One-Step Affinity Purification of Leucine-Rich α2-Glycoproteins from Snake Sera and Characterization of Their Phospholipase A2-Inhibitory Activities as β-Type Phospholipase A2 Inhibitors
Abstract
1. Introduction
2. Results
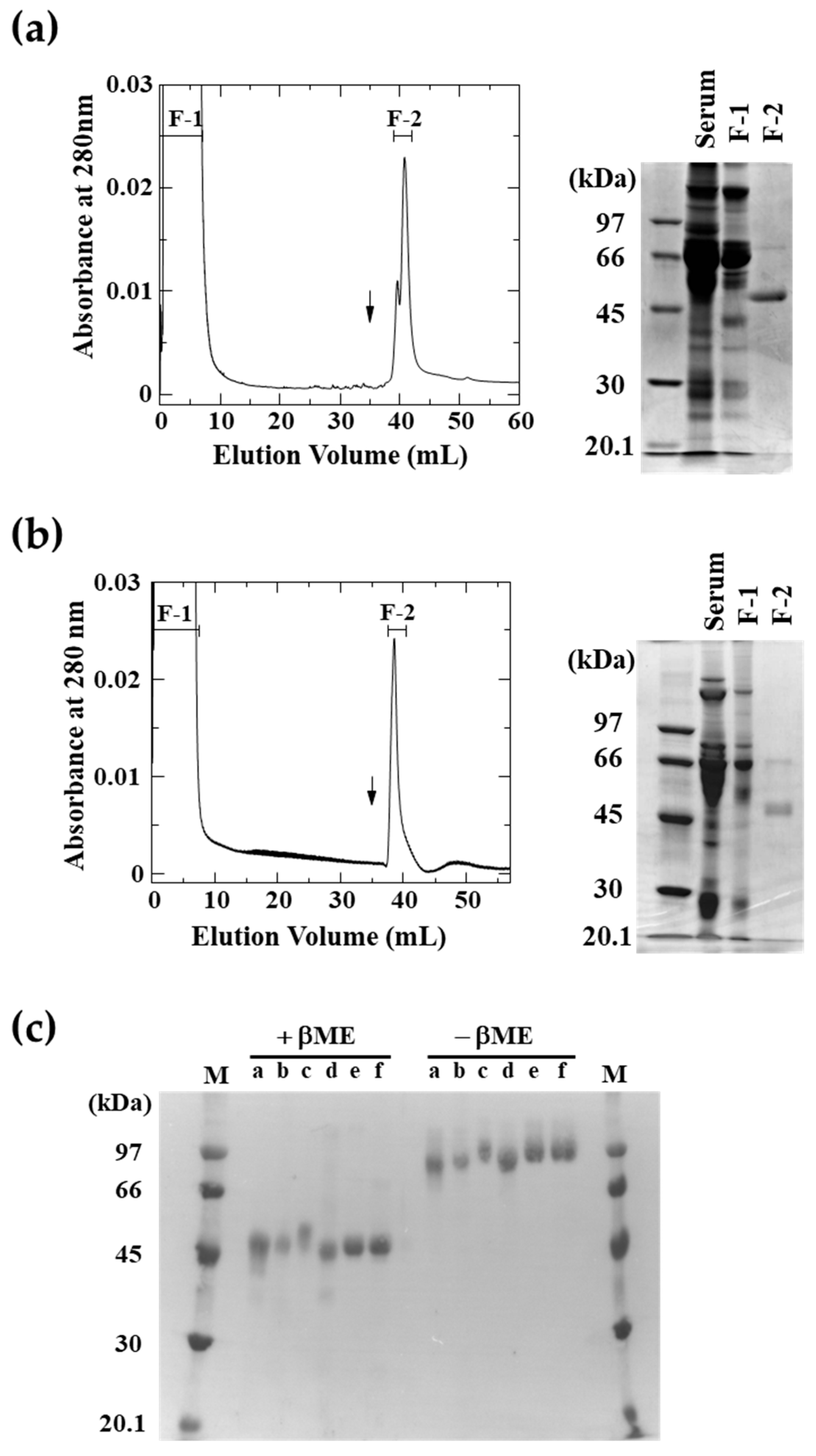
2.1. Purification of LRGs from Various Snake Sera
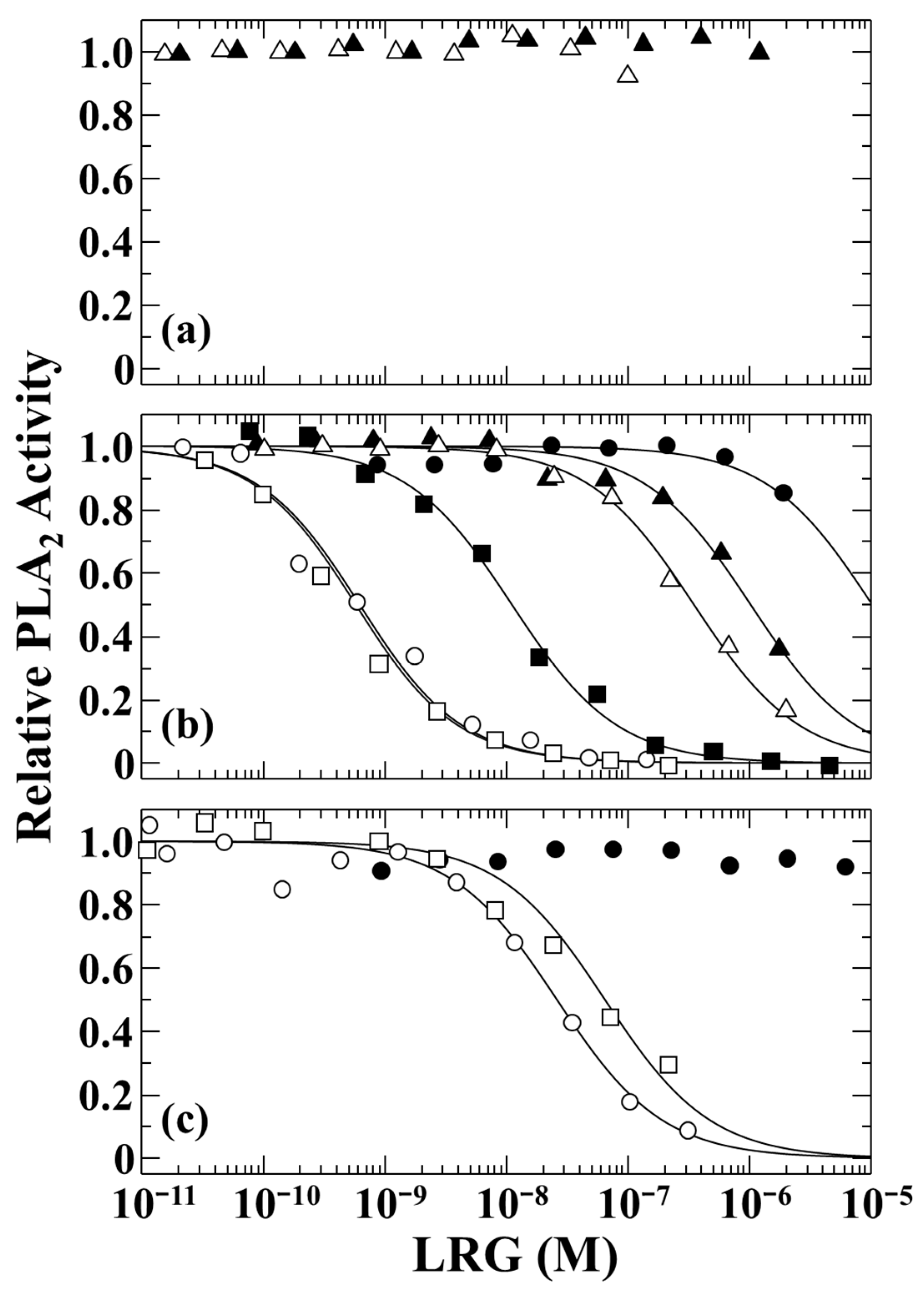
2.2. PLA2-Inhibitory Activities of the Snake LRGs
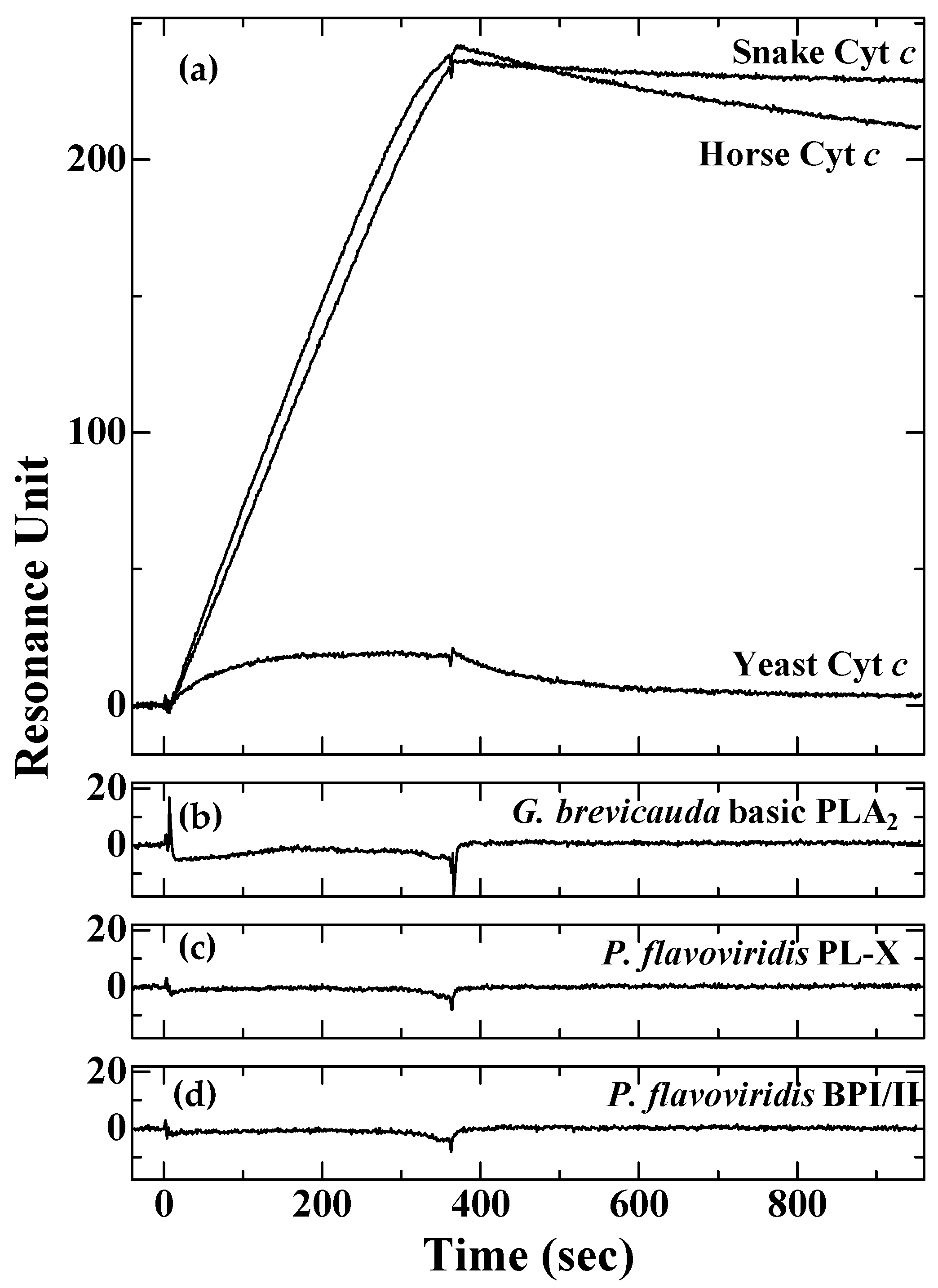
2.3. Surface Plasmon Resonance Analyses of the Interaction of P. flavoviridis LRG with Various Cyt c and PLA2
2.4. cDNA Cloning of P. flavoviridis and L. semifasciata LRGs
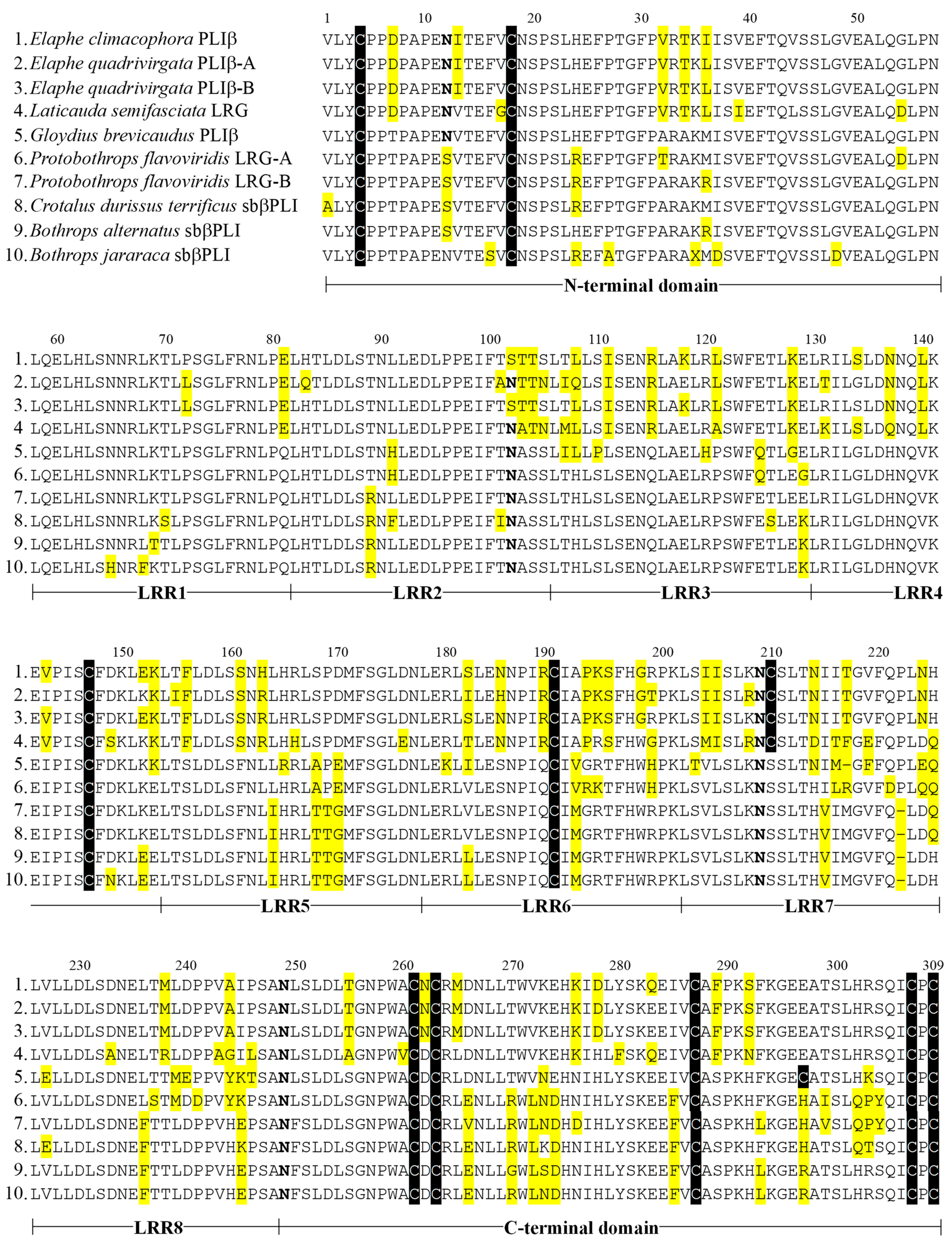
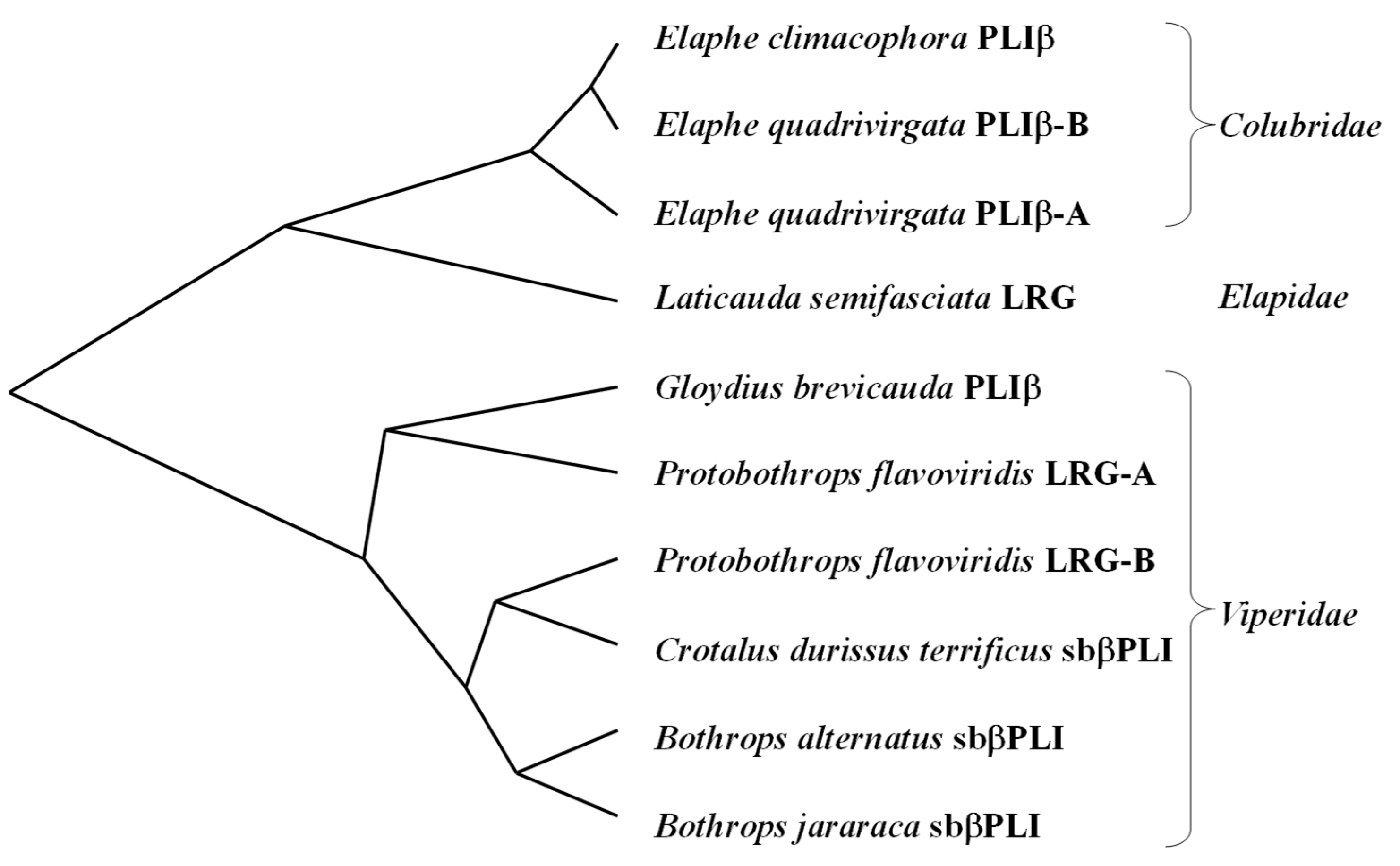
2.5. Sequence Comparison and Molecular Phylogenic Tree of Snake LRGs
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Purification of LRGs from Various Snake Sera
4.3. Inhibition of PLA2 Enzymatic Activity
4.4. Binding Analysis Using Surface Plasmon Resonance
4.5. cDNA Cloning and Sequence Analysis
4.6. Protein Structure Prediction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohkura, N.; Okuhara, H.; Inoue, S.; Ikeda, K.; Hayashi, K. Purification and characterization of three distinct types of phospholipase A2 inhibitors from the blood plasma of the Chinese mamushi, Agkistrodon blomhoffii siniticus. Biochem. J. 1997, 325, 527–531. [Google Scholar] [CrossRef]
- Campos, P.C.; de Melo, L.A.; Dias, G.L.F.; Fortes-Dias, C.L. Endogenous phospholipase A2 inhibitors in snakes: A brief overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 37. [Google Scholar] [CrossRef][Green Version]
- Lizano, S.; Domont, G.; Perales, J. Natural phospholipase A2myotoxin inhibitor proteins from snakes, mammals and plants. Toxicon 2003, 42, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Nobuhisa, I.; Chiwata, T.; Fukumaki, Y.; Hattori, S.; Shimohigashi, Y.; Ohno, M. Structural elements of Trimeresurus flavoviridis serum inhibitors for recognition of its venom phospholipase A2 isozymes. FEBS Lett. 1998, 429, 385–389. [Google Scholar] [CrossRef]
- Santos-Filho, N.A.; Santos, C.T. Alpha-type phospholipase A2 inhibitors from snake blood. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Shimada, A.; Ohkura, N.; Ikeda, K.; Samejima, Y.; Omori-Satoh, T.; Hayashi, K. Specificity of two types of phospholipase A2 inhibitors from the plasma of venomous snakes. Biochem. Mol. Biol. Int. 1997, 41, 529–537. [Google Scholar] [CrossRef]
- Quirós, S.; Alape-Girón, A.; Angulo, Y.; Lomonte, B. Isolation, characterization and molecular cloning of AnMIP, a new alpha-type phospholipase A2 myotoxin inhibitor from the plasma of the snake Atropoides nummifer (Viperidae: Crotalinae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hains, P.G.; Sung, K.L.; Tseng, A.; Broady, K.W. Functional characteristics of a phospholipase A(2) inhibitor from Notechis ater serum. J. Biol. Chem. 2000, 275, 983–991. [Google Scholar] [CrossRef]
- Dunn, R.D.; Broady, K.W. Snake inhibitors of phospholipase A(2) enzymes. Biochim. Biophys. Acta 2001, 1533, 29–37. [Google Scholar] [CrossRef]
- Lizano, S.; Lomonte, B.; Fox, J.W.; Gutiérrez, J.M. Biochemical characterization and pharmacological properties of a phospholipase A2 myotoxin inhibitor from the plasma of the snake Bothrops asper. Biochem. J. 1997, 326, 853–859. [Google Scholar] [CrossRef][Green Version]
- Okumura, K.; Ohkura, N.; Inoue, S.; Ikeda, K.; Hayashi, K. A novel phospholipase A2 inhibitor with leucine-rich repeats from the blood plasma of Agkistrodon blomhoffii siniticus. Sequence homologies with human leucine-rich alpha2-glycoprotein. J. Biol. Chem. 1998, 273, 19469–19475. [Google Scholar] [CrossRef]
- Camilli, C.; Hoeh, A.E.; De Rossi, G.; Moss, S.E.; Greenwood, J. LRG1: An emerging player in disease pathogenesis. J. Biomed. Sci. 2022, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Inoue, S.; Ikeda, K.; Hayashi, K. Identification of beta-type phospholipase A(2) inhibitor in a nonvenomous snake, Elaphe quadrivirgata. Arch. Biochem. Biophys. 2002, 408, 124–130. [Google Scholar] [CrossRef]
- Shirai, R.; Toriba, M.; Hayashi, K.; Ikeda, K.; Inoue, S. Identification and characterization of phospholipase A2 inhibitors from the serum of the Japanese rat snake, Elaphe climacophora. Toxicon 2009, 53, 685–692. [Google Scholar] [CrossRef]
- Lima, R.M.; Estevão-Costa, M.I.; Junqueira-de-Azevedo, I.L.; Ho, P.L.; Diniz, M.R.; Fortes-Dias, C.L. Phospholipase A2 inhibitors (βPLIs) are encoded in the venom glands of Lachesis muta (Crotalinae, Viperidae) snakes. Toxicon 2011, 57, 172–175. [Google Scholar] [CrossRef]
- Fortes-Dias, C.L.; Fernandes, C.A.H.; Ortolani, P.L.; Campos, P.C.; Melo, L.A.; Felicori, L.F.; Fontes, M.R.M. Identification, description and structural analysis of beta phospholipase A 2 inhibitors (sbβPLIs) from Latin American pit vipers indicate a binding site region for basic snake venom phospholipases A 2. Toxicon X 2019, 2, 100009. [Google Scholar] [CrossRef] [PubMed]
- Shirai, R.; Gotou, R.; Hirano, F.; Ikeda, K.; Inoue, S. Autologous extracellular cytochrome c is an endogenous ligand for leucine-rich alpha2-glycoprotein and beta-type phospholipase A2 inhibitor. J. Biol. Chem. 2010, 285, 21607–21614. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.; Walder, J.; Treeful, A.; Jemmerson, R. Serum leucine-rich alpha-2-glycoprotein-1 binds cytochrome c and inhibits antibody detection of this apoptotic marker in enzyme-linked immunosorbent assay. Apoptosis 2006, 11, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Heinrikson, R.L.; Krueger, E.T.; Keim, P.S. Amino acid sequence of phospholipase A2-alpha from the venom of Crotalus adamanteus. A new classification of phospholipases A2 based upon structural determinants. J. Biol. Chem. 1977, 252, 4913–4921. [Google Scholar] [CrossRef]
- Shibata, H.; Chijiwa, T.; Oda-Ueda, N.; Nakamura, H.; Yamaguchi, K.; Hattori, S.; Matsubara, K.; Matsuda, Y.; Yamashita, A.; Isomoto, A.; et al. The habu genome reveals accelerated evolution of venom protein genes. Sci. Rep. 2018, 8, 11300. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Van Der Hoorn, R.A.; Rivas, S.; Wulff, B.B.; Jones, J.D.; Joosten, M.H. Rapid migration in gel filtration of the Cf-4 and Cf-9 resistance proteins is an intrinsic property of Cf proteins and not because of their association with high-molecular-weight proteins. Plant J. 2003, 35, 305–315. [Google Scholar] [CrossRef]
- Mori, A.; Moriguchi, H. Food habits of the snakes in Japan: A critical review. Snake 1988, 20, 98–113. [Google Scholar]
- Kinkawa, K.; Shirai, R.; Watanabe, S.; Toriba, M.; Hayashi, K.; Ikeda, K.; Inoue, S. Up-regulation of the expressions of phospholipase A2 inhibitors in the liver of a venomous snake by its own venom phospholipase A2. Biochem. Biophys. Res. Commun. 2010, 395, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kudo, T.; Shinkal, W.; Tamiya, N. Phospholipase A of sea snake Laticauda semjfasciata venom: Isolation and properties of novel forms lacking tryptophan. J. Biochem. 1979, 85, 379–388. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yoshizumi, K.; Oda, N.; Ohno, M.; Tokunaga, F.; Iwanaga, S.; Kihara, H. Purification and amino acid sequence of basic protein II, a lysine-49-phospholipase A2 with low activity, from Trimeresurus flavoviridis venom. J. Biochem. 1990, 107, 400–408. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv 2021. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
| Snakes | LRG Amount (mg/10 mL Serum) |
|---|---|
| Laticauda semifasciata | 0.67 |
| Naja kaouthia | 0.48 |
| Elaphe climacophore | 0.40 |
| Gloydius brevicauda | 4.30 |
| Protobothrops flavoviridis | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirai, R.; Shibata, K.; Fujii, S.; Fukunaga, R.; Inoue, S. One-Step Affinity Purification of Leucine-Rich α2-Glycoproteins from Snake Sera and Characterization of Their Phospholipase A2-Inhibitory Activities as β-Type Phospholipase A2 Inhibitors. Toxins 2024, 16, 126. https://doi.org/10.3390/toxins16030126
Shirai R, Shibata K, Fujii S, Fukunaga R, Inoue S. One-Step Affinity Purification of Leucine-Rich α2-Glycoproteins from Snake Sera and Characterization of Their Phospholipase A2-Inhibitory Activities as β-Type Phospholipase A2 Inhibitors. Toxins. 2024; 16(3):126. https://doi.org/10.3390/toxins16030126
Chicago/Turabian StyleShirai, Ryoichi, Kana Shibata, Shinobu Fujii, Rikiro Fukunaga, and Seiji Inoue. 2024. "One-Step Affinity Purification of Leucine-Rich α2-Glycoproteins from Snake Sera and Characterization of Their Phospholipase A2-Inhibitory Activities as β-Type Phospholipase A2 Inhibitors" Toxins 16, no. 3: 126. https://doi.org/10.3390/toxins16030126
APA StyleShirai, R., Shibata, K., Fujii, S., Fukunaga, R., & Inoue, S. (2024). One-Step Affinity Purification of Leucine-Rich α2-Glycoproteins from Snake Sera and Characterization of Their Phospholipase A2-Inhibitory Activities as β-Type Phospholipase A2 Inhibitors. Toxins, 16(3), 126. https://doi.org/10.3390/toxins16030126





