Pierisin, Cytotoxic and Apoptosis-Inducing DNA ADP-Ribosylating Protein in Cabbage Butterfly
Abstract
1. Discovery and Characterization of Pierisin-1
2. Distribution of Pierisin-like Proteins in Various Kinds of Butterflies
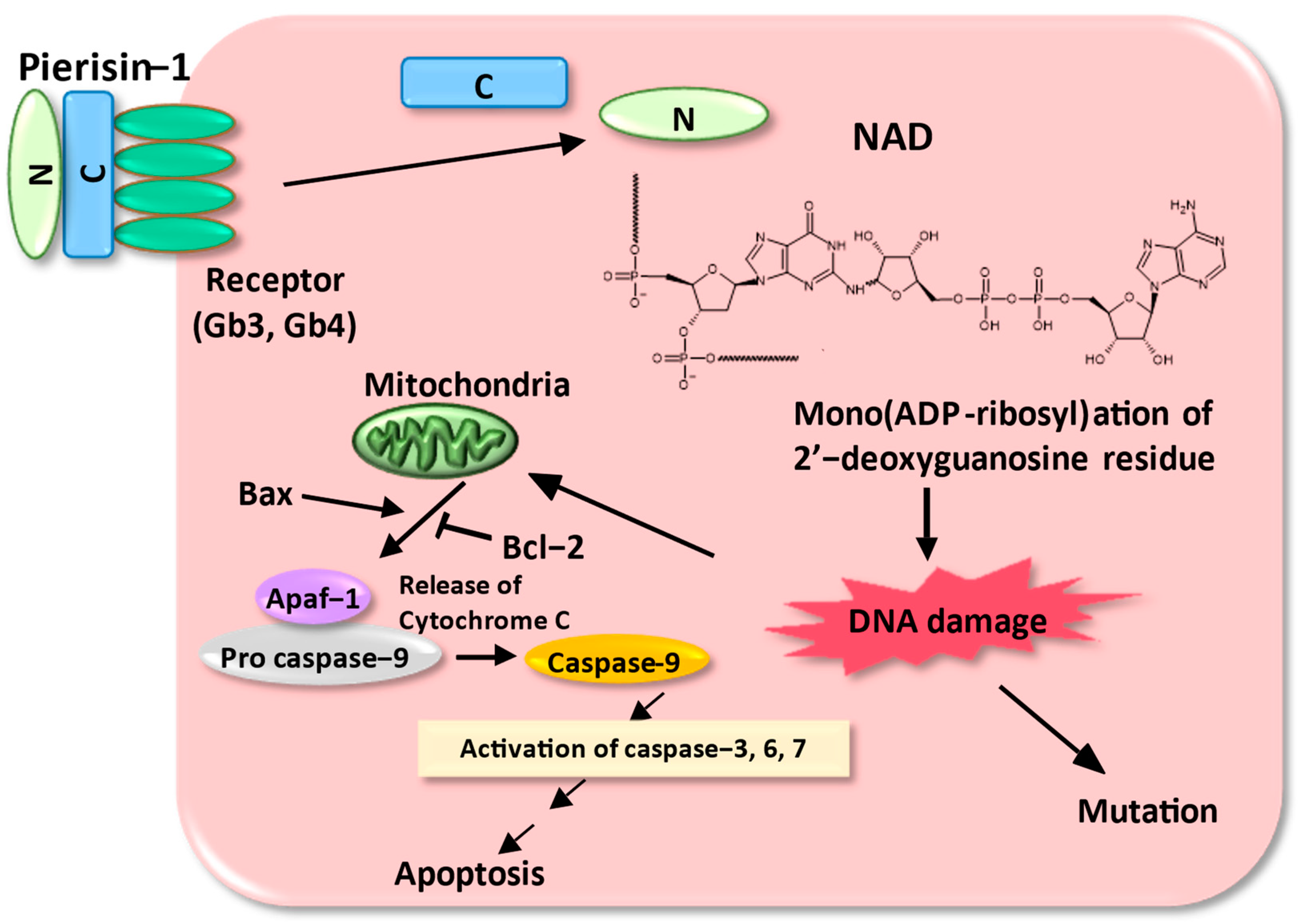
3. Biological Role of Pierisin-1 in Cabbage Butterfly
4. Distribution of Pierisin-like Proteins in Other Species Than Butterflies
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochiai, A.; Yasui, W.; Tahara, E. Growth-Promoting Effect of Gastrin on Human Gastric Carcinoma Cell Line Tmk-1. Jpn. J. Cancer Res. 1985, 76, 1064–1071. [Google Scholar] [PubMed]
- Koyama, K.; Wakabayashi, K.; Masutani, M.; Koiwai, K.; Watanabe, M.; Yamazaki, S.; Kono, T.; Miki, K.; Sugimura, T. Presence in Pieris rapae of Cytotoxic Activity against Human Carcinoma Cells. Jpn. J. Cancer Res. 1996, 87, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kono, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Purification of Pierisin, an Inducer of Apoptosis in Human Gastric Carcinoma Cells, from Cabbage Butterfly, Pieris rapae. Jpn. J. Cancer Res. 1998, 89, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Carpusca, I.; Jank, T.; Aktories, K. Bacillus sphaericus Mosquitocidal Toxin (MTX) and Pierisin: The Enigmatic Offspring from the Family of ADP-Ribosyltransferases. Mol. Microbiol. 2006, 62, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Thanabalu, T.; Hindley, J.; Jackson-Yap, J.; Berry, C. Cloning, Sequencing, and Expression of a Gene Encoding a 100-Kilodalton Mosquitocidal Toxin from Bacillus sphaericus SSII-1. J. Bacteriol. 1991, 173, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, J.; Wieden, H.J.; Rodnina, M.V.; Aktories, K. Inactivation of the Elongation Factor Tu by Mosquitocidal Toxin-Catalyzed Mono-ADP-Ribosylation. Appl. Environ. Microbiol. 2002, 68, 4894–4899. [Google Scholar] [CrossRef]
- Schirmer, J.; Just, I.; Aktories, K. The ADP-Ribosylating Mosquitocidal Toxin from Bacillus sphaericus. J. Biol. Chem. 2002, 277, 11941–11948. [Google Scholar] [CrossRef] [PubMed]
- Thanabalu, T.; Berry, C.; Hindley, J. Cytotoxicity and ADP-Ribosylating Activity of the Mosquitocidal Toxin from Bacillus sphaericus SSII-1: Possible Roles of the 27- and 70-Kilodalton Peptides. J. Bacteriol. 1993, 175, 2314–2320. [Google Scholar] [CrossRef]
- Honjo, T.; Nishizuka, Y.; Hayaishi, O. Diphtheria Toxin-Dependent Adenosine Diphosphate Ribosylation of Aminoacyl Transferase II and Inhibition of Protein Synthesis. J. Biol. Chem. 1968, 243, 3553–3555. [Google Scholar] [CrossRef]
- Kessel, M.; Klink, F. Archaebacterial Elongation Factor Is ADP-Ribosylated by Diphtheria Toxin. Nature 1980, 287, 250–251. [Google Scholar] [CrossRef]
- Parikh, S.L.; Schramm, V.L. Transition State Structure for ADP-Ribosylation of Eukaryotic Elongation Factor 2 Catalyzed by Diphtheria Toxin. Biochemistry 2004, 43, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.E.; Rodriguez, O.I.; Marquez, J.A.; Berghuis, A.M. An Entamoeba Histolytica ADP-Ribosyl Transferase from the Diphtheria Toxin Family Modifies the Bacterial Elongation Factor Tu. Mol. Biochem. Parasitol. 2016, 207, 68–74. [Google Scholar] [CrossRef] [PubMed]
- De Haan, L.; Hirst, T.R. Cholera Toxin: A Paradigm for Multi-Functional Engagement of Cellular Mechanisms (Review). Mol. Membr. Biol. 2004, 21, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Kimura, K.; Fujii, N.; Yokosawa, N.; Indoh, T.; Murakami, T.; Oguma, K. Cloning and Complete Nucleotide Sequence of the Gene for the Main Component of Hemagglutinin Produced by Clostridium botulinum Type C. Infect. Immun. 1990, 58, 3173–3177. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kono, T.; Matsushima-Hibiya, Y.; Kanazawa, T.; Nishisaka, N.; Kishimoto, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Molecular Cloning of an Apoptosis-Inducing Protein, Pierisin, from Cabbage Butterfly: Possible Involvement of ADP-Ribosylation in Its Activity. Proc. Natl. Acad. Sci. USA 1999, 96, 10608–10613. [Google Scholar] [CrossRef] [PubMed]
- Takamura-Enya, T.; Watanabe, M.; Totsuka, Y.; Kanazawa, T.; Matsushima-Hibiya, Y.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Mono(ADP-Ribosyl)Ation of 2’-Deoxyguanosine Residue in DNA by an Apoptosis-Inducing Protein Pierisin-1 from Cabbage Butterfly. Proc. Natl. Acad. Sci. USA 2001, 98, 12414–12419. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Watanabe, M.; Matsushima-Hibiya, Y.; Kono, T.; Tanaka, N.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Distinct Roles for the N- and C-Terminal Regions in the Cytotoxicity of Pierisin-1, a Putative ADP-Ribosylating Toxin from Cabbage Butterfly, against Mammalian Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 2226–2231. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Hirabayashi, H.; Shikauchi, G.; Takamura, R.; Hiraga, K.; Minami, H.; Hashimoto, H.; Yamamoto, M.; Wakabayashi, K.; Shimizu, T.; et al. Structural Basis of Autoinhibition and Activation of the DNA-Targeting ADP-Ribosyltransferase Pierisin-1. J. Biol. Chem. 2017, 292, 15445–15455. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Just, I.; Rosenthal, W. Different Types of ADP-Ribose Protein Bonds Formed by Botulinum C2 Toxin, Botulinum ADP-Ribosyltransferase C3 and Pertussis Toxin. Biochem. Biophys. Res. Commun. 1988, 156, 361–367. [Google Scholar] [CrossRef]
- Aktories, K.; Bärmann, M.; Ohishi, I.; Tsuyama, S.; Jakobs, K.H.; Habermann, E. Botulinum C2 Toxin ADP-Ribosylates Actin. Nature 1986, 322, 390–392. [Google Scholar] [CrossRef]
- Mauss, S.; Chaponnier, C.; Just, I.; Aktories, K.; Gabbiani, G. ADP-ribosylation of Actin Isoforms by Clostridium botulinum C2 Toxin and Clostridium Perfringens Iota Toxin. Eur. J. Biochem. 1990, 194, 237–241. [Google Scholar] [CrossRef]
- Weigt, C.; Just, I.; Wegner, A.; Aktories, K. Nonmuscle Actin ADP-Ribosylated by Botulinum C2 Toxin Caps Actin Filaments. FEBS Lett. 1989, 246, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, J.; Schering, B.; Barmann, M.; Aktories, K. Botulinum C2 Toxin ADP-Ribosylates Cytoplasmic β/γ-Actin in Arginine 177. J. Biol. Chem. 1988, 263, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Preiss, J.C.; Hofmann, F.; Aktories, K. Characterization of the Catalytic Site of the ADP-Ribosyltransferase Clostridium botulinum C2 Toxin by Site-Directed Mutagenesis. J. Biol. Chem. 1998, 273, 29506–29511. [Google Scholar] [CrossRef] [PubMed]
- Matsushima-Hibiya, Y.; Watanabe, M.; Hidari, K.I.-P.; Miyamoto, D.; Suzuki, Y.; Kasama, T.; Kasama, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Identification of Glycosphingolipid Receptors for Pierisin-1, a Guanine-Specific ADP-Ribosylating Toxin from the Cabbage Butterfly. J. Biol. Chem. 2003, 278, 9972–9978. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Boodhoo, A.; Hazes, B.; Cummings, M.D.; Armstrong, G.D.; Brunton, J.L.; Read, R.J. Structure of the Shiga-like Toxin I B-Pentamer Complexed with an Analogue of Its Receptor Gb3. Biochemistry 1998, 37, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.E.; Fujinaga, M.; Cherney, M.M.; Melton-Celsa, A.R.; Twiddy, E.M.; O’Brien, A.D.; James, M.N. Structure of shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J. Biol. Chem. 2004, 279, 27511–27517. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Kono, T.; Watanabe, M.; Matsushima-Hibiya, Y.; Nakano, T.; Koyama, K.; Tanaka, N.; Sugimura, T.; Wakabayashi, K. Bcl-2 Blocks Apoptosis Caused by Pierisin-1, a Guanine-Specific ADP-Ribosylating Toxin from the Cabbage Butterfly. Biochem. Biophys. Res. Commun. 2002, 296, 20–25. [Google Scholar] [CrossRef]
- Adelberg, E.A.; Mandel, M.; Ching Chen, G.C. Optimal Conditions for Mutagenesis by N-Methyl-N’-Nitro-N-Nitrosoguanidine in Escherichia coli K12. Biochem. Biophys. Res. Commun. 1965, 18, 788–795. [Google Scholar] [CrossRef]
- Heidelberger, C.; Brankow, D.W. Quantitative and Qualitative Studies of Chemical Transformation of Cloned C3H Mouse Embryo Cells Sensitive to Postconfluence Inhibition of Cell Division. Cancer Res. 1973, 33, 3239–3249. [Google Scholar]
- Yu, S.W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of Poly(ADP-Ribose) Polymerase-1—Dependent Cell Death by Apoptosis-Inducing Factor. Science 2002, 297, 5579. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, M.; Iishi, H.; Baba, M.; Nakaizumi, A.; Ichii, M.; Taniguchi, H. Inhibition by γ-Amino-n-Butyric Acid and Baclofen of Gastric Carcinogenesis Induced by N’-Methyl-N’-Nitro-N’-Nitrosoguanidine in Wistar Rats. Cancer Res. 1990, 50, 4931–4934. [Google Scholar] [PubMed]
- Cupples, C.G.; Cabrera, M.; Cruz, C.; Miller, J.H. A Set of LacZ Mutations in Escherichia coli That Allow Rapid Detection of Specific Frameshift Mutations. Genetics 1990, 125, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, H.G.; Kim, B.S.; Holsapple, M.P. Characterization of the Effects of Direct Alkylators on In Vitro Immune Responses. Mutat. Res./Genet. Toxicol. 1990, 242, 67–78. [Google Scholar] [CrossRef]
- Jones, M.J.; Epstein, L. Adhesion of Macroconidia to the Plant Surface and Virulence of Nectria haematococca. Appl. Environ. Microbiol. 1990, 56, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, Y.; Kawanishi, M.; Nishigaki, R.; Matsukawa, K.; Yagi, T.; Takamura-Enya, T.; Watanabe, M.; Sugimura, T.; Wakabayashi, K. Analysis of HPRT and SupF Mutations Caused by Pierisin-1, a Guanine Specific ADP-Ribosylating Toxin Derived from the Cabbage Butterfly. Chem. Res. Toxicol. 2003, 16, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Enomoto, S.; Takamura-Enya, T.; Nakano, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Enzymatic Properties of Pierisin-1 and Its N-Terminal Domain, a Guanine-Specific ADP-Ribosyltransferase from the Cabbage Butterfly. J. Biochem. 2004, 135, 471–477. [Google Scholar] [CrossRef]
- Sugimura, T. Serendipitous Discoveries from Sudden Inspirations and the Joy of Being a Scientist. Biochem. Biophys. Res. Commun. 2002, 296, 1037–1038. [Google Scholar] [CrossRef]
- Kono, T.; Watanabe, M.; Koyama, K.; Kishimoto, T.; Fukushima, S.; Sugimura, T.; Wakabayashi, K. Cytotoxic Activity of Pierisin, from the Cabbage Butterfly, Pieris rapae, in Various Human Cancer Cell Lines. Cancer Lett. 1999, 137, 75–81. [Google Scholar] [CrossRef]
- Kanazawa, T.; Watanabe, M.; Kanzawa, F.; Matsushima-Hibiya, Y.; Koyama, K.; Tanaka, N.; Sugimura, T.; Wakabayashi, K. Pierisin-1 from Cabbage Butterfly Suppresses HeLa Tumor Growth in Nude Mice. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2002, 78, 305–308. [Google Scholar] [CrossRef]
- Shiga, A.; Kakamu, S.; Sugiyama, Y.; Shibata, M.; Makino, E.; Enomoto, M. Acute Toxicity of Pierisin-1, a Cytotoxic Protein from Pieris rapae, in Mouse and Rat. J. Toxicol. Sci. 2006, 31, 123–137. [Google Scholar] [CrossRef]
- Siegall, C.B.; Chaudhary, V.K.; FitzGerald, D.J.; Pastan, I. Functional Analysis of Domains II, Ib, and III of Pseudomonas Exotoxin. J. Biol. Chem. 1989, 264, 14256–14261. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.K.; Jinno, Y.; FitzGerald, D.; Pastan, I. Pseudomonas Exotoxin Contains a Specific Sequence at the Carboxyl Terminus That Is Required for Cytotoxicity. Proc. Natl. Acad. Sci. USA 1990, 87, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Weldon, J.E.; Pastan, I. A Guide to Taming a Toxin—Recombinant Immunotoxins Constructed from Pseudomonas Exotoxin A for the Treatment of Cancer. FEBS J. 2011, 278, 4683–4700. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Obiri, N.I.; Pastan, I.; Puri, R.K. A Novel Chimeric Protein Composed of Interleukin 13 and Pseudomonas Exotoxin Is Highly Cytotoxic to Human Carcinoma Cells Expressing Receptors for Interleukin 13 and Interleukin 4. J. Biol. Chem. 1995, 270, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.J.; Jolliffe, N.A.; Marsden, C.J.; Pateman, C.S.C.; Smith, D.C.; Spooner, R.A.; Watson, P.D.; Roberts, L.M. Ricin: Mechanisms of Cytotoxicity. Toxicol. Rev. 2003, 22, 53–64. [Google Scholar] [CrossRef]
- Wu, Y.; Taisne, C.; Mahtal, N.; Forrester, A.; Lussignol, M.; Cintrat, J.C.; Esclatine, A.; Gillet, D.; Barbier, J. Autophagic Degradation Is Involved in Cell Protection against Ricin Toxin. Toxins 2023, 15, 304. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Watanabe, M.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Anti-Cancer Substance in Pieris brassicae. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1997, 73, 192–194. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsushima-Hibiya, Y.; Nakano, T.; Yamamoto, M.; Iwabuchi, K.; Sugimura, T.; Wakabayashi, K. Persistence of Pierisin-1 Activities in the Adult Cabbage White Butterfly, Pieris rapae, during Storage after Killing. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 175–178. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nakano, T.; Yamamoto, M.; Matsushima-Hibiya, Y.; Odagiri, K.-I.; Yata, O.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Distribution of Cytotoxic and DNA ADP-Ribosylating Activity in Crude Extracts from Butterflies among the Family Pieridae. Proc. Natl. Acad. Sci. USA 2008, 105, 2516–2520. [Google Scholar] [CrossRef]
- Matsushima-Hibiya, Y.; Watanabe, M.; Kono, T.; Kanazawa, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Purification and Cloning of Pierisin-2, an Apoptosis-Inducing Protein from the Cabbage Butterfly, Pieris brassicae. Fed. Eur. Biochem. Soc. J. 2000, 267, 5742–5750. [Google Scholar] [CrossRef]
- Takamura-Enya, T.; Watanabe, M.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Mono(ADP-Ribosyl)Ation of the N 2 Amino Groups of Guanine Residues in DNA by Pierisin-2, from the Cabbage Butterfly, Pieris brassicae. Biochem. Biophys. Res. Commun. 2004, 323, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nakano, T.; Matsushima-Hibiya, Y.; Totsuka, Y.; Takahashi-Nakaguchi, A.; Matsumoto, Y.; Sugimura, T.; Wakabayashi, K. Molecular Cloning of Apoptosis-Inducing Pierisin-like Proteins, from Two Species of White Butterfly, Pieris melete and Aporia crataegi. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2009, 154, 326–333. [Google Scholar] [CrossRef]
- Subbarayan, S.; Marimuthu, S.K.; Nachimuthu, S.K.; Zhang, W.; Subramanian, S. Characterization and Cytotoxic Activity of Apoptosis-Inducing Pierisin-5 Protein from White Cabbage Butterfly. Int. J. Biol. Macromol. 2016, 87, 16–27. [Google Scholar] [CrossRef]
- Sarathbabu, S.; Marimuthu, S.K.; Ghatak, S.; Vidyalakshmi, S.; Gurusubramanian, G.; Ghosh, S.K.; Subramanian, S.; Zhang, W.; Kumar, N.S. Induction of Apoptosis by Pierisin-6 in HPV Positive HeLa and HepG2 Cancer Cells Is Mediated by the Caspase-3 Dependent Mitochondrial Pathway. Anticancer. Agents Med. Chem. 2018, 19, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, G.; Casartelli, M. Cell Death during Complete Metamorphosis. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190065. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakano, T.; Shiotani, B.; Matsushima-Hibiya, Y.; Kiuchi, M.; Yukuhiro, F.; Kanazawa, T.; Koyama, K.; Sugimura, T.; Wakabayashi, K. Developmental Stage-Specific Expression and Tissue Distribution of Pierisin-1, a Guanine-Specific ADP-Ribosylating Toxin, in Pieris rapae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 139, 125–131. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi-Nakaguchi, A.; Matsushima-Hibiya, Y.; Nakano, T.; Totsuka, Y.; Imanishi, S.; Mitsuhashi, J.; Watanabe, M.; Nakagama, H.; Sugimura, T.; et al. Nucleotide Sequence and Chromosomal Localization of the Gene for Pierisin-1, a DNA ADP-Ribosylating Protein, in the Cabbage Butterfly Pieris rapae. Genetica 2011, 139, 1251–1258. [Google Scholar] [CrossRef]
- Truman, J.W. The Evolution of Insect Metamorphosis. Curr. Biol. 2019, 29, R1252–R1268. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the Insect Metamorphic Transition by Ecdysteroid Production and Secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef]
- Tettamanti, G.; Grimaldi, A.; Pennacchio, F.; De Eguileor, M. Erratum: Lepidopteran Larval Midgut during Prepupal Instar: Digestion or Self-Digestion? Autophagy 2007, 3, 630–631. [Google Scholar] [CrossRef]
- Rusconi, J.C.; Hays, R.; Cagan, R.L. Programmed Cell Death and Patterning in Drosophila. Cell Death Differ. 2000, 7, 1063–1070. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.H. Programmed Cell Death Reshapes the Central Nervous System during Metamorphosis in Insects. Curr. Opin. Insect Sci. 2021, 43, 39–45. [Google Scholar] [CrossRef]
- Steller, H. Mechanisms and Genes of Cellular Suicide. Science 1995, 267, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, R.; Srinivasan, R.; Rawdzah, M.A.; Malini, P. Mapping and Identification of Potential Target Genes from Short–RNA Seq for the Control of Pieris rapae Larvae. Genomics 2020, 112, 1464–1476. [Google Scholar] [CrossRef]
- Grishin, N.V.; Shen, J.; Cong, Q.; Kinch, L.N.; Borek, D.; Otwinowski, Z. Complete Genome of Pieris rapae, a Resilient Alien, a Cabbage Pest, and a Source of Anti-Cancer Proteins. F1000Research 2016, 5, 2631. [Google Scholar] [CrossRef]
- Kebede, M.; Fite, T. RNA Interference (RNAi) Applications to the Management of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae): Its Current Trends and Future Prospects. Front. Mol. Biosci. 2022, 9, 944774. [Google Scholar] [CrossRef]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.L.; Barthel, A.; et al. RNA Interference in Lepidoptera: An Overview of Successful and Unsuccessful Studies and Implications for Experimental Design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Ortolá, B.; Daròs, J.A. RNA Interference in Insects: From a Natural Mechanism of Gene Expression Regulation to a Biotechnological Crop Protection Promise. Biology 2024, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Chen, R.; Wang, J.J. RNA Interference in Insects: The Link between Antiviral Defense and Pest Control. Insect Sci. 2024, 31, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the Elements of Successful Insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef]
- Takahashi-Nakaguchi, A.; Matsumoto, Y.; Yamamoto, M.; Iwabuchi, K.; Totsuka, Y.; Sugimura, T.; Wakabayashi, K. Demonstration of Cytotoxicity against Wasps by Pierisin-1: A Possible Defense Factor in the Cabbage White Butterfly. PLoS ONE 2013, 8, e60539. [Google Scholar] [CrossRef]
- Nakano, T.; Takahashi-Nakaguchi, A.; Yamamoto, M.; Watanabe, M. Pierisins and CARP-1: ADP-Ribosylation of DNA by ARTCs in Butterflies and Shellfish. In Endogenous ADP-Ribosylation; Springer: Berlin/Heidelberg, Germany, 2014; Volume 384. [Google Scholar]
- Nakano, T.; Matsushima-Hibiya, Y.; Yamamoto, M.; Enomoto, S.; Matsumoto, Y.; Totsuka, Y.; Watanabe, M.; Sugimura, T.; Wakabayashi, K. Purification and Molecular Cloning of a DNA ADP-Ribosylating Protein, CARP-1, from the Edible Clam Meretrix lamarckii. Proc. Natl. Acad. Sci. USA 2006, 103, 13652–13657. [Google Scholar] [CrossRef]
- Nakano, T.; Matsushima-Hibiya, Y.; Yamamoto, M.; Takahashi-Nakaguchi, A.; Fukuda, H.; Ono, M.; Takamura-Enya, T.; Kinashi, H.; Totsuka, Y. ADP-Ribosylation of Guanosine by SCO5461 Protein Secreted from Streptomyces coelicolor. Toxicon 2013, 63, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Schuller, M.; Butler, R.E.; Ariza, A.; Tromans-Coia, C.; Jankevicius, G.; Claridge, T.D.W.; Kendall, S.L.; Goh, S.; Stewart, G.R.; Ahel, I. Molecular Basis for DarT ADP-Ribosylation of a DNA Base. Nature 2021, 596, 597–602. [Google Scholar] [CrossRef]
- Schuller, M.; Raggiaschi, R.; Mikolcevic, P.; Rack, J.G.; Ariza, A.; Zhang, Y.; Ledermann, R.; Tang, C.; Mikoc, A.; Ahel, I. Molecular basis for the reversible ADP-ribosylation of guanosine bases. Mol. Cell 2023, 83, 2303–2315.e6. [Google Scholar] [CrossRef]
- Krska, D.; Ravulapalli, R.; Fieldhouse, R.J.; Lugo, M.R.; Merrill, A.R. C3larvin Toxin, an ADP-Ribosyltransferase from Paenibacillus larvae. J. Biol. Chem. 2015, 290, 1639–1653. [Google Scholar] [CrossRef]
- Ebeling, J.; Fünfhaus, A.; Genersch, E. The Buzz about ADP-Ribosylation Toxins from Paenibacillus larvae, the Causative Agent of American Foulbrood in Honey Bees. Toxins 2021, 13, 151. [Google Scholar] [CrossRef]
- Turner, M.; Tremblay, O.; Heney, K.A.; Lugo, M.R.; Ebeling, J.; Genersch, E.; Rod Merrill, A. Characterization of C3larvinA, a Novel RhoA-Targeting ADP-Ribosyltransferase Toxin Produced by the Honey Bee Pathogen, Paenibacillus larvae. Biosci. Rep. 2020, 40, BSR20193405. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Heney, K.A.; Rod Merrill, A. The N-Terminus of Paenibacillus Larvae C3larvinA Modulates Catalytic Efficiency. Biosci. Rep. 2021, 41, BSR20203727, Erratum in Biosci Rep. 2021, 41, BSR-20203727_COR. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tsuge, H. Substrate N2 Atom Recognition Mechanism in Pierisin Family DNA-Targeting, Guanine-Specific ADP-Ribosyltransferase ScARP. J. Biol. Chem. 2018, 293, 13768–13774. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tsuge, H. Common Mechanism for Target Specificity of Protein-and DNA-Targeting ADP-Ribosyltransferases. Toxins 2021, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.M.; Barbieri, J.T. The family of bacterial ADP-ribosylating exotoxins. Clin. Microbiol. Rev. 1995, 8, 34–47. [Google Scholar] [CrossRef]
- Okazaki, I.J.; Moss, J. Characterization of glycosylphosphatidylinositiol-anchored, secreted, and intracellular vertebrate mono-ADP-ribosyltransferases. Annu. Rev. Nutr. 1999, 19, 485–509. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Nakaguchi, A.; Shishido, E.; Yahara, M.; Urayama, S.; Sakai, K.; Chibana, H.; Kamei, K.; Moriyama, H.; Gonoi, T. Analysis of an Intrinsic Mycovirus Associated with Reduced Virulence of the Human Pathogenic Fungus Aspergillus fumigatus. Front. Microbiol. 2020, 10, 3045. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- d’Herrelle, F.; Smith, G.H. The Bacteriophage and Its Behavior. Sci. News-Lett. 1926, 9, 10. [Google Scholar] [CrossRef]
- Otsuki, R.; Yamamoto, M.; Matsumoto, E.; Iwamoto, S.I.; Sezutsu, H.; Suzui, M.; Takaki, K.; Wakabayashi, K.; Mori, H.; Kotani, E. Bioengineered Silkworms with Butterfly Cytotoxinmodified Silk Glands Produce Sericin Cocoons with a Utility for a New Biomaterial. Proc. Natl. Acad. Sci. USA 2017, 114, 6740–6745. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi-Nakaguchi, A.; Horiuchi, Y.; Yamamoto, M.; Totsuka, Y.; Wakabayashi, K. Pierisin, Cytotoxic and Apoptosis-Inducing DNA ADP-Ribosylating Protein in Cabbage Butterfly. Toxins 2024, 16, 270. https://doi.org/10.3390/toxins16060270
Takahashi-Nakaguchi A, Horiuchi Y, Yamamoto M, Totsuka Y, Wakabayashi K. Pierisin, Cytotoxic and Apoptosis-Inducing DNA ADP-Ribosylating Protein in Cabbage Butterfly. Toxins. 2024; 16(6):270. https://doi.org/10.3390/toxins16060270
Chicago/Turabian StyleTakahashi-Nakaguchi, Azusa, Yu Horiuchi, Masafumi Yamamoto, Yukari Totsuka, and Keiji Wakabayashi. 2024. "Pierisin, Cytotoxic and Apoptosis-Inducing DNA ADP-Ribosylating Protein in Cabbage Butterfly" Toxins 16, no. 6: 270. https://doi.org/10.3390/toxins16060270
APA StyleTakahashi-Nakaguchi, A., Horiuchi, Y., Yamamoto, M., Totsuka, Y., & Wakabayashi, K. (2024). Pierisin, Cytotoxic and Apoptosis-Inducing DNA ADP-Ribosylating Protein in Cabbage Butterfly. Toxins, 16(6), 270. https://doi.org/10.3390/toxins16060270




