabi, F.; Jafari, H. Differential venom gland gene expression analysis of juvenile and adult scorpions Androctonus crassicauda. BMC Genom. 2022, 23, 636. [Google Scholar] [CrossRef]
- Khamtorn, P.; Rungsa, P.; Jangpromma, N.; Klaynongsruang, S.; Daduang, J.; Tessiri, T.; Daduang, S. Partial proteomic analysis of brown widow spider (Latrodectus geometricus) venom to determine the biological activities. Toxicon X 2020, 8, 100062. [Google Scholar] [CrossRef]
- Liang, S. Proteome and peptidome profiling of spider venoms. Expert Rev. Proteom. 2008, 5, 731–746. [Google Scholar] [CrossRef]
- Dai, C.; Ma, Y.; Zhao, Z.; Zhao, R.; Wang, Q.; Wu, Y.; Cao, Z.; Li, W. Mucroporin, the First Cationic Host Defense Peptide from the Venom of Lychas mucronatus. Antimicrob. Agents Chemother. 2008, 52, 3967–3972. [Google Scholar] [CrossRef]
- Zobel-Thropp, P.A.; Bulger, E.A.; Cordes, M.H.J.; Binord, G.J.; Gillespie, R.G.; Brewer, M.S. Sexually dimorphic venom proteins in long-jawed orb-weaving spiders (Tetragnatha) comprise novel gene families. PeerJ 2018, 6, e4691. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Dutra, A.A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.C.; Kjeldsen, F. Moving Pieces in a Venomic Puzzle: Unveiling Post-translationally Modified Toxins from Tityus serrulatus. J. Proteome Res. 2013, 12, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.; Bodiga, S. Renin-Angiotensin System in Cognitive Function and Dementia. Asian J. Neurosci. 2013, 2013, 102602. [Google Scholar] [CrossRef]
- Isaac, R.E.; Bland, N.D.; Shirras, A.D. Neuropeptides and metabolic inactivation of insect neuropeptides. Gen. Comp. Endocrinol. 2009, 162, 8–17. [Google Scholar] [CrossRef]
- Rastogi, A.; Sarkar, A.; Chakrabarty, D. Partial purification and identification of a metalloproteinase with anticoagulant activity from Rhizostoma pulmo (Barrel Jellyfish). Toxicon 2017, 132, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Murgas, I.; Bermúdez, S.; Miranda, R. First report of accidental envenomation by Ananteris platnicki Lourenço, 1993 (Scorpiones: Buthidae) in Panama. Rev. Med. Pan. 2020, 40, 163–164. [Google Scholar] [CrossRef]
- Cevallos, M.A.; Navarro-Duque, C.; Varela-Julia, M.; Alagon, A.C. Molecular mass determination and assay of venom hyaluronidases by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Toxicon 1992, 30, 925–930. [Google Scholar] [CrossRef]
- Delafontaine, M.; Villas-Boas, I.M.; Mathiew, L.; Josset, P.; Blomet, J.; Tambourgi, D.V. Enzymatic and pro-inflammatory activities of Bothrops lanceolatus venom: Relevance for envenomation. Toxins 2017, 9, 244. [Google Scholar] [CrossRef]
- Araújo Tenorio, H.; da Costa Marques, M.E.; Salgueiro Machado, S.; Juárez Vieira Pereira, H. Angiotensin processing activities in the venom of Thalassophryne nattereri. Toxicon 2015, 98, 49–53. [Google Scholar] [CrossRef]
- Lomonte, B.; Fernández, J. Solving the microheterogeneity of Bothrops asper myotoxin-II by high-resolution mass spectrometry: Insights into C-terminal region variability in Lys49-phospholipase A2 homologs. Toxicon 2022, 210, 123–1314. [Google Scholar] [CrossRef]


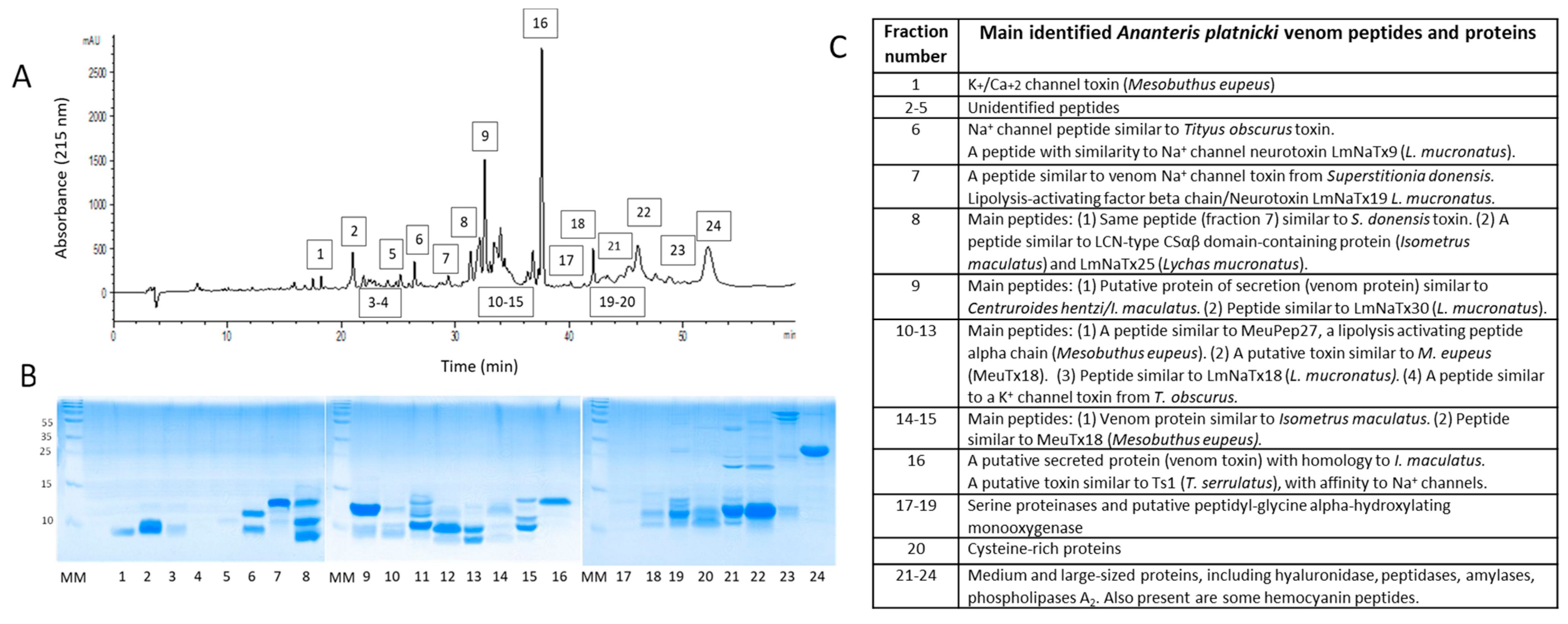
| # | Matching Protein, Species | Protein Family | Accession |
|---|---|---|---|
| 1 | Toxin, Mesobuthus eupeus | K+ channel/Ca+2 channel | A0A5P8U2N2 |
| 2 | Toxin, Mesobuthus eupeus | K+ channel | A0A5P8U2Q6 |
| 3 | Toxin, Tityus obscurus | K+ channel | A0A1E1WVV4 |
| 4 | Neurotoxin LmNaTx9, Lychas mucronatus | Na+ channel | A0A0U1S5U0 |
| 5 | Neurotoxin LmNaTx12, Lychas mucronatus | Na+ channel | A0A0U1SJ71 |
| 6 | Neurotoxin LmNaTx18, Lychas mucronatus | Na+ channel | A0A0U1SN99 |
| 7 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus/LmNaTx19, Lychas mucronatus | Na+ channel, LAPβ | A0A0U1S617 |
| 8 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus/ LmNaTx25 Lychas mucronatus | Na+ channel, LAPα | A0A0U1SPD0 |
| 9 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus/LmNaTx30, Lychas mucronatus | Na+ channel | A0A0U1TZ19 |
| 10 | LCN-type CS-alpha/beta domain-containing protein, Isometrus maculatus | Na+ channel | A0A0U1S617 |
| 11 | Venom peptide meuPep27, Mesobuthus eupeus | Na+ channel, LAPα | A0A146CJ25 |
| 12 | Putative Na+ channel toxin, Superstitionia donensis | Na+ channel | A0A1V1WBQ9 |
| 13 | Sodium channel toxin Ts1, Tityus serrulatus | Na+ channel | A0A7S8RFZ4 |
| 14 | Neurotoxin, Tityus obscurus | Na+ channel | A0A1E1WW03 |
| 15 | Alpha-amylase, Tityus obscurus | Amylase | A0A1E1WVL9 |
| 16 | Alpha-amylase, Hadrurus spadix | Amylase | A0A1W7RB82 |
| 17 | Cysteine-rich secretory protein, Centruroides hentzi | CRISP | A0A2I9LNV7 |
| 18 | CAP-Lyc-1, Lychas buchari | CRISP | T1DPC1 |
| 19 | Hyaluronidase, Androctonus crassicauda | Hyaluronidase | A0A7T9L322 |
| 20 | Glyceraldehyde-3-phosphate dehydrogenase, Hadrurus spadix | Oxidorreductase | A0A1W7RAH3 |
| 21 | Putative peptidyl-glycine alpha-hydroxylating monooxygenase, Tityus serrulatus | Oxidase | A0A7S8RGB6 |
| 22 | Angiotensin-converting enzyme, Tityus serrulatus | Peptidase | A0A1E1WWB8 |
| 23 | Neprilysn, Hadrurus spadix | Peptidase | A0A1W7RA38 |
| 24 | Metalloproteinase, Centruroides hentzi | Metalloproteinase | A0A2I9LP89 |
| 25 | Metalloproteinase, Centruroides hentzi | Metalloproteinase | A0A2I9LP68 |
| 26 | Metalloproteinase, Tityus obscurus | Metalloproteinase | A0A1E1WVW3 |
| 27 | Peptidase M14, Isometrus maculatus | Carboxypeptidase | A0A0U1SF04 |
| 28 | Cathepsin spartic protease, Centruroides hentzi | Aspartic protease | A0A2I9LNV0 |
| 29 | Peptidase S1 domain-containing protein, Isometrus maculatus | Serine protease | A0A0U1S633 |
| 30 | Aminopeptidase, Hadrurus spadix | Aminopeptidase | A0A1W7RAL3 |
| 31 | Phospholipase A2 Tityus obscurus | Phospholipase A2 | A0A1E1WVV6 |
| 32 | Phospholipase A2, Hadrurus spadix | Phospholipase A2 | A0A1W7RA16 |
| 33 | Venom protein, Centruroides hentzi/Isometrus maculatus | unclassified | A0A2I9LPX8 |
| 34 | Venom protein, Isometrus maculatus | unclassified | A0A0U1S614 |
| 35 | Venom protein, Mesobuthus eupeus | unclassified | E4VP36 |
| 36 | Ectonucleotide pyrophosphatase/phosphodiesterase, Tityus obscurus | Phosphodiesterase | A0A1E1WVL0 |
| 37 | Hemocyanins, Tityus obscurus/Hadrurus spadix/Pandinus imperator | Hemocyanin | A0A1E1WWC5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, C.; Lomonte, B.; Chang-Castillo, A.; Bonilla, F.; Alfaro-Chinchilla, A.; Triana, F.; Angulo, D.; Fernández, J.; Sasa, M. Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama. Toxins 2024, 16, 327. https://doi.org/10.3390/toxins16080327
Díaz C, Lomonte B, Chang-Castillo A, Bonilla F, Alfaro-Chinchilla A, Triana F, Angulo D, Fernández J, Sasa M. Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama. Toxins. 2024; 16(8):327. https://doi.org/10.3390/toxins16080327
Chicago/Turabian StyleDíaz, Cecilia, Bruno Lomonte, Arturo Chang-Castillo, Fabián Bonilla, Adriana Alfaro-Chinchilla, Felipe Triana, Diego Angulo, Julián Fernández, and Mahmood Sasa. 2024. "Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama" Toxins 16, no. 8: 327. https://doi.org/10.3390/toxins16080327
APA StyleDíaz, C., Lomonte, B., Chang-Castillo, A., Bonilla, F., Alfaro-Chinchilla, A., Triana, F., Angulo, D., Fernández, J., & Sasa, M. (2024). Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama. Toxins, 16(8), 327. https://doi.org/10.3390/toxins16080327






