Environmental Toxin Biliatresone-Induced Biliary Atresia-like Abnormal Cilia and Bile Duct Cell Development of Human Liver Organoids
Abstract
1. Introduction
2. Results
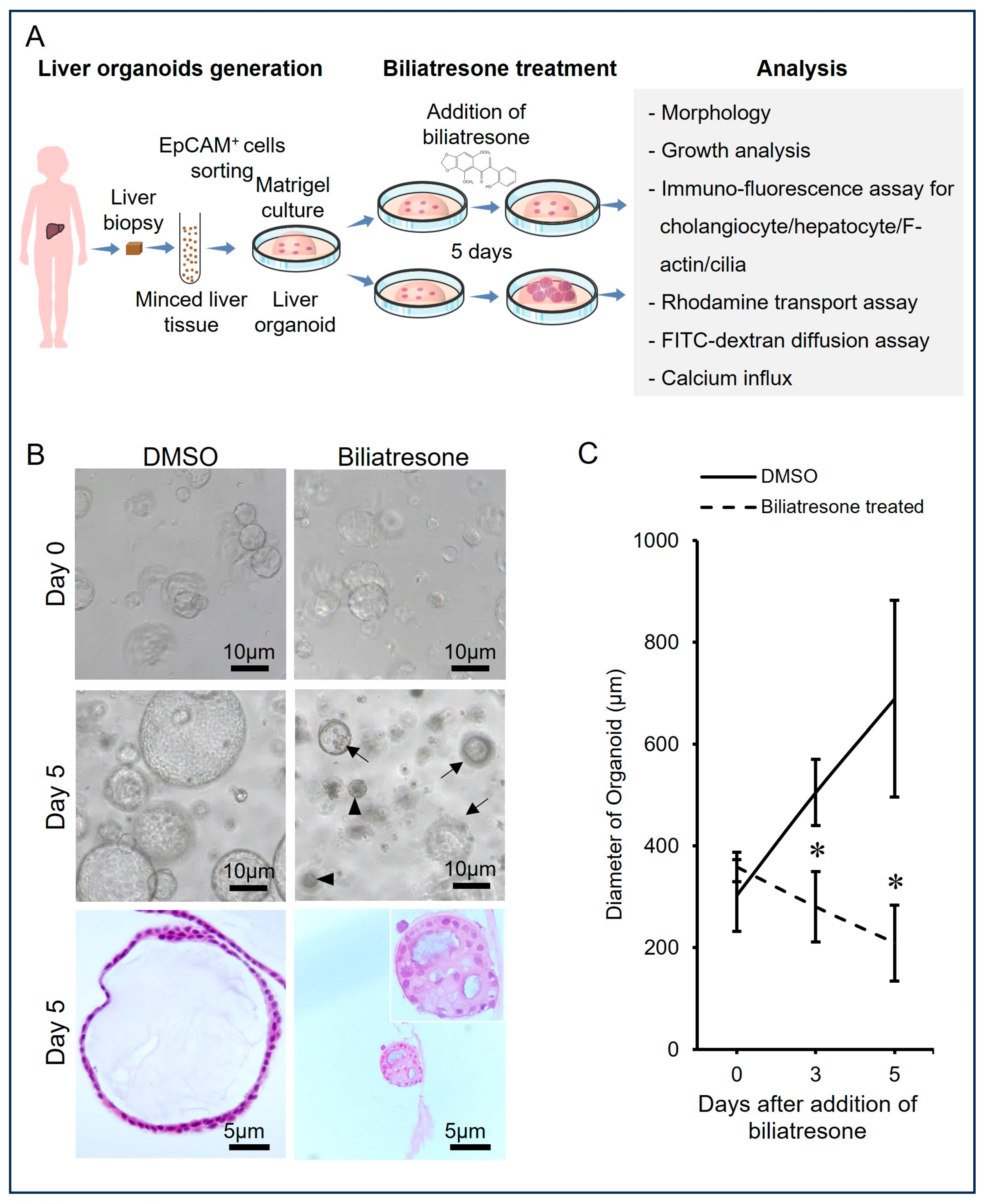
2.1. Biliatresone-Induced Aberrant Growth of Human Liver Organoids
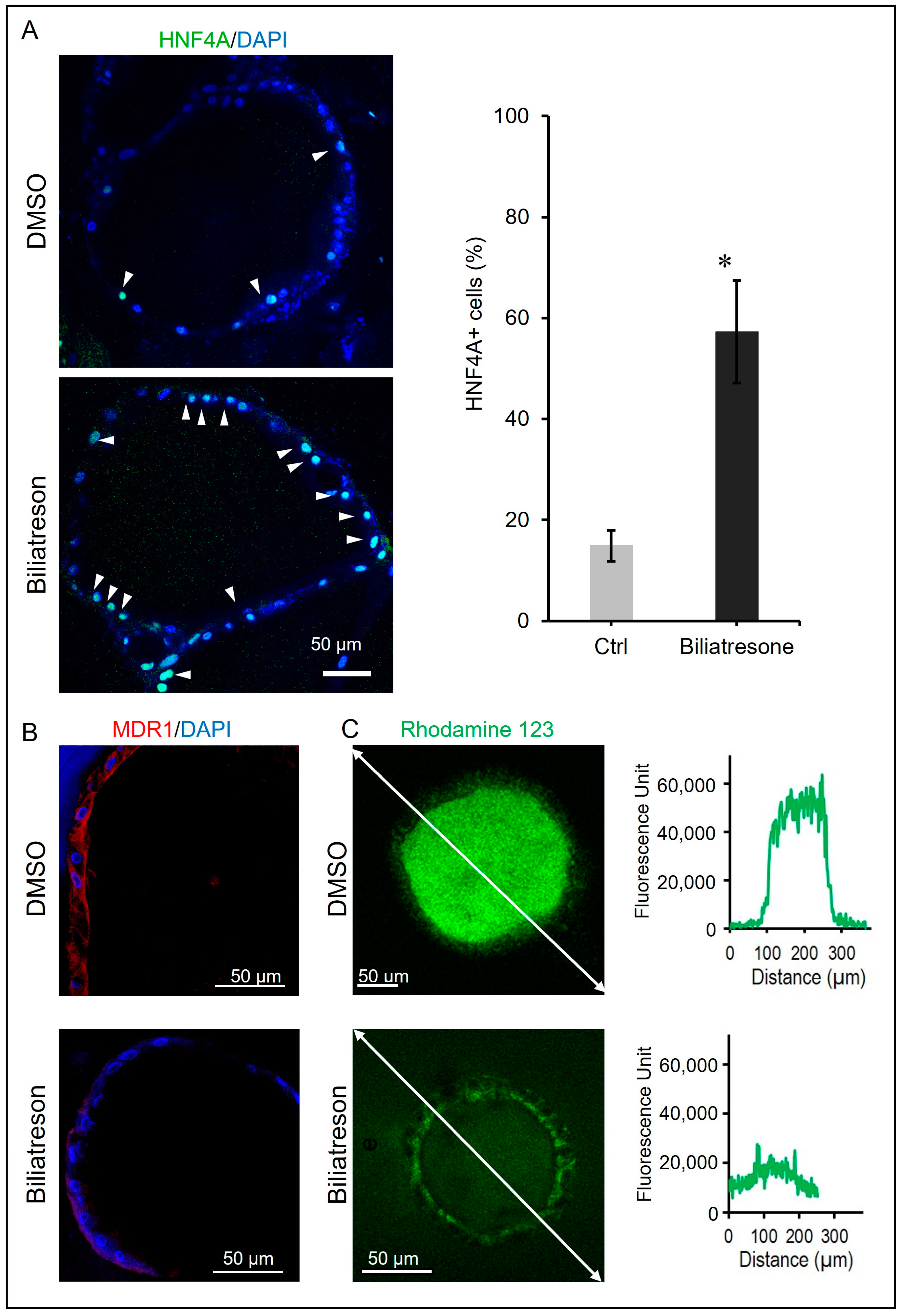
2.2. Biliatresone-Induced Hepatocytic Differentiation of Human Liver Organoids
2.3. Biliatresone-Induced Apical-Basal Polarity Defect in Human Liver Organoids
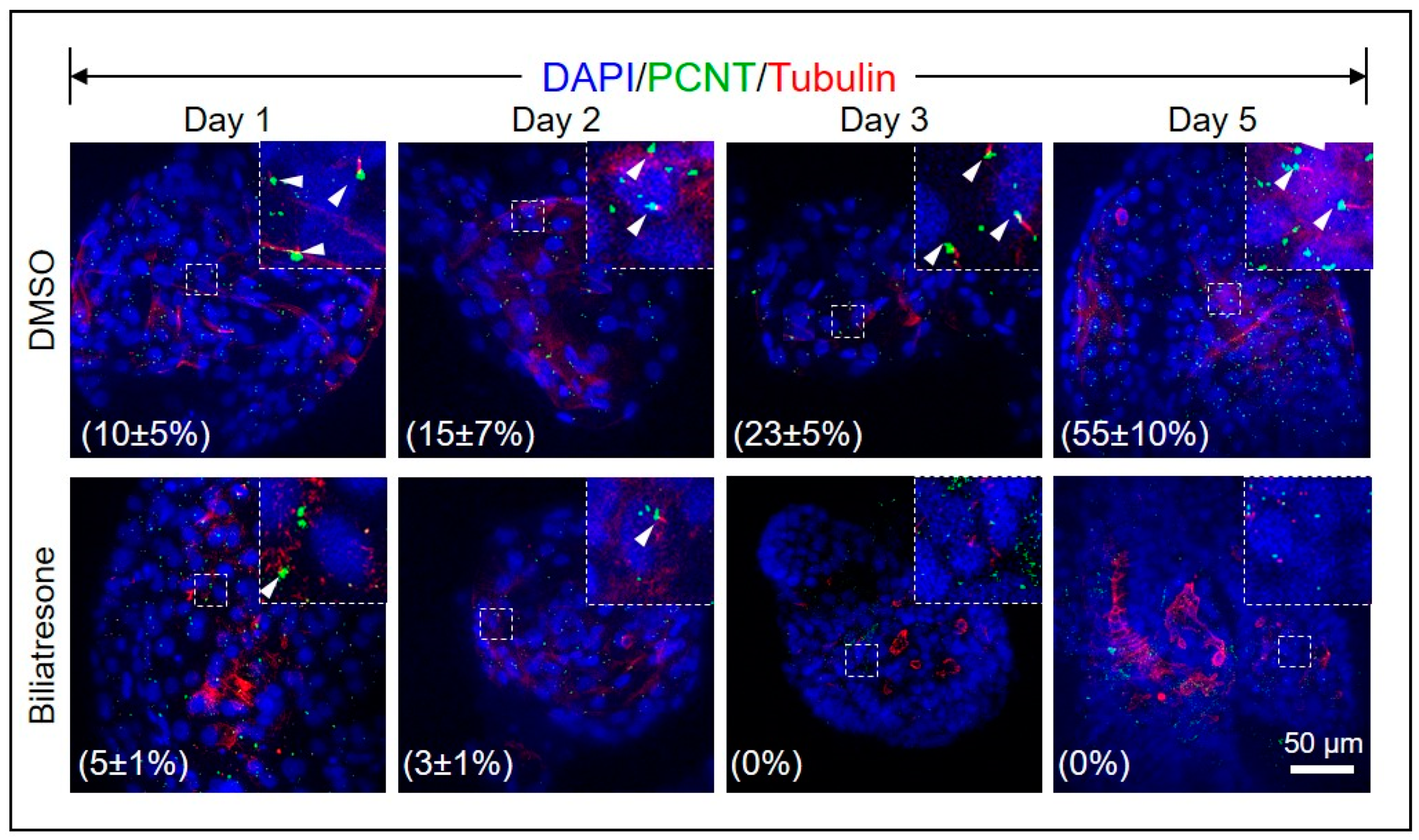
2.4. Biliatresone Decreased the Number of Ciliated Cholangiocytes in Human Organoids
3. Discussion
4. Materials and Methods
4.1. Human Ethics
4.2. Human Liver Organoids
4.3. Treatment of Biliatresone
4.4. Measurement of Organoid Growth
4.5. Immunostaining and Confocal Imaging
4.6. Confocal Imaging and Analysis
4.7. Rhodamine 123 Transport Assay
4.8. FITC-Labeled Dextran Diffusion Tests
4.9. Calcium Imaging
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez-Valle, A.; Kassira, N.; Varela, V.C.; Radu, S.C.; Paidas, C.; Kirby, R.S. Biliary Atresia: Epidemiology, Genetics, Clinical Update, and Public Health Perspective. Adv. Pediatr. 2017, 64, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.L.; Davenport, M.; Kelly, D.A. Biliary atresia. Lancet 2009, 374, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.K.H.; Yiu, R.S.; Lendahl, U.; Andersson, E.R. Cholangiopathies—Towards a molecular understanding. EBioMedicine 2018, 35, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Burford, C.; Alexander, E.C.; Sutton, H.; Dhawan, A.; Joshi, D.; Davenport, M.; Heaton, N.; Hadzic, N.; Samyn, M. Prognostic markers at adolescence in patients requiring liver transplantation for biliary atresia in adulthood. J. Hepatol. 2019, 71, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Panasyuk, G. Genetics in biliary atresia. Curr. Opin. Gastroenterol. 2019, 35, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barcelo, M.M.; Yeung, M.Y.; Miao, X.P.; Tang, C.S.; Cheng, G.; So, M.T.; Ngan, E.S.; Lui, V.C.; Chen, Y.; Liu, X.L.; et al. Genome-wide association study identifies a susceptibility locus for biliary atresia on 10q24.2. Hum. Mol. Genet. 2010, 19, 2917–2925. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Vega, M.; Gerfen, J.; Thiel, B.D.; Jurkiewicz, D.; Rand, E.B.; Pawlowska, J.; Kaminska, D.; Russo, P.; Gai, X.; Krantz, I.D.; et al. Genomic alterations in biliary atresia suggest region of potential disease susceptibility in 2q37.3. Am. J. Med. Genet. A 2010, 152A, 886–895. [Google Scholar] [CrossRef]
- Cheng, G.; Tang, C.S.; Wong, E.H.; Cheng, W.W.; So, M.T.; Miao, X.; Zhang, R.; Cui, L.; Liu, X.; Ngan, E.S.; et al. Common genetic variants regulating ADD3 gene expression alter biliary atresia risk. J. Hepatol. 2013, 59, 1285–1291. [Google Scholar] [CrossRef]
- Chen, Y.; Gilbert, M.A.; Grochowski, C.M.; McEldrew, D.; Llewellyn, J.; Waisbourd-Zinman, O.; Hakonarson, H.; Bailey-Wilson, J.E.; Russo, P.; Wells, R.G.; et al. A genome-wide association study identifies a susceptibility locus for biliary atresia on 2p16.1 within the gene EFEMP1. PLoS Genet. 2018, 14, e1007532. [Google Scholar] [CrossRef]
- Berauer, J.P.; Mezina, A.I.; Okou, D.T.; Sabo, A.; Muzny, D.M.; Gibbs, R.A.; Hegde, M.R.; Chopra, P.; Cutler, D.J.; Perlmutter, D.H.; et al. Identification of Polycystic Kidney Disease 1 Like 1 Gene Variants in Children with Biliary Atresia Splenic Malformation Syndrome. Hepatology 2019, 70, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Lui, V.C.H.; Chung, P.H.Y.; Tam, P.K.H. Biliary Atresia—Emerging diagnostic and therapy opportunities. EBioMedicine 2021, 74, 103689. [Google Scholar] [CrossRef]
- Brindley, S.M.; Lanham, A.M.; Karrer, F.M.; Tucker, R.M.; Fontenot, A.P.; Mack, C.L. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology 2012, 55, 1130–1138. [Google Scholar] [CrossRef]
- Lorent, K.; Gong, W.; Koo, K.A.; Waisbourd-Zinman, O.; Karjoo, S.; Zhao, X.; Sealy, I.; Kettleborough, R.N.; Stemple, D.L.; Windsor, P.A.; et al. Identification of a plant isoflavonoid that causes biliary atresia. Sci. Transl. Med. 2015, 7, 286ra267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Chen, Z.; Liang, J.; Lin, Z.; Liang, H.; Xu, Y.; Wu, Q.; Guo, X.; Nie, J.; et al. Liver Immune Profiling Reveals Pathogenesis and Therapeutics for Biliary Atresia. Cell 2020, 183, 1867–1883.e26. [Google Scholar] [CrossRef] [PubMed]
- Pack, M.; Wells, R.G. Toxins and Biliary Atresia. In The Liver: Biology and Pathobiology; Arias, I.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 1000–1006. [Google Scholar]
- Harper, P.; Plant, J.W.; Unger, D.B. Congenital biliary atresia and jaundice in lambs and calves. Aust. Vet. J. 1990, 67, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.; Gilboa, D.; Har-Zahav, A.; Lavrut, P.M.; Du, Y.; Karjoo, S.; Russo, P.; Shamir, R.; Wells, R.G.; Waisbourd-Zinman, O. Extrahepatic cholangiocyte obstruction is mediated by decreased glutathione, Wnt and Notch signaling pathways in a toxic model of biliary atresia. Sci. Rep. 2020, 10, 7599. [Google Scholar] [CrossRef] [PubMed]
- Waisbourd-Zinman, O.; Koh, H.; Tsai, S.; Lavrut, P.M.; Dang, C.; Zhao, X.; Pack, M.; Cave, J.; Hawes, M.; Koo, K.A.; et al. The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17. Hepatology 2016, 64, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.C.; Hagens, J.; Schuppert, P.; Appl, B.; Raluy, L.P.; Trochimiuk, M.; Philippi, C.; Li, Z.; Reinshagen, K.; Tomuschat, C. Biliatresone induces cholangiopathy in C57BL/6J neonates. Sci. Rep. 2023, 13, 10574. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Zhan, Y.; Chen, G.; Shen, Z.; Zheng, S.; Dong, R. The synthetic toxin biliatresone causes biliary atresia in mice. Lab. Investig. 2020, 100, 1425–1435. [Google Scholar] [CrossRef]
- Estrada, M.A.; Zhao, X.; Lorent, K.; Kriegermeier, A.; Nagao, S.A.; Berritt, S.; Wells, R.G.; Pack, M.; Winkler, J.D. Synthesis and Structure-Activity Relationship Study of Biliatresone, a Plant Isoflavonoid That Causes Biliary Atresia. ACS Med. Chem. Lett. 2018, 9, 61–64. [Google Scholar] [CrossRef]
- Koo, K.A.; Lorent, K.; Gong, W.; Windsor, P.; Whittaker, S.J.; Pack, M.; Wells, R.G.; Porter, J.R. Biliatresone, a Reactive Natural Toxin from Dysphania glomulifera and D. littoralis: Discovery of the Toxic Moiety 1,2-Diaryl-2-Propenone. Chem. Res. Toxicol. 2015, 28, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.A.; Waisbourd-Zinman, O.; Wells, R.G.; Pack, M.; Porter, J.R. Reactivity of Biliatresone, a Natural Biliary Toxin, with Glutathione, Histamine, and Amino Acids. Chem. Res. Toxicol. 2016, 29, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lorent, K.; Wilkins, B.J.; Marchione, D.M.; Gillespie, K.; Waisbourd-Zinman, O.; So, J.; Koo, K.A.; Shin, D.; Porter, J.R.; et al. Glutathione antioxidant pathway activity and reserve determine toxicity and specificity of the biliary toxin biliatresone in zebrafish. Hepatology 2016, 64, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Dorrell, C.; Boj, S.F.; van Es, J.H.; Li, V.S.; van de Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; de Sousa Lopes, S.M.C.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606 e1519. [Google Scholar] [CrossRef]
- Babu, R.O.; Lui, V.C.H.; Chen, Y.; Yiu, R.S.W.; Ye, Y.; Niu, B.; Wu, Z.; Zhang, R.; Yu, M.O.N.; Chung, P.H.Y.; et al. Beta-amyloid deposition around hepatic bile ducts is a novel pathobiological and diagnostic feature of biliary atresia. J. Hepatol. 2020, 73, 1391–1403. [Google Scholar] [CrossRef]
- Andersson, E.R.; Chivukula, I.V.; Hankeova, S.; Sjoqvist, M.; Tsoi, Y.L.; Ramskold, D.; Masek, J.; Elmansuri, A.; Hoogendoorn, A.; Vazquez, E.; et al. Mouse Model of Alagille Syndrome and Mechanisms of Jagged1 Missense Mutations. Gastroenterology 2018, 154, 1080–1095. [Google Scholar] [CrossRef]
- Amarachintha, S.P.; Mourya, R.; Ayabe, H.; Yang, L.; Luo, Z.; Li, X.; Thanekar, U.; Shivakumar, P.; Bezerra, J.A. Biliary organoids uncover delayed epithelial development and barrier function in biliary atresia. Hepatology 2022, 75, 89–103. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef]
- Karjoo, S.; Hand, N.J.; Loarca, L.; Russo, P.A.; Friedman, J.R.; Wells, R.G. Extrahepatic cholangiocyte cilia are abnormal in biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 96–101. [Google Scholar] [CrossRef]
- Frassetto, R.; Parolini, F.; Marceddu, S.; Satta, G.; Papacciuoli, V.; Pinna, M.A.; Mela, A.; Secchi, G.; Galleri, G.; Manetti, R.; et al. Intrahepatic bile duct primary cilia in biliary atresia. Hepatol. Res. 2018, 48, 664–674. [Google Scholar] [CrossRef]
- Chu, A.S.; Russo, P.A.; Wells, R.G. Cholangiocyte cilia are abnormal in syndromic and non-syndromic biliary atresia. Mod. Pathol. 2012, 25, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Masyuk, T.V.; Splinter, P.L.; Huang, B.Q.; Stroope, A.J.; LaRusso, N.F. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006, 131, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Larusso, N.F.; Masyuk, T.V. The role of cilia in the regulation of bile flow. Dig. Dis. 2011, 29, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; Masyuk, A.I.; Splinter, P.L.; Banales, J.M.; Huang, B.Q.; Tietz, P.S.; Masyuk, T.V.; Larusso, N.F. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc. Natl. Acad. Sci. USA 2007, 104, 19138–19143. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.M.; Beales, P.L. Ciliopathies: An expanding disease spectrum. Pediatr. Nephrol. 2011, 26, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Tang, C.S.; So, M.T.; Yue, H.; Hsu, J.S.; Chung, P.H.; Nicholls, J.M.; Yeung, F.; Lee, C.D.; Ngo, D.N.; et al. Identification of a wide spectrum of ciliary gene mutations in nonsyndromic biliary atresia patients implicates ciliary dysfunction as a novel disease mechanism. EBioMedicine 2021, 71, 103530. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Huang, B.Q.; Radtke, B.N.; Gajdos, G.B.; Splinter, P.L.; Masyuk, T.V.; Gradilone, S.A.; LaRusso, N.F. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1013–G1024. [Google Scholar] [CrossRef]
- Hall, C.; Sato, K.; Wu, N.; Zhou, T.; Kyritsi, K.; Meng, F.; Glaser, S.; Alpini, G. Regulators of Cholangiocyte Proliferation. Gene Expr. 2017, 17, 155–171. [Google Scholar] [CrossRef]
- Hartley, J.L.; O’Callaghan, C.; Rossetti, S.; Consugar, M.; Ward, C.J.; Kelly, D.A.; Harris, P.C. Investigation of primary cilia in the pathogenesis of biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 485–488. [Google Scholar] [CrossRef]
- Uemura, M.; Hara, K.; Shitara, H.; Ishii, R.; Tsunekawa, N.; Miura, Y.; Kurohmaru, M.; Taya, C.; Yonekawa, H.; Kanai-Azuma, M.; et al. Expression and function of mouse Sox17 gene in the specification of gallbladder/bile-duct progenitors during early foregut morphogenesis. Biochem. Biophys. Res. Commun. 2010, 391, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kanai-Azuma, M.; Kanai, Y.; Gad, J.M.; Tajima, Y.; Taya, C.; Kurohmaru, M.; Sanai, Y.; Yonekawa, H.; Yazaki, K.; Tam, P.P.; et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 2002, 129, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Chen, D.; Wells, R.G. Microcystin-RR is a biliary toxin selective for neonatal cholangiocytes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Kotb, M.A.; Kotb, A. Glutathione S Transferase M1 Polymorphism in Extrahepatic Biliary Atresia. Med. J. Cairo Univ. 2015, 83, 109–112. [Google Scholar]
- Kotb, M.A. Aflatoxins in infants with extrahepatic biliary atresia. Med. J. Cairo Univ. 2015, 83, 207–210. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai-Bing, Y.; Sivasankaran, M.S.; Ottakandathil, B.R.; Zhong-Luan, W.; Man-Ting, S.; Ho-Yu, C.; Kak-Yuen, W.; Kwong-Hang, T.; Chi-Hang, L. Environmental Toxin Biliatresone-Induced Biliary Atresia-like Abnormal Cilia and Bile Duct Cell Development of Human Liver Organoids. Toxins 2024, 16, 144. https://doi.org/10.3390/toxins16030144
Hai-Bing Y, Sivasankaran MS, Ottakandathil BR, Zhong-Luan W, Man-Ting S, Ho-Yu C, Kak-Yuen W, Kwong-Hang T, Chi-Hang L. Environmental Toxin Biliatresone-Induced Biliary Atresia-like Abnormal Cilia and Bile Duct Cell Development of Human Liver Organoids. Toxins. 2024; 16(3):144. https://doi.org/10.3390/toxins16030144
Chicago/Turabian StyleHai-Bing, Yue, Menon Sudheer Sivasankaran, Babu Rosana Ottakandathil, Wu Zhong-Luan, So Man-Ting, Chung (Patrick) Ho-Yu, Wong (Kenneth) Kak-Yuen, Tam (Paul) Kwong-Hang, and Lui (Vincent) Chi-Hang. 2024. "Environmental Toxin Biliatresone-Induced Biliary Atresia-like Abnormal Cilia and Bile Duct Cell Development of Human Liver Organoids" Toxins 16, no. 3: 144. https://doi.org/10.3390/toxins16030144
APA StyleHai-Bing, Y., Sivasankaran, M. S., Ottakandathil, B. R., Zhong-Luan, W., Man-Ting, S., Ho-Yu, C., Kak-Yuen, W., Kwong-Hang, T., & Chi-Hang, L. (2024). Environmental Toxin Biliatresone-Induced Biliary Atresia-like Abnormal Cilia and Bile Duct Cell Development of Human Liver Organoids. Toxins, 16(3), 144. https://doi.org/10.3390/toxins16030144





