Effects of Scallop Mantle Toxin on Intestinal Microflora and Intestinal Barrier Function in Mice
Abstract
1. Introduction
2. Results and Discussion
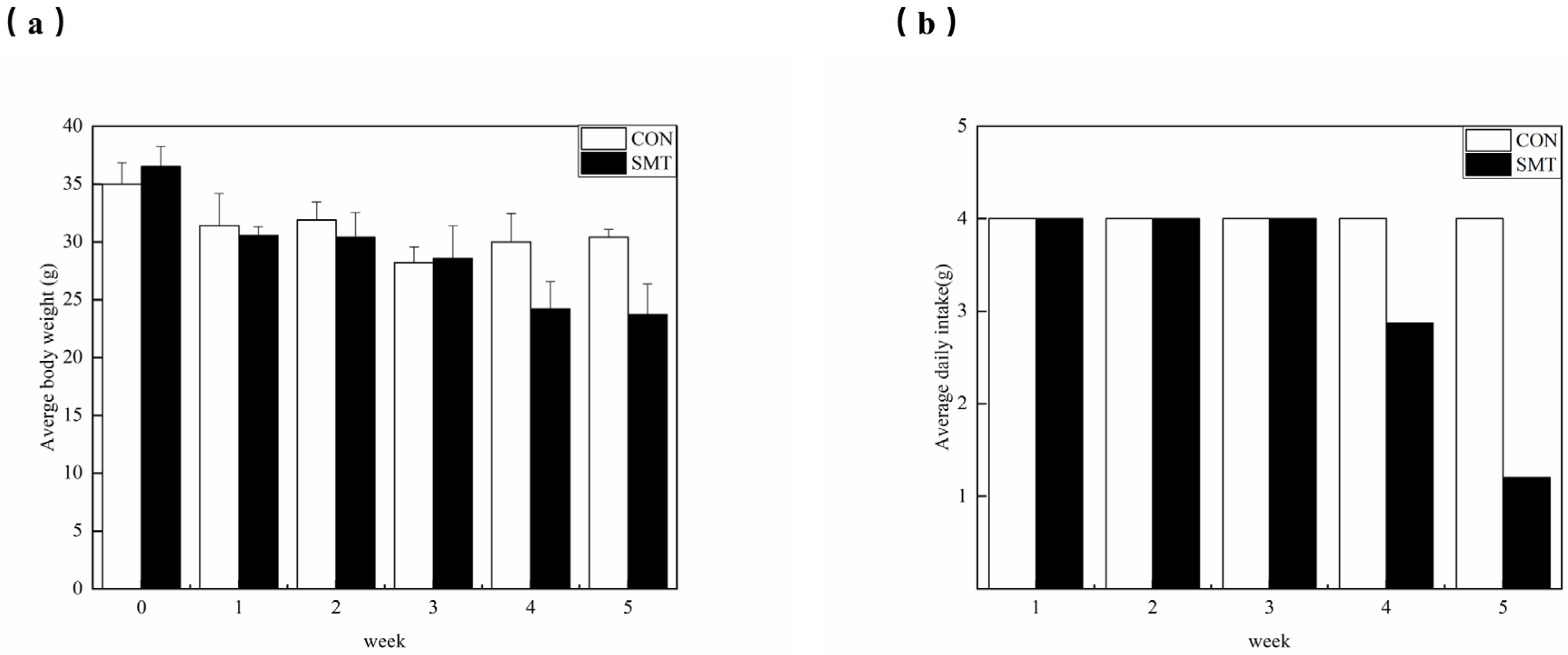
2.1. Changes in Body Weight and Food Intake
2.2. Effects of Scallop Mantle Toxin on Liver Damage
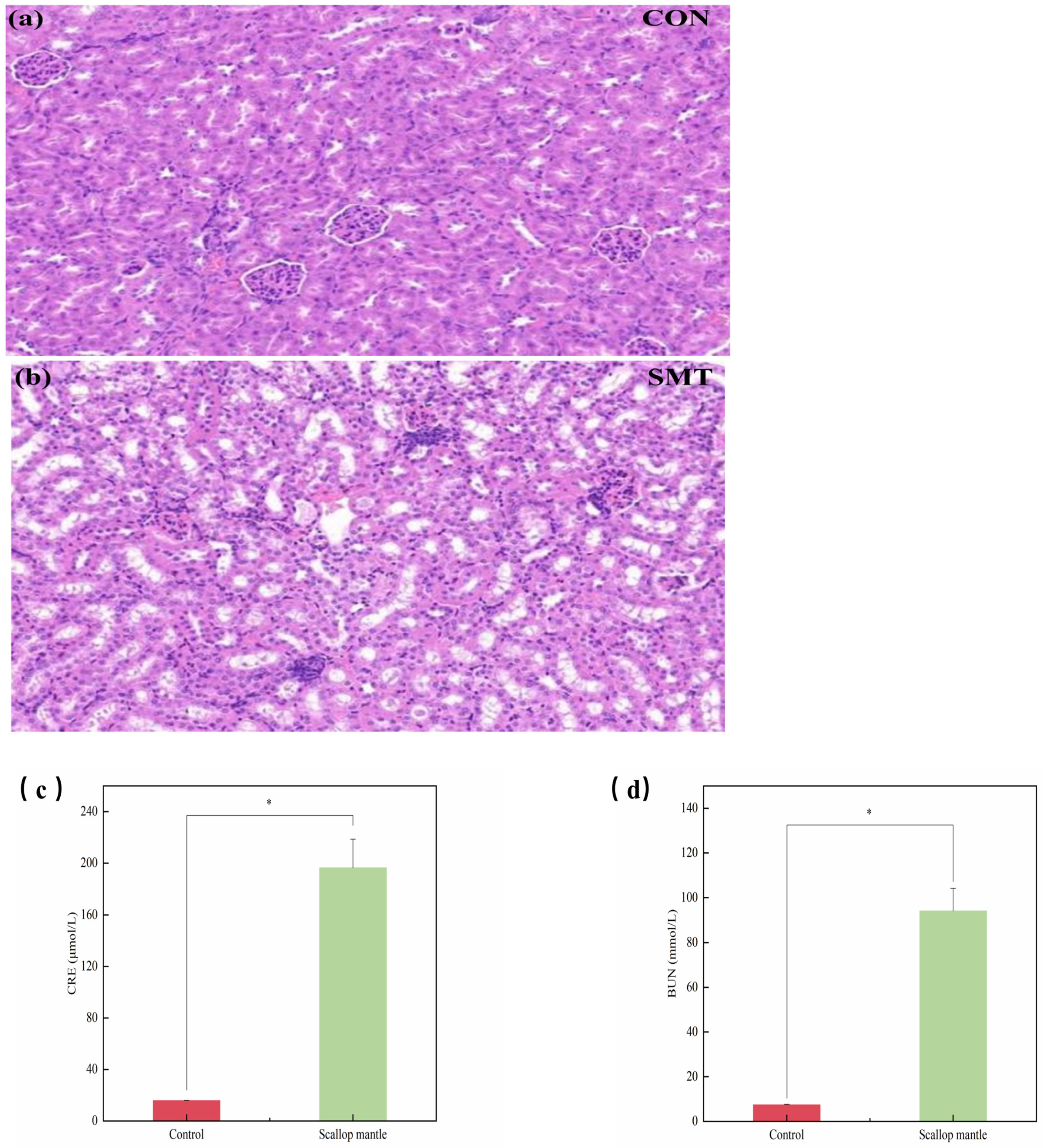
2.3. Effects of Scallop Mantle Toxin on Nephridial Tissue Damage
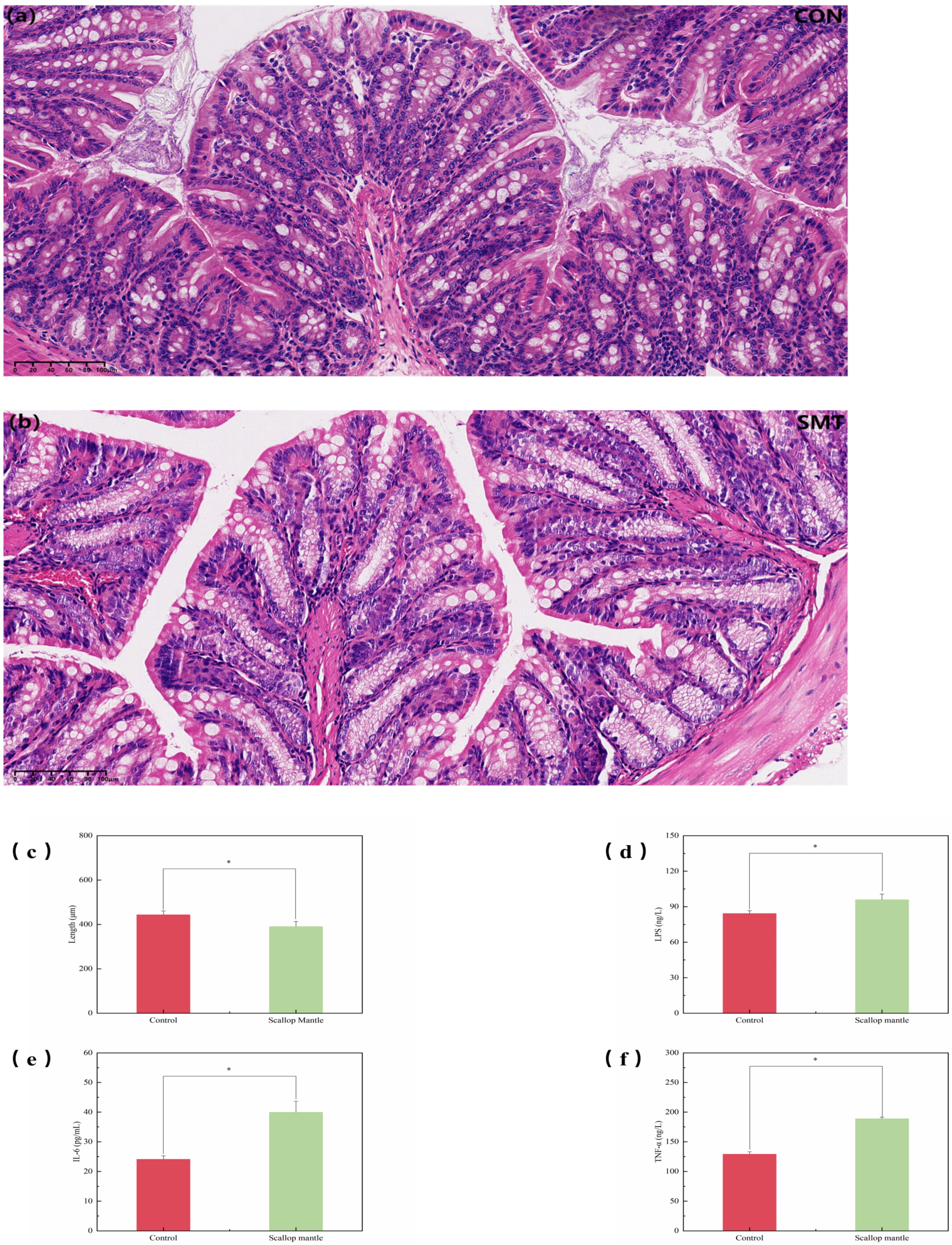
2.4. Effects of Scallop Mantle Toxin on Intestinal Tissue Damage
2.5. Effect of Scallop Mantle Toxin on the Alpha and Beta Diversity
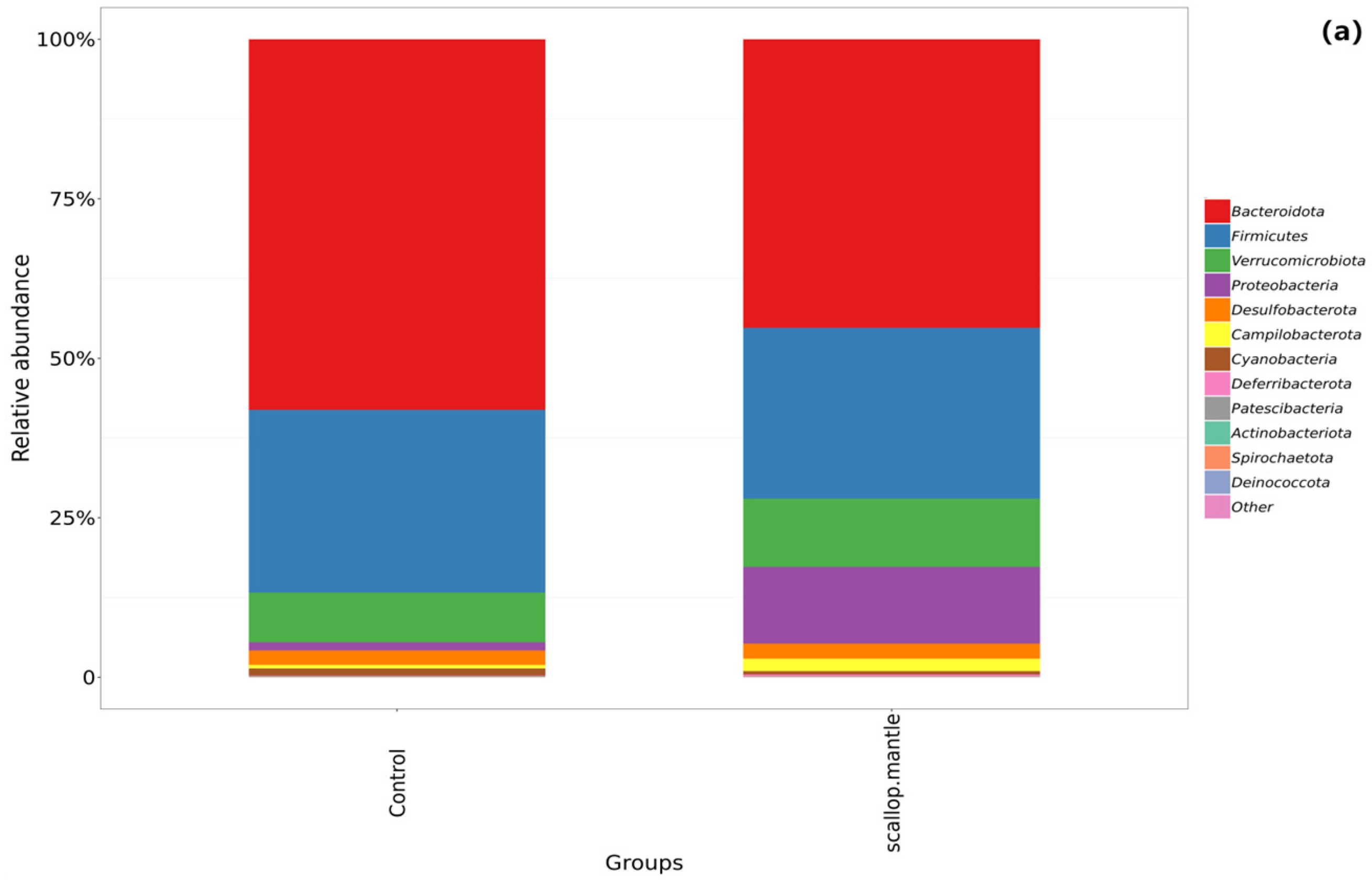
2.6. Effect of Scallop Mantle Toxin on Intestinal Microbial Composition
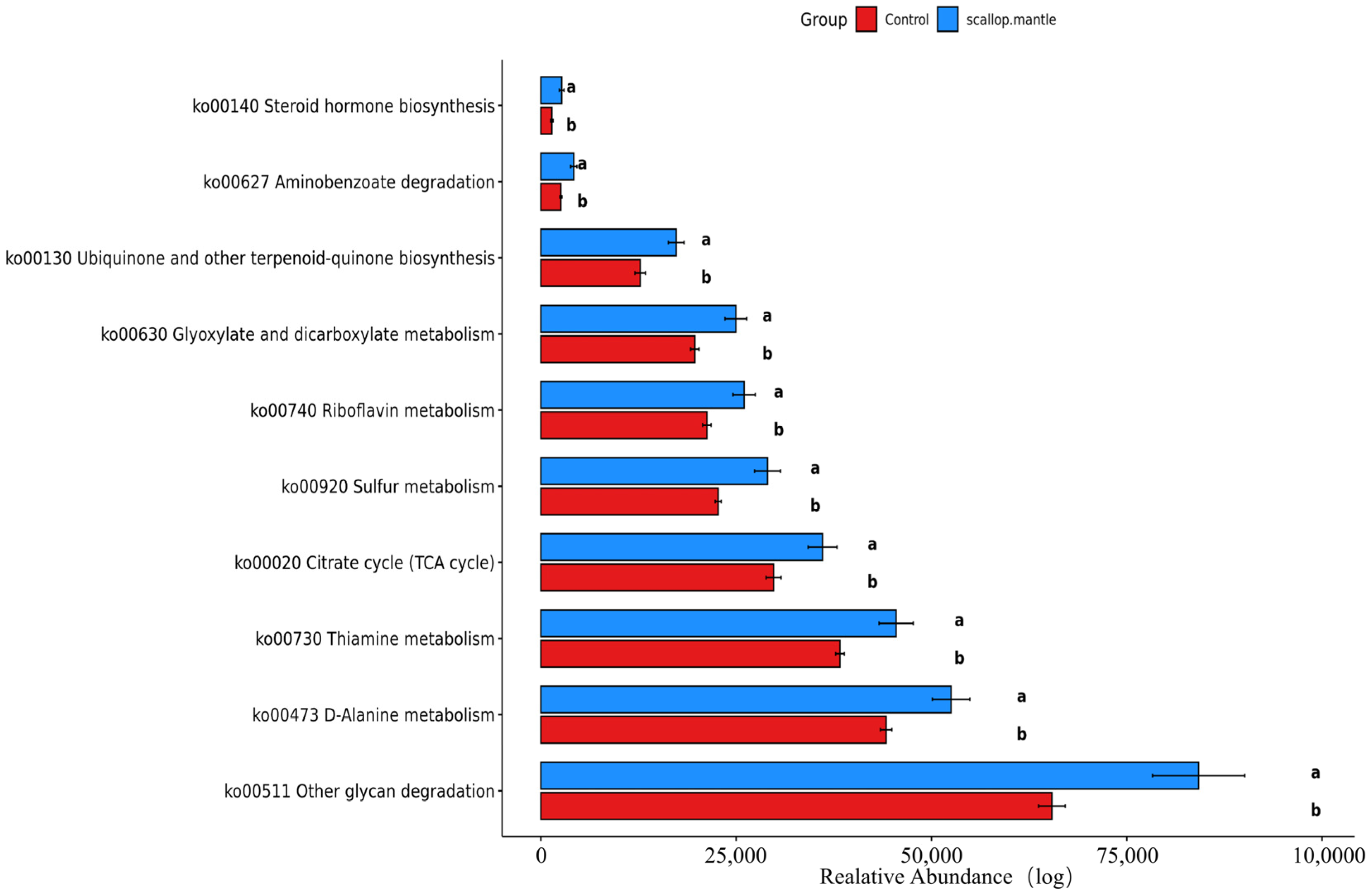
2.7. Analysis and Prediction of Intestinal Flora Metabolism
3. Conclusions
4. Materials and Methods
4.1. Collection and Preparation Scallop Mantle Toxin
4.2. Animal Treatment
4.3. Biochemical Assays of the Serum
4.4. Histology Examination
4.5. Inflammatory Cytokines Examination
4.6. 16S Sequencing and Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMT | Scallop mantle toxin |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| TBA | Total bile acid |
| TG | Triglyceride |
| TC | Total cholesterol |
| γ-GT | γ-Glutamyl transferase |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| CRE | Creatinine |
| BUN | Blood urea nitrogen |
| LPS | Lipopolysaccharides |
| CON | Control |
References
- Peng, D.; Hou, X.; Li, Y.; Mu, Y. The difference in development level of marine shellfish industry in 10 major producing countries. Mar. Policy 2019, 106, 103516. [Google Scholar] [CrossRef]
- Zhou, J. Marine Fish Farming in China: Development Path and Technological Changes. J. Agric. 2020, 10, 88–96. [Google Scholar] [CrossRef]
- Huang, C.; Liu, S.; Zhang, J.; Liu, Z.; Zhong, W.; Yu, S. A study of the extract of scallop skirt on anti-atherosclerotic action and its related mechanisms. Zhongguo Hai Yang Yao Wu—Chin. J. Mar. Drugs 2002, 21, 12–19. [Google Scholar]
- Qin, Y.; Zhang, C.; Xin, R. Preparation technology of seafood scallop skirt soy sauce by high-salt dilute fermentation. China Brew. 2021, 40, 122–126. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Qi, J. Processing formula research of scallop skirt sausage. Food Sci. Technol. 2013, 38, 160–164. [Google Scholar] [CrossRef]
- Kobayashi, K.; Umehara, J.; Pataky, T.C.; Yagi, M.; Hirono, T.; Ueda, Y.; Ichihashi, N. Application of statistical parametric mapping for comparison of scapular kinematics and EMG. J. Biomech. 2022, 145, 111357. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Itagaki, D.; Konno, K.; Hasegawa, C. Feeding of scallop mantle epithelial cell layer causes subacute toxicity against rodents. Fish. Sci. 2017, 84, 91–100. [Google Scholar] [CrossRef]
- Maeda, N.; Yumoto, T.; Xiong, G.; Hasegawa, Y. Characterization and Stability of a Novel Toxin in Scallop Mantle Tissue. Foods 2023, 12, 3224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Wang, L.; Zhu, C.L.; Xue, X.H.; Xia, X.J.; Wu, X.L.; Wu, Y.D.; Liu, S.Q.; Zhang, G.P.; Bai, Y.Y.; et al. Oral Administration of the Antimicrobial Peptide Mastoparan X Alleviates Enterohemorrhagic Escherichia coli–Induced Intestinal Inflammation and Regulates the Gut Microbiota. Probiotics Antimicrob. Proteins 2024, 16, 138–151. [Google Scholar] [CrossRef]
- Huang, Z.; Li, N.; Tang, Y.; Jin, J.; Liu, W.; Xu, H.; Yu, F.; Hao, L.; Zhang, Q.; Ding, Y.; et al. Neoadjuvant radiotherapy for soft tissue sarcoma in China: A preliminary result. Ann. Transl. Med. 2022, 10, 452. [Google Scholar] [CrossRef]
- Sun, X.; Shi, J.; Kong, L.; Shen, Q.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Recent insights into the hepatoprotective effects of lactic acid bacteria in alcoholic liver disease. Trends Food Sci. Technol. 2022, 125, 91–99. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Chen, Y.; Guan, W.; Zhang, J.; Yu, H.; Wang, Y.; Wang, W. The ameliorative effects of probiotic-fermented soymilk on acute alcoholic liver injury. J. Food Process. Preserv. 2021, 45, e16026. [Google Scholar] [CrossRef]
- Dong, Q.; Chu, F.; Wu, C.; Huo, Q.; Gan, H.; Li, X.; Liu, H. Scutellaria baicalensis Georgi extract protects against alcohol-induced acute liver injury in mice and affects the mechanism of ER stress. Mol. Med. Rep. 2016, 13, 3052–3062. [Google Scholar] [CrossRef]
- Hao, J.; Ye, L.; Meng, G.; Song, Y.; Fu, J.; Wu, X. The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics. Food Sci. Hum. Wellness 2021, 10, 480–489. [Google Scholar] [CrossRef]
- Reddy, J.K.; Sambasiva Rao, M. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, W.; Qiu, Y.; Wang, S.; Zhang, B.; Sun, N.; Zhou, Q. Bergapten exerts a chondroprotective effect in temporomandibular joint osteoarthritis by combining intestinal flora alteration and reactive oxygen species reduction. Biomed. Pharmacother. 2023, 167, 115525. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Liu, Y.; Zhang, X.; Dai, L. 16S rDNA profiling of Loach (Misgurnus anguillicus) fed with soybean fermented powder intestinal flora in response to Lipopolysaccharide (LPS) infection. Heliyon 2023, 9, e22369. [Google Scholar] [CrossRef]
- Bu, F.; Zhang, S.; Duan, Z.; Ding, Y.; Chen, T.; Wang, R.; Feng, Z.; Shi, G.; Zhou, J.; Chen, Y. A critical review on the relationship of herbal medicine, Akkermansia muciniphila, and human health. Biomed. Pharmacother. 2020, 128, 110352. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wang, C.; Chen, H.; Liu, T.; Chen, L.; Huang, Y.; Zeng, F.; Liu, B. Luteolin cooperated with metformin hydrochloride alleviates lipid metabolism disorders and optimizes intestinal flora compositions of high-fat diet mice. Food Funct. 2020, 11, 10033–10046. [Google Scholar] [CrossRef]
- Liang, H.; Song, H.; Zhang, X.; Song, G.; Wang, Y.; Ding, X.; Duan, X.; Li, L.; Sun, T.; Kan, Q. Metformin attenuated sepsis-related liver injury by modulating gut microbiota. Emerg. Microbes Infect. 2022, 11, 815–828. [Google Scholar] [CrossRef]
- Ochoa, S.; Collado, L. Enterohepatic Helicobacter species—Clinical importance, host range, and zoonotic potential. Crit. Rev. Microbiol. 2021, 47, 728–761. [Google Scholar] [CrossRef] [PubMed]
- Chichlowski, M.; Hale, L.P. Effects of Helicobacter infection on research: The case for eradication of Helicobacter from rodent research colonies. Comp. Med. 2009, 59, 10–17. [Google Scholar]
- Park, J.M.; Shin, Y.; Kim, S.H.; Jin, M.; Choi, J.J. Dietary epigallocatechin-3-gallate alters the gut microbiota of obese diabetic db/db mice: Lactobacillus is a putative target. J. Med. Food 2020, 23, 1033–1042. [Google Scholar] [CrossRef]
- Chen, M.; Liao, Z.; Lu, B.; Wang, M.; Lin, L.; Zhang, S.; Li, Y.; Liu, D.; Liao, Q.; Xie, Z. Huang-Lian-Jie-Du-decoction ameliorates hyperglycemia and insulin resistant in association with gut microbiota modulation. Front. Microbiol. 2018, 9, 2380. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, F.-Q.; Tang, P.; Gao, T.-H.; Yang, C.-X.; Tan, L.; Yue, P.; Hua, Y.-N.; Liu, S.-J.; Guo, J.-L. Regulation of the intestinal flora: A potential mechanism of natural medicines in the treatment of type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 151, 113091. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: A multicenter, randomized, open label clinical trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef]
- Proffitt, C.; Bidkhori, G.; Moyes, D.; Shoaie, S. Disease, drugs and dysbiosis: Understanding microbial signatures in metabolic disease and medical interventions. Microorganisms 2020, 8, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Li, S.; Perveen, A.; Shen, J.; Li, C. Effects of maternal T-2 toxin exposure on microorganisms and intestinal barrier function in young mice. Ecotoxicol. Environ. Saf. 2022, 247, 114252. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liang, M.; Jiang, Q.; Jie, Y.; Chen, L.; Zhang, F. Proteomic analysis of neonatal mouse hearts shows PKA functions as a cardiomyocyte replication regulator. Proteome Sci. 2023, 21, 16. [Google Scholar] [CrossRef]
- Zhang, Z.; Yeung, W.K.; Huang, Y.; Chen, Z.-Y. Effect of squalene and shark liver oil on serum cholesterol level in hamsters. Int. J. Food Sci. Nutr. 2002, 53, 411–418. [Google Scholar] [CrossRef]
- Liu, Z.-N.; Jia, W.-Q.; Jiang, T.; Dai, J.-W.; Shuai, C.; Lv, X.-W. Regulation of CD39 expression in ATP-P2Y2R-mediated alcoholic liver steatosis and inflammation. Int. Immunopharmacol. 2019, 77, 105915. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.-L.; Ma, T.-H.; Tang, J.-H.; Xia, T.-F.; Ge, J.; Yuan, W.-J.; Chen, L.; Wan, R.; Cheng, J.-X.; Chen, Z.-K.; et al. Hesperitin enhances the ability of daunorubicin by co-loading with MPEG-PCL nanoparticles to induce apoptosis in gastric cancer. Oncotarget 2017, 5. [Google Scholar] [CrossRef]
- Dong, W.; Jin, X.; Chen, M.; Mo, S.; Liu, Y. Gut microorganisms among Chinese School-Age Children in Two Different Areas in North and South China: A Cross-Sectional Study. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J.; Fadrosh, D.W.; Ma, B.; Gajer, P.; et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 2016, 081257. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Hou, H.; Zhang, J.; Qi, S.; Li, Y.; Alotaibi, N.H.; Alharbi, K.S.; Bukhari, S.N.A. Effects of berberine on intestinal flora of non-alcoholic fatty liver induced by high-fat diet through 16S rRNA gene segmentation. J. King Saud Univ.-Sci. 2020, 32, 2603–2609. [Google Scholar] [CrossRef]




| Control | Scallop Mantle | |
|---|---|---|
| Liver | 7.0 ± 0.3 | 6.5 ± 0.57 |
| Kidney | 1.6 ± 0.01 | 2.0 ± 0.08 |
| Intestinal tract | 2.21 ± 0.13 | 2.24 ± 0.15 |
| Control Diet (%) | Scallop Mantle Diet (%) | |
|---|---|---|
| Casein | 20% | 20% |
| Corn starch | 15% | 15% |
| Cellulose | 5% | 5% |
| Mineral mixture | 3.5% | 3.5% |
| Vitamins mixture | 1% | 1% |
| Freebase L-cysteine | 0.3% | 0.3% |
| Choline bitartrate | 0.2% | 0.2% |
| Sucrose | 50% | 48% |
| Soybean oil | 5% | 5% |
| Scallop mantle | 0% | 2% |
| Total | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Lin, R.; Hasegawa, Y.; Chao, L.; Shang, H.; Yang, J.; Tian, W.; Ma, W.; Zhuang, M.; Li, J. Effects of Scallop Mantle Toxin on Intestinal Microflora and Intestinal Barrier Function in Mice. Toxins 2024, 16, 247. https://doi.org/10.3390/toxins16060247
Geng X, Lin R, Hasegawa Y, Chao L, Shang H, Yang J, Tian W, Ma W, Zhuang M, Li J. Effects of Scallop Mantle Toxin on Intestinal Microflora and Intestinal Barrier Function in Mice. Toxins. 2024; 16(6):247. https://doi.org/10.3390/toxins16060247
Chicago/Turabian StyleGeng, Xiong, Ran Lin, Yasushi Hasegawa, Luomeng Chao, Huayan Shang, Jingjing Yang, Weina Tian, Wenting Ma, Miaomiao Zhuang, and Jianrong Li. 2024. "Effects of Scallop Mantle Toxin on Intestinal Microflora and Intestinal Barrier Function in Mice" Toxins 16, no. 6: 247. https://doi.org/10.3390/toxins16060247
APA StyleGeng, X., Lin, R., Hasegawa, Y., Chao, L., Shang, H., Yang, J., Tian, W., Ma, W., Zhuang, M., & Li, J. (2024). Effects of Scallop Mantle Toxin on Intestinal Microflora and Intestinal Barrier Function in Mice. Toxins, 16(6), 247. https://doi.org/10.3390/toxins16060247




