The Effect of Combined Exposure of Fusarium Mycotoxins on Lipid Peroxidation, Antioxidant Defense, Fatty Acid Profile, and Histopathology in Laying Hens’ Liver
Abstract
1. Introduction
2. Results
2.1. Body and Liver Weight
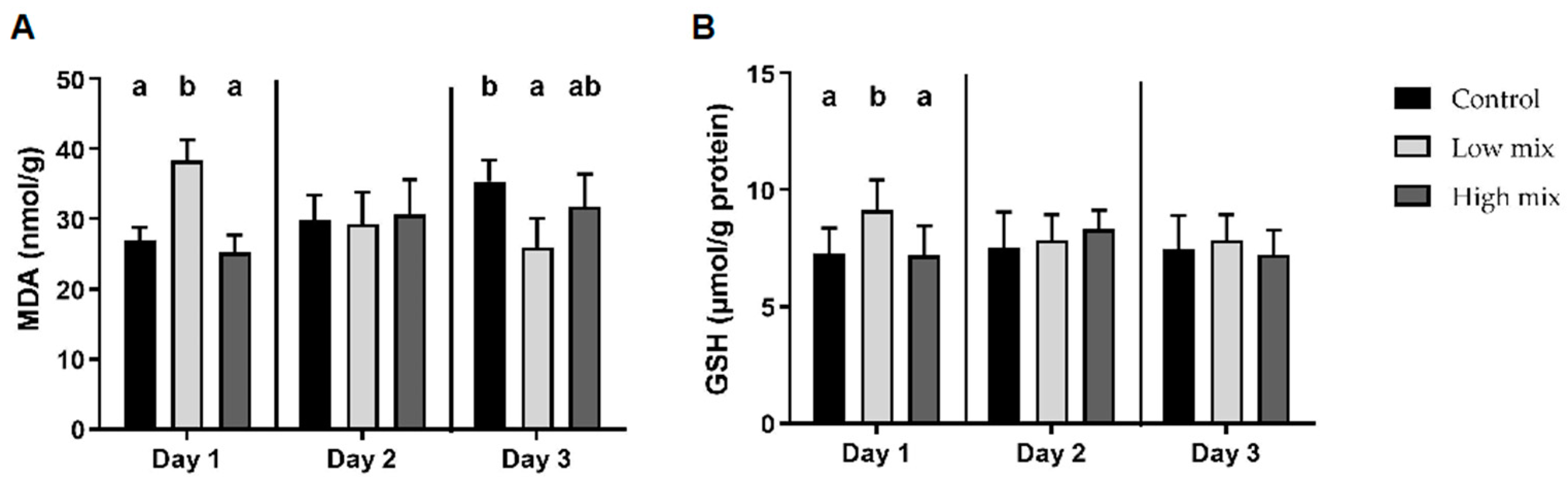
2.2. Liver Oxidative and Antioxidant Parameters
2.3. Hepatic Phospholipid Fatty Acid Profile
2.3.1. Saturated Fatty Acids
2.3.2. Monounsaturated Fatty Acids
2.3.3. Polyunsaturated Fatty Acids
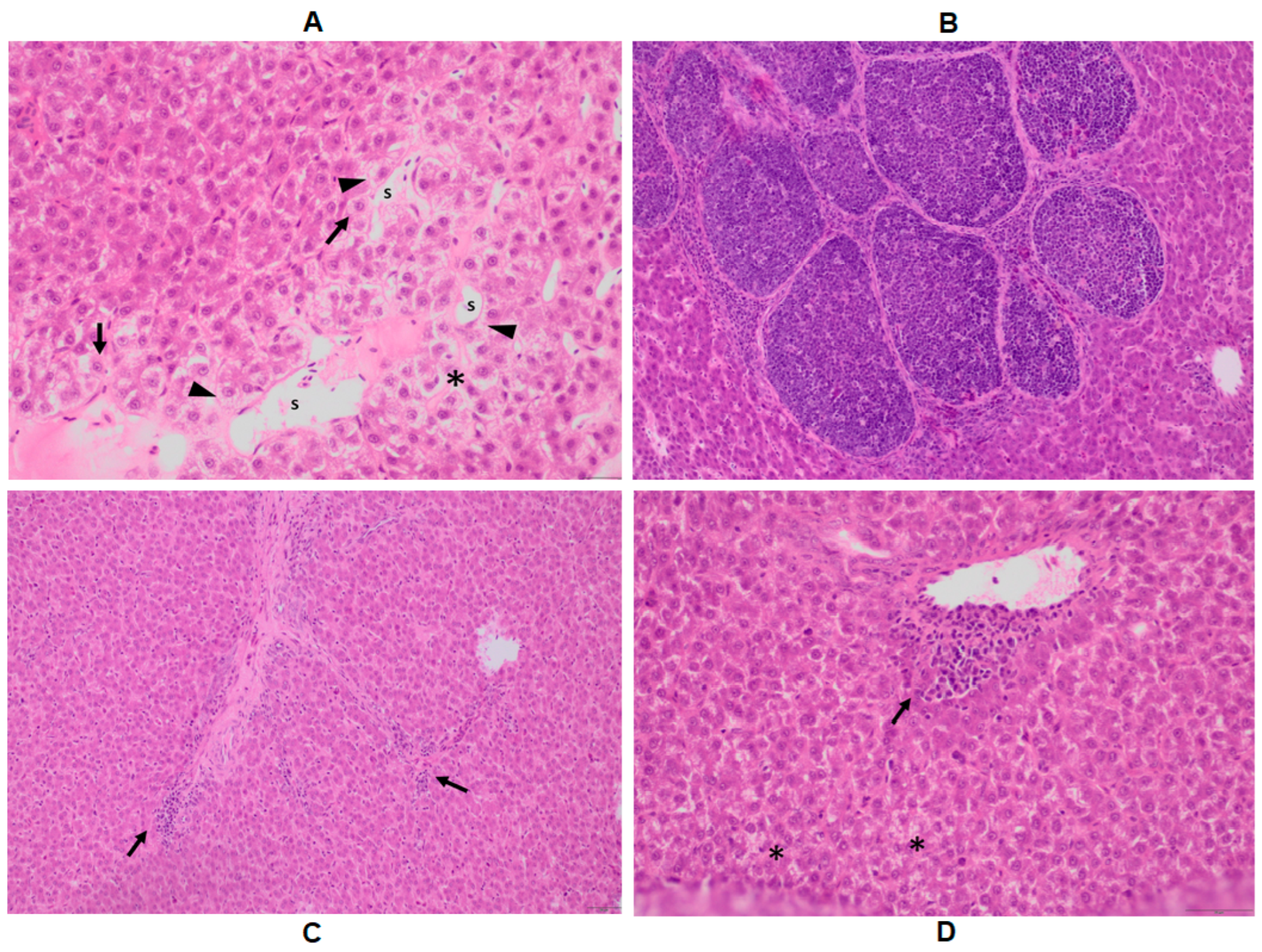
2.4. Histopathology of the Liver
2.5. Interrelationship between Histopathological Findings and Phospholipid Fatty Acids
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Setup and Conditions
5.2. Mycotoxin Production and Analysis
5.3. Measurement of the Antioxidant and Oxidative Markers
5.4. Lipid Analysis
5.5. Histological Sample Preparation
5.6. Data Analysis
5.7. Ethical Allowance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fatty Acid | Exp. Day | Control | Low Mix | High Mix | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C12:0 | D_0 | 0.01 | ± | 0.01 | b | 0.01 | ± | 0.01 | b | 0.01 | ± | 0.01 | b |
| D_1 | 0.01 | ± | 0.01 | ab | 0.01 | ± | 0.00 | b | 0.00 | ± | 0.00 | a | |
| D_2 | 0.00 | ± | 0.00 | a | 0.00 | ± | 0.00 | a | 0.00 | ± | 0.00 | a | |
| D_3 | 0.00 | ± | 0.00 | a | 0.00 | ± | 0.00 | a | 0.00 | ± | 0.00 | a | |
| C14:0 | D_0 | 0.15 | ± | 0.04 | 0.15 | ± | 0.04 | b | 0.15 | ± | 0.04 | b | |
| D_1 | 0.14 | ± | 0.03 | AB | 0.15 | ± | 0.03 | bB | 0.11 | ± | 0.01 | aA | |
| D_2 | 0.12 | ± | 0.02 | B | 0.11 | ± | 0.01 | aAB | 0.11 | ± | 0.01 | aA | |
| D_3 | 0.12 | ± | 0.02 | B | 0.15 | ± | 0.02 | bA | 0.15 | ± | 0.01 | bA | |
| C16:0 | D_0 | 21.4 | ± | 2.07 | a | 21.4 | ± | 2.07 | a | 21.4 | ± | 2.07 | a |
| D_1 | 24.1 | ± | 0.81 | bB | 23.1 | ± | 1.63 | aAB | 22.5 | ± | 1.08 | aA | |
| D_2 | 24.0 | ± | 2.24 | bB | 22.0 | ± | 2.38 | aAB | 21.0 | ± | 1.02 | aA | |
| D_3 | 22.7 | ± | 2.81 | abA | 28.9 | ± | 1.50 | bB | 27.2 | ± | 1.61 | bB | |
| C18:0 | D_0 | 21.8 | ± | 2.67 | b | 21.8 | ± | 2.67 | b | 21.8 | ± | 2.67 | b |
| D_1 | 19.2 | ± | 0.76 | aA | 20.6 | ± | 0.71 | bB | 20.7 | ± | 1.32 | bB | |
| D_2 | 18.6 | ± | 1.37 | aA | 21.1 | ± | 2.42 | bB | 21.1 | ± | 1.25 | bB | |
| D_3 | 19.3 | ± | 2.33 | bB | 14.8 | ± | 0.88 | aA | 15.9 | ± | 1.16 | aA | |
| C20:0 | D_0 | 0.09 | ± | 0.07 | 0.09 | ± | 0.07 | 0.09 | ± | 0.07 | b | ||
| D_1 | 0.04 | ± | 0.01 | 0.05 | ± | 0.01 | 0.04 | ± | 0.01 | ab | |||
| D_2 | 0.05 | ± | 0.01 | 0.06 | ± | 0.03 | 0.04 | ± | 0.00 | a | |||
| D_3 | 0.08 | ± | 0.04 | B | 0.05 | ± | 0.02 | A | 0.03 | ± | 0.01 | aA | |
| C22:0 | D_0 | 0.03 | ± | 0.02 | b | 0.03 | ± | 0.02 | 0.03 | ± | 0.02 | b | |
| D_1 | 0.02 | ± | 0.00 | aA | 0.02 | ± | 0.01 | AB | 0.03 | ± | 0.01 | abB | |
| D_2 | 0.02 | ± | 0.00 | ab | 0.03 | ± | 0.02 | 0.02 | ± | 0.01 | a | ||
| D_3 | 0.03 | ± | 0.01 | b | 0.02 | ± | 0.02 | 0.01 | ± | 0.00 | a | ||
| C24:0 | D_0 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | ab | ||
| D_1 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | a | |||
| D_2 | 0.01 | ± | 0.00 | 0.02 | ± | 0.02 | 0.02 | ± | 0.01 | b | |||
| D_3 | 0.02 | ± | 0.03 | 0.02 | ± | 0.02 | 0.01 | ± | 0.00 | a | |||
| total saturated | D_0 | 43.5 | ± | 0.81 | b | 43.5 | ± | 0.81 | 43.5 | ± | 0.81 | b | |
| D_1 | 43.5 | ± | 0.48 | b | 43.9 | ± | 1.00 | 43.4 | ± | 0.55 | b | ||
| D_2 | 42.7 | ± | 0.97 | abAB | 43.3 | ± | 0.37 | B | 42.3 | ± | 0.37 | aA | |
| D_3 | 42.2 | ± | 0.79 | aA | 44.0 | ± | 0.89 | B | 43.4 | ± | 0.67 | bB | |
| Fatty Acid | Exp. Day | Control | Low Mix | High Mix | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C16:1n7 | D_0 | 0.94 | ± | 0.43 | a | 0.94 | ± | 0.43 | a | 0.94 | ± | 0.43 | |
| D_1 | 1.42 | ± | 0.45 | b | 1.07 | ± | 0.29 | ab | 1.25 | ± | 0.36 | ||
| D_2 | 1.25 | ± | 0.22 | abB | 1.10 | ± | 0.25 | abAB | 0.93 | ± | 0.25 | A | |
| D_3 | 1.12 | ± | 0.26 | ab | 1.52 | ± | 0.61 | b | 1.16 | ± | 0.28 | ||
| C18:1n7 | D_0 | 1.10 | ± | 0.24 | 1.10 | ± | 0.24 | 1.10 | ± | 0.24 | a | ||
| D_1 | 1.22 | ± | 0.17 | 1.12 | ± | 0.24 | 1.25 | ± | 0.14 | ab | |||
| D_2 | 1.12 | ± | 0.17 | 1.24 | ± | 0.07 | 1.16 | ± | 0.22 | ab | |||
| D_3 | 1.17 | ± | 0.18 | 1.31 | ± | 0.16 | 1.36 | ± | 0.14 | b | |||
| C18:1n9 | D_0 | 19.4 | ± | 5.25 | 19.4 | ± | 5.25 | a | 19.4 | ± | 5.25 | a | |
| D_1 | 22.2 | ± | 1.09 | 21.5 | ± | 2.27 | a | 21.4 | ± | 2.39 | a | ||
| D_2 | 23.1 | ± | 1.39 | 21.9 | ± | 1.45 | a | 21.2 | ± | 1.96 | a | ||
| D_3 | 23.0 | ± | 4.89 | A | 30.4 | ± | 2.60 | bB | 30.6 | ± | 0.86 | bB | |
| C20:1n9 | D_0 | 0.15 | ± | 0.02 | 0.15 | ± | 0.02 | ab | 0.15 | ± | 0.02 | ||
| D_1 | 0.15 | ± | 0.02 | 0.13 | ± | 0.02 | a | 0.15 | ± | 0.02 | |||
| D_2 | 0.14 | ± | 0.02 | 0.17 | ± | 0.02 | b | 0.15 | ± | 0.03 | |||
| D_3 | 0.16 | ± | 0.02 | 0.14 | ± | 0.02 | a | 0.15 | ± | 0.01 | |||
| C22:1n9 | D_0 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | a | 0.02 | ± | 0.00 | b | |
| D_1 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | bc | 0.02 | ± | 0.00 | b | ||
| D_2 | 0.02 | ± | 0.01 | 0.02 | ± | 0.01 | ab | 0.02 | ± | 0.01 | b | ||
| D_3 | 0.02 | ± | 0.00 | B | 0.02 | ± | 0.00 | cA | 0.01 | ± | 0.00 | aA | |
| total | D_0 | 21.6 | ± | 5.8 | 21.6 | ± | 5.8 | a | 21.6 | ± | 5.8 | a | |
| monounsaturated | D_1 | 25.0 | ± | 1.6 | 23.8 | ± | 2.7 | a | 24.1 | ± | 2.7 | a | |
| D_2 | 25.6 | ± | 1.3 | 24.4 | ± | 1.6 | a | 23.5 | ± | 2.4 | a | ||
| D_3 | 25.4 | ± | 5.1 | A | 33.4 | ± | 3.1 | bB | 33.3 | ± | 0.8 | bB | |
| Fatty Acid | Exp. Day | Control | Low | High | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C18:2n6 | D_0 | 12.39 | ± | 3.08 | 12.39 | ± | 3.08 | 12.39 | ± | 3.08 | |||
| D_1 | 11.56 | ± | 0.80 | 11.60 | ± | 1.13 | 11.62 | ± | 1.15 | ||||
| D_2 | 11.41 | ± | 0.89 | A | 11.50 | ± | 0.66 | AB | 12.45 | ± | 0.62 | B | |
| D_3 | 12.13 | ± | 0.89 | B | 11.38 | ± | 1.31 | AB | 10.50 | ± | 0.61 | A | |
| C18:3n3 | D_0 | 0.03 | ± | 0.03 | 0.03 | ± | 0.03 | 0.03 | ± | 0.03 | b | ||
| D_1 | 0.02 | ± | 0.01 | 0.03 | ± | 0.01 | 0.02 | ± | 0.01 | ab | |||
| D_2 | 0.02 | ± | 0.00 | 0.02 | ± | 0.01 | 0.02 | ± | 0.01 | ab | |||
| D_3 | 0.03 | ± | 0.00 | B | 0.03 | ± | 0.01 | B | 0.02 | ± | 0.00 | aA | |
| C18:3n6 | D_0 | 0.08 | ± | 0.03 | 0.08 | ± | 0.03 | a | 0.08 | ± | 0.03 | a | |
| D_1 | 0.10 | ± | 0.03 | 0.10 | ± | 0.03 | ab | 0.11 | ± | 0.01 | b | ||
| D_2 | 0.09 | ± | 0.02 | 0.09 | ± | 0.02 | ab | 0.08 | ± | 0.01 | a | ||
| D_3 | 0.10 | ± | 0.02 | 0.11 | ± | 0.02 | b | 0.10 | ± | 0.02 | ab | ||
| C20:2n6 | D_0 | 0.24 | ± | 0.07 | 0.24 | ± | 0.07 | b | 0.24 | ± | 0.07 | b | |
| D_1 | 0.22 | ± | 0.03 | 0.24 | ± | 0.05 | b | 0.24 | ± | 0.04 | b | ||
| D_2 | 0.22 | ± | 0.05 | A | 0.26 | ± | 0.02 | bAB | 0.30 | ± | 0.03 | Bc | |
| D_3 | 0.28 | ± | 0.05 | B | 0.18 | ± | 0.03 | aA | 0.17 | ± | 0.04 | Aa | |
| C20:3n3 | D_0 | 0.01 | ± | 0.01 | 0.01 | ± | 0.01 | 0.01 | ± | 0.01 | ab | ||
| D_1 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | ab | |||
| D_2 | 0.01 | ± | 0.00 | A | 0.01 | ± | 0.01 | B | 0.01 | ± | 0.00 | aB | |
| D_3 | 0.01 | ± | 0.01 | B | 0.01 | ± | 0.00 | AB | 0.01 | ± | 0.00 | aA | |
| C20:3n6 | D_0 | 0.75 | ± | 0.19 | a | 0.75 | ± | 0.19 | ab | 0.75 | ± | 0.19 | a |
| D_1 | 0.97 | ± | 0.13 | ab | 0.87 | ± | 0.18 | b | 0.88 | ± | 0.24 | ab | |
| D_2 | 0.95 | ± | 0.09 | ab | 0.97 | ± | 0.30 | b | 1.08 | ± | 0.20 | c | |
| D_3 | 0.98 | ± | 0.27 | bB | 0.51 | ± | 0.08 | aA | 0.46 | ± | 0.07 | aA | |
| C20:3n9 | D_0 | 0.30 | ± | 0.09 | 0.30 | ± | 0.09 | b | 0.30 | ± | 0.09 | b | |
| D_1 | 0.30 | ± | 0.08 | 0.25 | ± | 0.10 | ab | 0.31 | ± | 0.10 | b | ||
| D_2 | 0.29 | ± | 0.06 | 0.29 | ± | 0.17 | b | 0.26 | ± | 0.07 | b | ||
| D_3 | 0.24 | ± | 0.09 | B | 0.14 | ± | 0.05 | Aa | 0.12 | ± | 0.04 | aA | |
| C20:4n6 | D_0 | 14.63 | ± | 1.97 | 14.63 | ± | 1.97 | b | 14.63 | ± | 1.97 | b | |
| D_1 | 12.97 | ± | 0.95 | 13.05 | ± | 1.39 | b | 13.52 | ± | 1.52 | b | ||
| D_2 | 12.80 | ± | 1.34 | 13.17 | ± | 0.90 | b | 14.23 | ± | 1.26 | b | ||
| D_3 | 12.92 | ± | 3.30 | B | 6.70 | ± | 1.08 | aA | 8.13 | ± | 0.88 | aA | |
| C20:5n3 | D_0 | 0.03 | ± | 0.02 | 0.03 | ± | 0.02 | 0.03 | ± | 0.02 | b | ||
| D_1 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | ab | |||
| D_2 | 0.02 | ± | 0.01 | 0.03 | ± | 0.02 | 0.02 | ± | 0.01 | ab | |||
| D_3 | 0.03 | ± | 0.00 | B | 0.01 | ± | 0.00 | A | 0.01 | ± | 0.00 | aA | |
| C22:4n6 | D_0 | 1.02 | ± | 0.61 | 1.02 | ± | 0.61 | b | 1.02 | ± | 0.61 | b | |
| D_1 | 0.66 | ± | 0.05 | 0.79 | ± | 0.14 | ab | 0.79 | ± | 0.20 | ab | ||
| D_2 | 0.70 | ± | 0.11 | A | 0.83 | ± | 0.06 | bB | 0.79 | ± | 0.11 | bAB | |
| D_3 | 0.74 | ± | 0.23 | B | 0.43 | ± | 0.07 | aA | 0.45 | ± | 0.03 | aA | |
| C22:5n3 | D_0 | 2.94 | ± | 0.81 | 2.94 | ± | 0.81 | b | 2.94 | ± | 0.81 | b | |
| D_1 | 2.74 | ± | 0.56 | 3.12 | ± | 0.69 | b | 2.83 | ± | 0.58 | b | ||
| D_2 | 3.05 | ± | 0.51 | 2.86 | ± | 0.54 | b | 2.72 | ± | 0.32 | b | ||
| D_3 | 2.67 | ± | 0.84 | B | 1.85 | ± | 0.34 | aA | 1.78 | ± | 0.40 | aA | |
| C22:5n6 | D_0 | 0.16 | ± | 0.07 | 0.16 | ± | 0.07 | ab | 0.16 | ± | 0.07 | ||
| D_1 | 0.13 | ± | 0.01 | 0.15 | ± | 0.02 | ab | 0.15 | ± | 0.04 | |||
| D_2 | 0.16 | ± | 0.02 | 0.17 | ± | 0.02 | b | 0.15 | ± | 0.02 | |||
| D_3 | 0.15 | ± | 0.05 | B | 0.12 | ± | 0.02 | aAB | 0.11 | ± | 0.02 | A | |
| C22:6n3 | D_0 | 2.23 | ± | 0.59 | 2.23 | ± | 0.59 | b | 2.23 | ± | 0.59 | b | |
| D_1 | 1.78 | ± | 0.32 | 2.02 | ± | 0.46 | b | 2.03 | ± | 0.54 | b | ||
| D_2 | 1.95 | ± | 0.30 | 2.15 | ± | 0.36 | b | 2.10 | ± | 0.26 | b | ||
| D_3 | 2.07 | ± | 0.60 | B | 1.21 | ± | 0.27 | aA | 1.49 | ± | 0.24 | aA | |
| total polyunsaturated | D_0 | 34.68 | ± | 5.07 | 34.68 | ± | 5.07 | b | 34.68 | ± | 5.07 | b | |
| D_1 | 31.35 | ± | 1.34 | 32.10 | ± | 2.21 | b | 32.37 | ± | 2.31 | b | ||
| D_2 | 31.52 | ± | 1.49 | A | 32.19 | ± | 1.25 | bAB | 34.06 | ± | 2.12 | bB | |
| D_3 | 32.20 | ± | 5.47 | B | 22.56 | ± | 2.71 | aA | 23.23 | ± | 1.14 | aA | |
| total n3 | D_0 | 5.24 | ± | 1.14 | 5.24 | ± | 1.14 | b | 5.24 | ± | 1.14 | b | |
| D_1 | 4.56 | ± | 0.72 | 5.19 | ± | 1.05 | b | 4.91 | ± | 0.91 | b | ||
| D_2 | 5.05 | ± | 0.72 | 5.07 | ± | 0.59 | b | 4.88 | ± | 0.52 | b | ||
| D_3 | 4.81 | ± | 1.14 | B | 3.11 | ± | 0.46 | aA | 3.31 | ± | 0.49 | aA | |
| total n6 | D_0 | 29.11 | ± | 5.36 | 29.11 | ± | 5.36 | b | 29.11 | ± | 5.36 | b | |
| D_1 | 26.49 | ± | 1.14 | 26.66 | ± | 1.57 | b | 27.15 | ± | 1.95 | b | ||
| D_2 | 26.18 | ± | 0.84 | A | 26.83 | ± | 1.53 | bA | 28.93 | ± | 1.88 | bB | |
| D_3 | 27.15 | ± | 4.48 | B | 19.31 | ± | 2.40 | aA | 19.80 | ± | 1.05 | aA | |
| Histopathological Symptoms | Symptom Description |
|---|---|
| S1 | diffuse vacuolization |
| S2 | the boundary of cells is blurry around the portal vessels |
| S3 | multifocal discrete mononuclear cell infiltration around the portal vessels |
| S4 | the cytoplasm of hepatocytes is faintly staining around the portal vessels |
| S5 | the cytoplasm of hepatocytes around the portal vessels is faintly staining |
| S6 | blurry cell boundaries |
| S7 | dilatated sinusoids |
| S8 | focal mononuclear cell infiltration of the parenchyma |
| S9 | focal mononuclear cell infiltration around the ductile |
| S10 | the cytoplasm of hepatocytes is faintly staining in the portal area |
| S11 | the nucleus is faintly staining in the portal area |
| S12 | hydropic degeneration of the hepatocytes limited to the portal area |
| S13 | hydropic degeneration of the hepatocytes |
| S14 | hepatocyte detachment in the portal area |
| S15 | diffuse mononuclear and, to a lesser extent, heterophil cellular infiltration |
References
- DSM. 2023. Available online: https://www.dsm.com/content/dam/dsm/anh/en/documents/REP_MTXsurvey_Q3_2023_EN_1123_AUE.pdf (accessed on 10 January 2024).
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239. [Google Scholar] [CrossRef]
- Awad, W.; Ghareeb, K.; Böhm, J.; Zentek, J. The toxicological impacts of the Fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins 2013, 5, 912–925. [Google Scholar] [CrossRef]
- Lee, J.T.; Jessen, K.A.; Beltran, R.; Starkl, V.; Schatzmayr, G.; Borutova, R.; Caldwell, D.J. Effects of mycotoxin-contaminated diets and deactivating compound in laying hens: 2. Effects on white shell egg quality and characteristics. Poult. Sci. 2012, 91, 2096–2104. [Google Scholar] [CrossRef]
- Laurain, J.; Tardieu, D.; Matard-Mann, M.; Rodriguez, M.A.; Guerre, P. Fumonisin B1 accumulates in chicken tissues over time and this accumulation was reduced by feeding algo-clay. Toxins 2021, 13, 701. [Google Scholar] [CrossRef]
- Dazuk, V.; Boiago, M.M.; Rolim, G.; Paravisi, A.; Copetti, P.M.; Bissacotti, B.F.; Morsch, V.M.; Vedovatto, M.; Gazoni, F.L.; Matte, F.; et al. Laying hens fed mycotoxin-contaminated feed produced by Fusarium fungi (T-2 toxin and fumonisin B1) and Saccharomyces cerevisiae lysate: Impacts on poultry health, productive efficiency, and egg quality. Microb. Pathog. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Keck, B.B.; Bodine, A.B. The effects of fumonisin B1 on viability and mitogenic response of avian immune cells. Poult. Sci. 2006, 85, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U.; Adler, A.; Clasen, P.E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Appell, M.; Bosma, W.B. Assessment of the electronic structure and properties of trichothecene toxins using density functional theory. J. Hazard. Mater. 2015, 288, 113–123. [Google Scholar] [CrossRef]
- Wu, Q.H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.Y.; Kuča, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- Riley, R.T.; Enongene, E.; Voss, K.A.; Norred, W.P.; Meredith, F.I.; Sharma, R.P.; Spitzbergák, J.; Williams, D.E.; Carlson, D.B.; Merrill, A.H., Jr. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 2001, 109 (Suppl. 2), 301–308. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Martínez-Larranaga, M.R.; Martínez, M.A.; Anadón, A.; Yuan, Z. Fumonisins: Oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch. Toxicol. 2016, 90, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; Galli, G.M.; Bottari, N.B.; Alba, D.F.; Leal, K.W.; Lopes, T.F.; Druzian, L.; Schetinger, M.R.; Gloria, E.M.; Mendes, R.E.; et al. Fumonisin-(Fusarium verticillioides)-contaminated feed causes hepatic oxidative stress and negatively affects broiler performance in the early stage: Does supplementation with açai flour residues (Euterpe oleracea) minimize these problems? Microb. Pathog. 2020, 146, 104237. [Google Scholar] [CrossRef] [PubMed]
- Poersch, A.B.; Trombetta, F.; Braga, A.C.M.; Boeira, S.P.; Oliveira, M.S.; Dilkin, P.; Mallmann, C.A.; Fighera, M.R.; Royes, L.F.F.; Oliveira, M.S.; et al. Involvement of oxidative stress in subacute toxicity induced by fumonisin B1 in broiler chicks. Vet. Microbiol. 2014, 174, 180–185. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Bajzik, G.; Kovács, M. Oral administration of fumonisin B 1 and T-2 individually and in combination affects hepatic total and mitochondrial membrane lipid profile of rabbits. Physiol. Int. 2016, 103, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and combined effects of fumonisin B1, deoxynivalenol and zearalenone on the hepatic and renal membrane lipid integrity of rats. Toxins 2017, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Safety evaluation of certain mycotoxins in food. Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Int. Program. Chem. Saf. 2001, 47, 420–555. [Google Scholar]
- Pelyhe, C.; Kövesi, B.; Zándoki, E.; Kovács, B.; Erdélyi, M.; Kulcsár, S.; Mézes, M.; Balogh, K. Multi-trichothecene mycotoxin exposure activates glutathione-redox system in broiler chicken. Toxicon 2018, 153, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kulcsár, S.; Kövesi, B.; Balogh, K.; Zándoki, E.; Ancsin, Z.; Erdélyi, M.; Mézes, M. Effects of Fusarium mycotoxin exposure on lipid peroxidation and glutathione redox system in the liver of laying hens. Antioxidants 2021, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 toxin—The most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef]
- Riahi, I.; Marquis, V.; Pérez-Vendrell, A.M.; Brufau, J.; Esteve-Garcia, E.; Ramos, A.J. Effects of deoxynivalenol-contaminated diets on metabolic and immunological parameters in broiler chickens. Animals 2021, 11, 147. [Google Scholar] [CrossRef]
- Antonissen, G.; De Baere, S.; Novak, B.; Schatzmayr, D.; den Hollander, D.; Devreese, M.; Croubels, S. Toxicokinetics of hydrolyzed fumonisin B1 after single oral or intravenous bolus to broiler chickens fed a control or a fumonisins-contaminated diet. Toxins 2020, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Han, S.; Chen, Y.; Wang, Y.; Li, D.; Zhu, Q. T-2 Toxin Induces Oxidative Stress, Apoptosis and Cytoprotective Autophagy in Chicken Hepatocytes. Toxins 2020, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A.; Bracarense, A.P.F. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon 2020, 185, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Tessari, E.N.C.; Oliveira, C.A.F.D.; Cardoso, A.L.S.P.; Ledoux, D.R.; Rot-tinghaus, G.E. Effects of aflatoxin B1 and fumonisin B1 on body weight, anti-body titres and histology of broiler chicks. Br. Poult. Sci. 2006, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Escriva, C.; Dromant, M.; Borras, C.; Vina, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Kulcsár, S.; Kövesi, B.; Balogh, K.; Zándoki, E.; Ancsin, Z.; Erdélyi, M.; Mézes, M. The co-occurrence of T-2 toxin, deoxynivalenol, and fumonisin B1 activated the glutathione redox system in the EU-limiting doses in laying hens. Toxins 2023, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.T.; Kauffman, P.; Kotha, S.R.; Yenigalla, A.; Veeraraghavan, R.; Pannu, S.R.; Hund, T.J.; Satoskar, A.R.; McDaniel, J.C.; Maddipati, R.K.; et al. Oxidative lipidomics: Analysis of oxidized lipids and lipid peroxidation in biological systems with relevance to health and disease. In Measuring Oxidants and Oxidative Stress in Biological Systems. Biological Magnetic Resonance; Berliner, L., Parinandi, N., Eds.; Springer: Berlin, Germany, 2020; Volume 34, pp. 61–92. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuča, K.; Yuan, Z. Metabolic pathways of trichothecenes. Drug Metab. Rev. 2010, 42, 250–267. [Google Scholar] [CrossRef]
- Lassallette, E.; Collén, P.N.; Guerre, P. Targeted sphingolipidomics indicates increased C22-C24: 16 ratios of virtually all assayed classes in liver, kidney, and plasma of fumonisin-fed chickens. Ecotoxicol. Environ. Saf. 2023, 268, 115697. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Ding, S.T.; Chang, M.H. Effect of fumonisins on macrophage immune functions and gene expression of cytokines in broilers. Arch. Anim. Nutr. 2006, 60, 267–276. [Google Scholar] [CrossRef]
- Bócsai, A.; Ancsin, Z.S.; Fernye, C.S.; Zándoki, E.; Szabó-Fodor, J.; Erdélyi, M.; Mézes, M.; Balogh, K. Dose-dependent short-term effects of T-2 toxin exposure on lipid peroxidation and antioxidant parameters of laying hens. Eur. Poult. Sci. 2015, 79, 115. [Google Scholar] [CrossRef]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kaninathan, A.; Subramaniyam, S.; Marimuthu, S.; D’souza, P. Impact of phytogenic formulation on performance and fatty liver disease of broiler chickens. Online J. Anim. Feed. Res. 2023, 13, 89–96. [Google Scholar] [CrossRef]
- Nagy, N.; Oláh, I.; Vervelde, L. Structure of the avian lymphoid system. In Avian Immunology; Academic Press: New York, NY, USA, 2022; pp. 11–44. [Google Scholar] [CrossRef]
- Garcia, A.R.; Avila, E.; Rosiles, R.; Petrone, V.M. Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T-2 toxin contaminated grain. Avian Dis. 2003, 47, 691–699. [Google Scholar] [CrossRef] [PubMed]
- European Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2007, L 118M, 1111–1113.
- AOAC. Official Methods of Analysis (28.054), 14th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984; p. 243. [Google Scholar]
- Al-Fawaeir, S.; Akgul, E.O.; Cayci, T.; Demirin, H.; Kurt, Y.G.; Aydin, I.; Agilli, M.; Özkan, E.; Yaman, H.; Cakir, E.; et al. Comparison of two methods for malondialdehyde measurement. J. Clin. Anal. Med. 2011, 2, 11–14. [Google Scholar]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl diadehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound and non-protein sulfhydryl groups in tissues with Ellmann’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Leray, C.; Andriamampandry, M.; Gutbier, G.; Cavadenti, J.; Klein-Soyer, C.; Gachet, C.; Cazenave, J.P. Quantitative analysis of vitamin E, cholesterol and phospholipid fatty acids in a single aliquot of human platelets and cultured endothelial cells. J. Chromatogr. B 1997, 696, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef] [PubMed]

| Exp. Day | Control | Low Mix | High Mix | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (g) | 0 | 1645.8 | ± | 317.6 | 1645.8 | ± | 317.6 | 1645.8 | ± | 317.6 |
| 1 | 1590.8 | ± | 209.5 | 1537.5 | ± | 138.9 | 1709.2 | ± | 158.1 | |
| 2 | 1699.5 | ± | 73.4 | 1597.0 | ± | 120.3 | 1615.8 | ± | 123.7 | |
| 3 | 1652.8 | ± | 172.6 | 1626.2 | ± | 77.2 | 1677.3 | ± | 105.2 | |
| Relative liver weight (% of BW) | 0 | 1.54 | ± | 0.14 | 1.54 | ± | 0.14 | 1.54 | ± | 0.14 |
| 1 | 1.79 | ± | 0.14 | 1.74 | ± | 0.25 | 1.89 | ± | 0.16 | |
| 2 | 1.83 | ± | 0.14 | 1.90 | ± | 0.32 | 1.62 | ± | 0.18 | |
| 3 | 1.92 | ± | 0.34 | 1.95 | ± | 0.48 | 1.77 | ± | 0.20 | |
| Exp. Time | Control | Low Mix | High Mix |
|---|---|---|---|
| Liver | |||
| Day 1 | 2.17 ± 1.60 ab | 1.83 ± 1.47 a | 2.17 ± 0.75 ab |
| Day 2 | 1.67 ± 1.21 a | 2.17 ± 1.47 ab | 1.67 ± 0.81 a |
| Day 3 | 4.00 ± 2.28 b | 4.00 ± 1.67 b | 3.50 ± 1.37 b |
| Symptom/FA | C12:0 | C14:0 | C18:0 | C18:1 n9c | C18:3 n3 | C20:3 n6 | C20:4 n6 | C22:5 n6 | C24:0 | |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Correlation Coefficient | −0.57 | ||||||||
| Sig. (2-tailed) | 0.04 | |||||||||
| S5 | Correlation Coefficient | −0.50 | 0.49 | 0.58 | ||||||
| Sig. (2-tailed) | 0.04 | 0.04 | 0.02 | |||||||
| S6 | Correlation Coefficient | 0.46 | −0.46 | |||||||
| Sig. (2-tailed) | 0.04 | 0.04 | ||||||||
| S15 | Correlation Coefficient | −0.91 | ||||||||
| Sig. (2-tailed) | 0.03 | |||||||||
| total lesion score | Correlation Coefficient | 0.38 | −0.30 | −0.38 | ||||||
| Sig. (2-tailed) | 0.00 | 0.02 | 0.00 |
| Group | T-2/HT-2 | DON/3-AcDON/15-AcDON | FB1 |
|---|---|---|---|
| Control | <0.01 | <0.01 | <0.01 |
| Low mix | 0.13/- | 0.67/0.56/0.01 | 20.00 |
| High mix | 0.29/- | 2.70/0.89/0.05 | 40.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulcsár, S.; Turbók, J.; Kövér, G.; Balogh, K.; Zándoki, E.; Gömbös, P.; Ali, O.; Szabó, A.; Mézes, M. The Effect of Combined Exposure of Fusarium Mycotoxins on Lipid Peroxidation, Antioxidant Defense, Fatty Acid Profile, and Histopathology in Laying Hens’ Liver. Toxins 2024, 16, 179. https://doi.org/10.3390/toxins16040179
Kulcsár S, Turbók J, Kövér G, Balogh K, Zándoki E, Gömbös P, Ali O, Szabó A, Mézes M. The Effect of Combined Exposure of Fusarium Mycotoxins on Lipid Peroxidation, Antioxidant Defense, Fatty Acid Profile, and Histopathology in Laying Hens’ Liver. Toxins. 2024; 16(4):179. https://doi.org/10.3390/toxins16040179
Chicago/Turabian StyleKulcsár, Szabina, Janka Turbók, György Kövér, Krisztián Balogh, Erika Zándoki, Patrik Gömbös, Omeralfaroug Ali, András Szabó, and Miklós Mézes. 2024. "The Effect of Combined Exposure of Fusarium Mycotoxins on Lipid Peroxidation, Antioxidant Defense, Fatty Acid Profile, and Histopathology in Laying Hens’ Liver" Toxins 16, no. 4: 179. https://doi.org/10.3390/toxins16040179
APA StyleKulcsár, S., Turbók, J., Kövér, G., Balogh, K., Zándoki, E., Gömbös, P., Ali, O., Szabó, A., & Mézes, M. (2024). The Effect of Combined Exposure of Fusarium Mycotoxins on Lipid Peroxidation, Antioxidant Defense, Fatty Acid Profile, and Histopathology in Laying Hens’ Liver. Toxins, 16(4), 179. https://doi.org/10.3390/toxins16040179







