Duration of Zearalenone Exposure Has Implications on Health Parameters of Lactating Cows
Abstract
1. Introduction
2. Results
2.1. Feed Intake and Chewing Activity
2.2. Rumen Fluid pH and Osmolality
2.3. Rumen Fluid and Fecal SCFA
2.4. Milk Yield and Composition
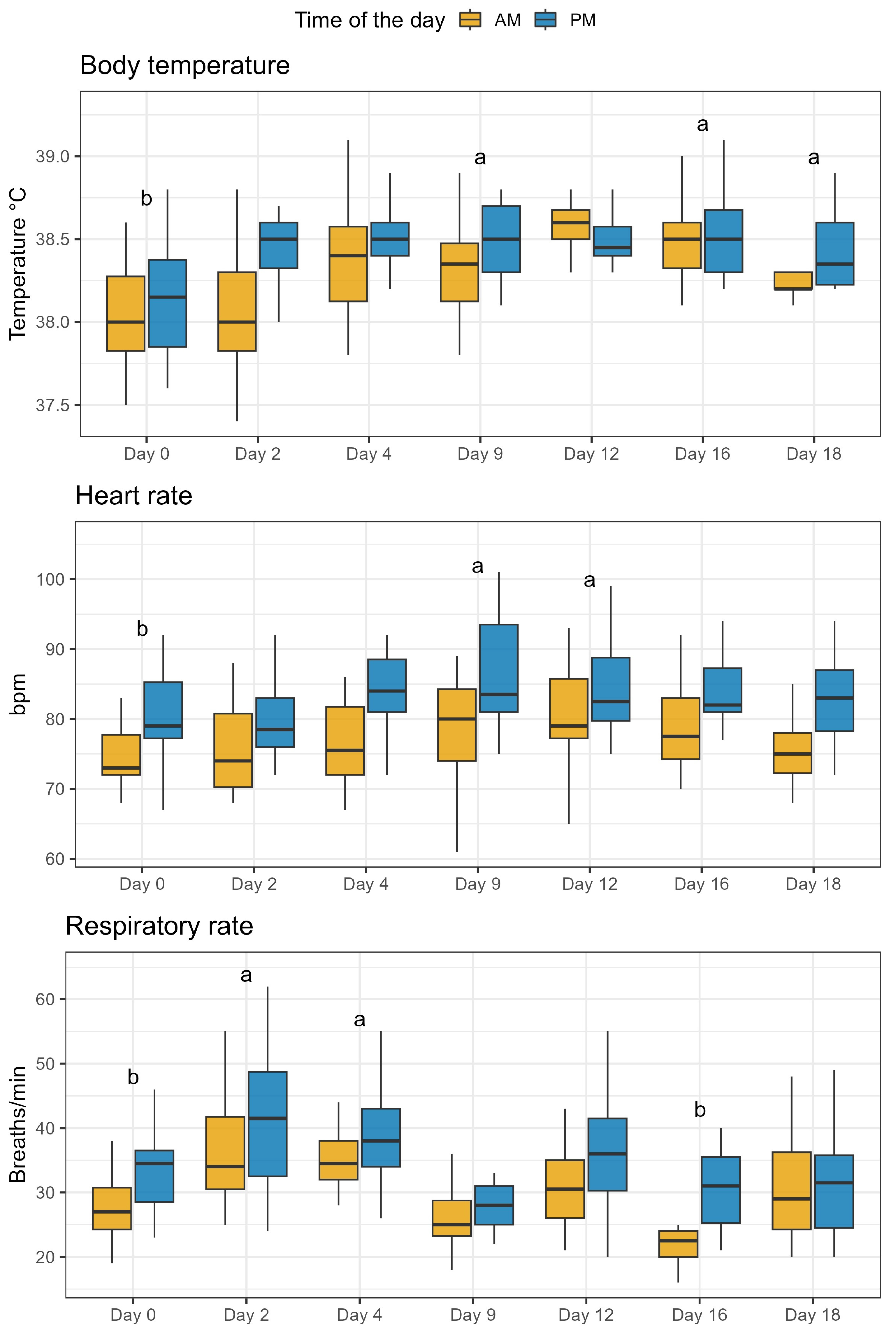
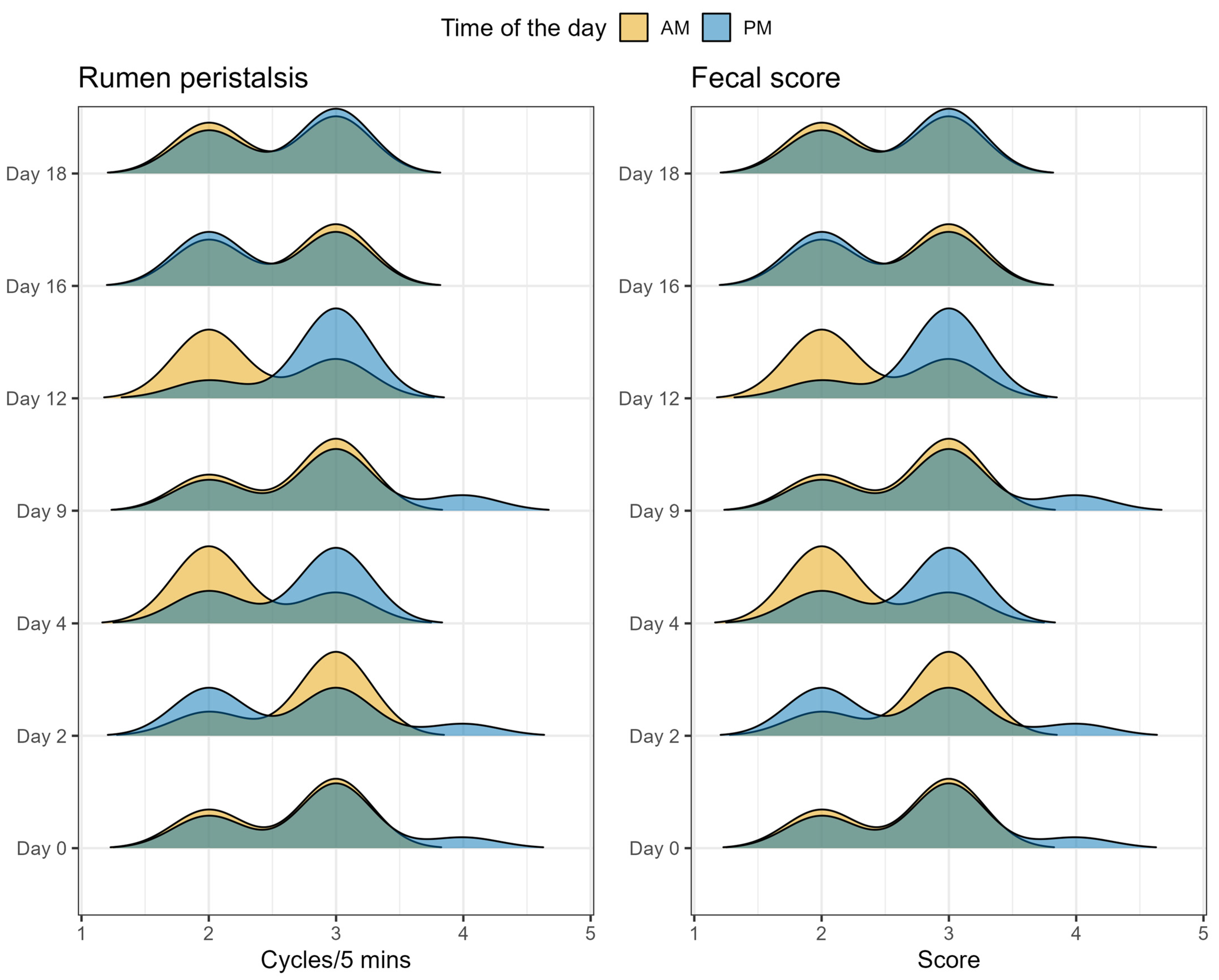
2.5. Animal Health Parameters
2.6. Blood Parameters
3. Discussion
4. Conclusions
5. Materials and Methods
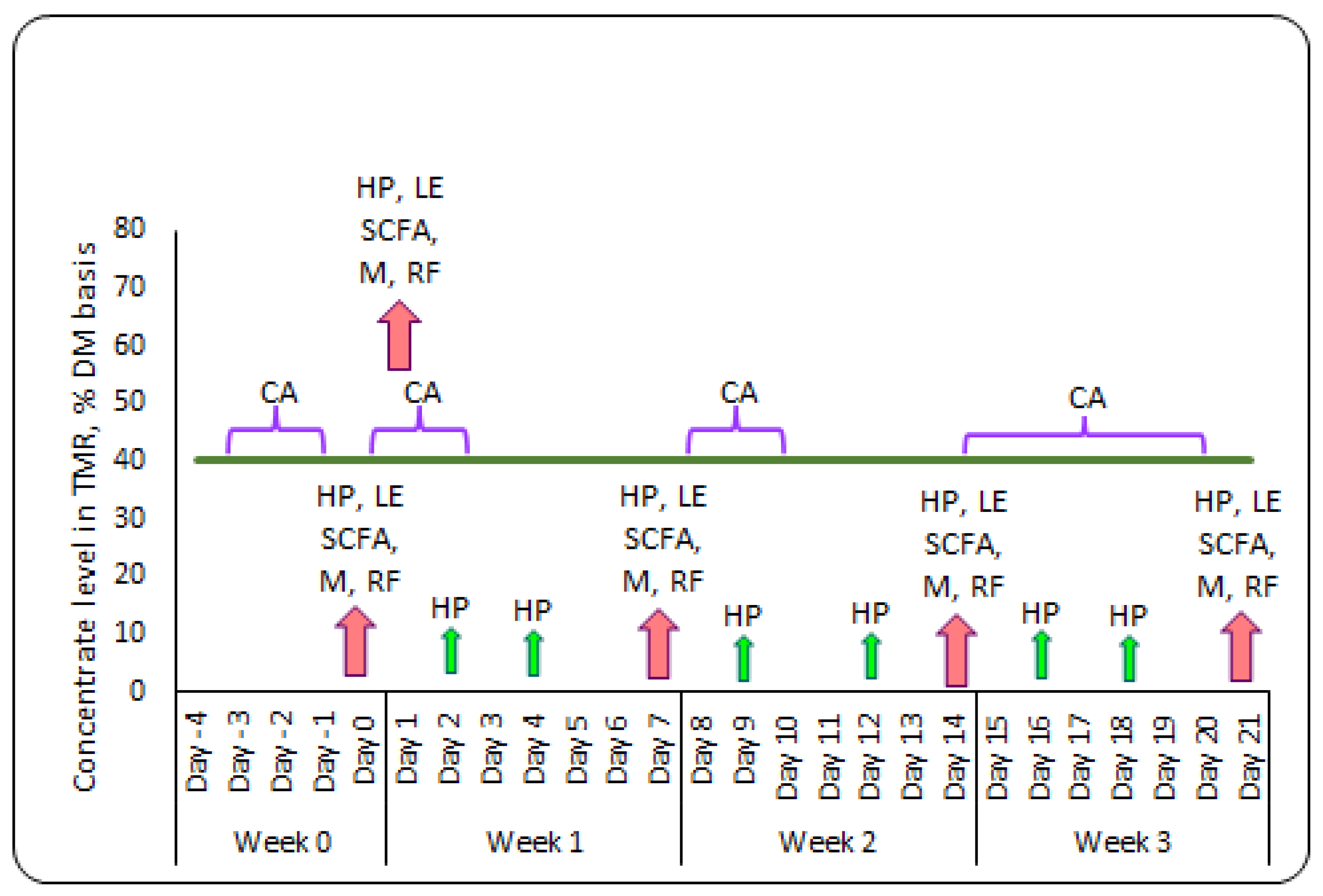
5.1. Animals, Experimental Design and Management
5.2. Sampling and Chemical Analysis of Feed
5.3. Monitoring of DMI and Chewing Activity
5.4. Collection of Rumen Fluid for Osmolality, pH, SCFA, and Feces for SCFA Analysis
5.5. Milk Sampling and Analysis
5.6. Measurement of Health Parameters and Fecal Score
5.7. Blood Sampling and Liver Enzyme Analysis
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- van Egmond, H.P. Mycotoxins in dairy products. Food Chem. 1983, 11, 289–307. [Google Scholar] [CrossRef]
- Bosco, F.; Mollea, C. Mycotoxins in Food. In Food Industrial Processes—Methods and Equipment; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Viegas, S.; Assunção, R.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Viegas, C. Mycotoxins Feed Contamination in a Dairy Farm—Potential Implications for Milk Contamination and Workers’ Exposure in a One Health Approach. J. Sci. Food Agric. 2020, 100, 1118–1123. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and Its Metabolites: Effect on Human Health, Metabolism and Neutralisation Methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Guevel, R.L.; Pakdel, F. Assessment of Oestrogenic Potency of Chemicals Used as Growth Promoter by In-Vitro Methods. Hum. Reprod. 2001, 16, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Zheng, N.; Zheng, B.Q.; Wen, F.; Cheng, J.B.; Han, R.W.; Xu, X.M.; Li, S.L.; Wang, J.Q. Simultaneous Determination of Aflatoxin M1, Ochratoxin A, Zearalenone and α-Zearalenol in Milk by UHPLC–MS/MS. Food Chem. 2014, 146, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Becker-Algeri, T.A.; Castagnaro, D.; de Bortoli, K.; de Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef]
- Tanaka, T.; Hasegawa, A.; Yamamoto, S.; Lee, U.S.; Sugiura, Y.; Ueno, Y. Worldwide Contamination of Cereals by the Fusarium Mycotoxins Nivalenol, Deoxynivalenol, and Zearalenone. 1. Survey of 19 Countries. J. Agric. Food Chem. 1988, 36, 979–983. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- El-Hoshy, S.M. Occurrence of Zearalenone in Milk, Meat and Their Products with Emphasis on Influence of Heat Treatments on Its Level. Arch. Fur Leb. 1999, 50, 140–143. [Google Scholar]
- Sándor, G. Occurrence of Mycotoxins in Feeds, Animal Organs and Secretions. Acta Vet. Hung. 1984, 32, 57–69. [Google Scholar]
- Vidnes, A.; Bergsten, C.; Birgitte, P. Subtask II: Zearalenone. In SCOOP Task 3.2.10 Collection of Occurrence Data of Fusarium Toxins in Food and Assessment of Dietary Intake by the Population of EU Member States; Directorate-General Health and Consumer Protection: Brussels, Belgium, 2003; pp. 241–264. [Google Scholar]
- EC (European Commission). Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zeara-Lenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:229:0007:0009:EN:PDF (accessed on 13 October 2023).
- Wu, F. Measuring the Economic Impacts of Fusarium Toxins in Animal Feeds. Anim. Feed Sci. Technol. 2007, 137, 363–374. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Olsen, M. Metabolism of Aflatoxin, Ochratoxin, Zearalenone, and Three Trichothecenes by Intact Rumen Fluid, Rumen Protozoa, and Rumen Bacteria. Appl. Environ. Microbiol. 1984, 47, 1070–1073. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Faas, J.; Doupovec, B.; Aleschko, M.; Stoiber, C.; Höbartner-Gußl, A.; Schöndorfer, K.; Killinger, M.; Zebeli, Q.; Schatzmayr, D. Metabolism of Zearalenone in the Rumen of Dairy Cows with and without Application of a Zearalenone-Degrading Enzyme. Toxins 2021, 13, 84. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Appropriateness to Set a Group Health-Based Guidance Value for Zearalenone and Its Modified Forms. EFSA J. 2016, 14, e04425. [Google Scholar] [CrossRef]
- Zada, S.; Alam, S.; Ayoubi, S.A.; Shakeela, Q.; Nisa, S.; Niaz, Z.; Khan, I.; Ahmed, W.; Bibi, Y.; Ahmed, S.; et al. Biological Transformation of Zearalenone by Some Bacterial Isolates Associated with Ruminant and Food Samples. Toxins 2021, 13, 712. [Google Scholar] [CrossRef]
- Dong, M.; Tulayakul, P.; Li, J.-Y.; Dong, K.-S.; Manabe, N.; Kumagai, S. Metabolic Conversion of Zearalenone to α-Zearalenol by Goat Tissues. J. Vet. Med. Sci. 2010, 72, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, T.; Grabher, L.; Pacífico, C.; Angelmayr, B.; Faas, J.; Zebeli, Q. Short-Term Exposure to the Mycotoxins Zearalenone or Fumonisins Affects Rumen Fermentation and Microbiota, and Health Variables in Cattle. Food Chem. Toxicol. 2022, 162, 112900. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Gädeken, D.; Ueberschär, K.-H.; Meyer, U.; Scholz, H. Effects of Fusarium Toxin Contaminated Wheat and of a Detoxifying Agent on Performance of Growing Bulls, on Nutrient Digestibility in Wethers and on the Carry Over of Zearalenone. Arch. Für Tierernaehrung 2002, 56, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Seeling, K.; Dänicke, S.; Lebzien, P.; Valenta, H.; Ueberschär, K.H.; Flachowsky, G. On the Effects of Fusarium-Contaminated Wheat and the Feed Intake Level on Ruminal Fermentation and Toxin-Turnover of Cows. Mycotox Res. 2005, 21, 132–135. [Google Scholar] [CrossRef]
- Seeling, K.; Dänicke, S.; Ueberschär, K.H.; Lebzien, P.; Flachowsky, G. On the Effects of Fusarium Toxin-Contaminated Wheat and the Feed Intake Level on the Metabolism and Carry over of Zearalenone in Dairy Cows. Food Addit. Contam. 2005, 22, 847–855. [Google Scholar] [CrossRef]
- Keese, C.; Meyer, U.; Rehage, J.; Spilke, J.; Boguhn, J.; Breves, G.; Dänicke, S. Ruminal Fermentation Patterns and Parameters of the Acid Base Metabolism in the Urine as Influenced by the Proportion of Concentrate in the Ration of Dairy Cows with and without Fusarium Toxin-Contaminated Triticale. Arch. Anim. Nutr. 2008, 62, 287–302. [Google Scholar] [CrossRef]
- Marin, D.; Motiu, M.; Taranu, I. Food Contaminant Zearalenone and Its Metabolites Affect Cytokine Synthesis and Intestinal Epithelial Integrity of Porcine Cells. Toxins 2015, 7, 1979–1988. [Google Scholar] [CrossRef]
- Abassi, H. The Mycotoxin Zearalenone Enhances Cell Proliferation, Colony Formation and Promotes Cell Migration in the Human Colon Carcinoma Cell Line HCT116. Toxicol. Lett. 2016, 254, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Noller, C.H.; Stob, M.; Tuite, J. Effects of Feeding Gibberella Zeae-Lnfected Corn on Feed Intake, Body Weight Gain, and Milk Production of Dairy Cows. J. Dairy Sci. 1979, 62, 1003–1006. [Google Scholar] [CrossRef]
- Vanyi, A.; Szemerédi, G.; Quarini, L.; Szailer, E.R. Fusariotoxicosis Egy Szarvasmarha-Allomanyban. Magy. Allatorv. Lapja 1974, 29, 544–546. [Google Scholar]
- Whitlow, L.W.; Nebel, R.L.; Hagler, W.M. The Association of Deoxynivalenol in Grain with Milk Production Loss in Dairy Cows. In Mycotoxins, Wood Decay, Plant Stress, Biocorrosion, and General Biodeterioration; Biodeterioration Research: Boston, MA, USA, 1994; Volume 4. [Google Scholar]
- Khamis, Y.; Hammad, H.A.; Hemeida, N. Mycotoxicosis with Oestrogenic Effect in Cattle. Zuchthyg 1986, 21, 233. [Google Scholar]
- Coppock, R.W.; Mostrom, M.S.; Sparling, C.G.; Jacobsen, B.; Ross, S.C. Apparent Zearalenone Intoxication in a Dairy Herd from Feeding Spoiled Acid-Treated Corn. Vet. Hum. Toxicol. 1990, 32, 246–248. [Google Scholar]
- Minervini, F.; Dell’Aquila, M.E.; Maritato, F.; Minoia, P.; Visconti, A. Toxic Effects of the Mycotoxin Zearalenone and Its Derivatives on In Vitro Maturation of Bovine Oocytes and 17b-Estradiol Levels in Mural Granulosa Cell Cultures. Toxicol. In Vitro 2001, 15, 489–495. [Google Scholar] [CrossRef]
- Silva, L.D.A.; De Mello, M.R.B.; Oliveira Pião, D.D.; Silenciato, L.N.; De Quadros, T.C.O.; De Souza, A.H.; Barbero, R.P. Effects of Experimental Exposure to Zearalenone on Reproductive System Morphometry, Plasma Oestrogen Levels, and Oocyte Quality of Beef Heifer. Reprod Domest. Anim. 2021, 56, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Sulzberger, S.A.; Melnichenko, S.; Cardoso, F.C. Effects of Clay after an Aflatoxin Challenge on Aflatoxin Clearance, Milk Production, and Metabolism of Holstein Cows. J. Dairy Sci. 2017, 100, 1856–1869. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.K.; Smith, T.K.; Karrow, N.A.; Boermans, H.J. Effects of Foodborne Fusarium Mycotoxins with and without a Polymeric Glucomannan Mycotoxin Adsorbent on Food Intake and Nutrient Digestibility, Body Weight, and Physical and Clinicopathologic Variables of Mature Dogs. Am. J. Vet. Res. 2007, 68, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Bol-Schoenmakers, M.; Braber, S.; Akbari, P.; De Graaff, P.; Van Roest, M.; Kruijssen, L.; Smit, J.J.; Van Esch, B.C.A.M.; Jeurink, P.V.; Garssen, J.; et al. The Mycotoxin Deoxynivalenol Facilitates Allergic Sensitization to Whey in Mice. Mucosal Immunol. 2016, 9, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Quanz, S.T.; Griswold, K.E.; Mamedova, L.K.; Kvidera, S.K.; Brouk, M.J.; Fry, R.S.; Bradford, B.J. Case Study: Combined risk Factors and Digestive Disorders in Mid-Lactation Holstein Cows. Appl. Anim. Sci. 2022, 38, 505–517. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, N.; Fan, C.; Cheng, M.; Wang, S.; Jabar, A.; Wang, J.; Cheng, J. Effects of Aflatoxin B1 Combined with Ochratoxin A and/or Zearalenone on Metabolism, Immune Function, and Antioxidant Status in Lactating Dairy Goats. Asian-Australas. J. Anim. Sci. 2018, 31, 505–513. [Google Scholar] [CrossRef]
- McKay, Z.C.; Averkieva, O.; Rajauria, G.; Pierce, K.M. The Effect of Feedborne Fusarium Mycotoxins on dry Matter Intake, Milk Production and Blood Metabolites of Early Lactation Dairy Cows. Anim. Feed. Sci. Technol. 2019, 253, 39–44. [Google Scholar] [CrossRef]
- Winkler, J.; Kersten, S.; Meyer, U.; Engelhardt, U.; Dänicke, S. Residues of Zearalenone (ZEN), Deoxynivalenol (DON) and Their Metabolites in Plasma of Dairy Cows Fed FUSARIUM Contaminated Maize and Their Relationships to Performance Parameters. Food Chem. Toxicol. 2014, 65, 196–204. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to Deoxynivalenol (DON) as Undesirable Substance in Animal Feed. EFSA J. 2004, 2, 73. [Google Scholar] [CrossRef]
- Kallela, K.; Vasenius, L. The Effects of Rumen Fluid on the Content of Zearalenone in Animal Fodder. Nord. Vet. Med. 1982, 34, 336–339. [Google Scholar]
- Debevere, S.; De Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In Vitro Rumen Simulations Show a Lower Disappearance of Deoxynivalenol, Nivalenol, Zearalenone and Enniatin B at Conditions of Rumen Acidosis and at Dry Conditions. In Proceedings of the 41st Mycotoxin Workshop, Lisbon, Portugal, 6–8 May 2019; p. 61. [Google Scholar]
- Dänicke, S.; Matthäus, K.; Lebzien, P.; Valenta, H.; Stemme, K.; Ueberschär, K.-H.; Razzazi-Fazeli, E.; Böhm, J.; Flachowsky, G. Effects of Fusarium Toxin-Contaminated Wheat Grain on Nutrient Turnover, Microbial Protein Synthesis and Metabolism of Deoxynivalenol and Zearalenone in the Rumen of Dairy Cows. Anim. Physiol. Nutr. 2005, 89, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Korosteleva, S.N.; Smith, T.K.; Boermans, H.J. Effects of Feedborne Fusarium Mycotoxins on the Performance, Metabolism, and Immunity of Dairy Cows. J. Dairy Sci. 2007, 90, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Korosteleva, S.N.; Smith, T.K.; Boermans, H.J. Effects of Feed Naturally Contaminated with Fusarium Mycotoxins on Metabolism and Immunity of Dairy Cows. J. Dairy Sci. 2009, 92, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Winkler, J. Invited Review: Diagnosis of Zearalenone (ZEN) Exposure of Farm Animals and Transfer of Its Residues into Edible Tissues (Carry Over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Breton, J.; Panic, G.; Cogan, T.A.; Bailey, M.; Swann, J.R.; Lee, M.R.F. The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro. Toxins 2019, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Pałubicki, J.; Kosicki, R.; Twaruzek, M.; Ałtyn, I.; Grajewski, J. Concentrations of Zearalenone and Its Metabolites in Female Wild Boars from Woodlands and Farmlands. Toxicon 2021, 196, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Seeling, K.; Lebzien, P.; Dänicke, S.; Spilke, J.; Südekum, K.-H.; Flachowsky, G. Effects of Level of Feed Intake and Fusarium Toxin-contaminated Wheat on Rumen Fermentation as Well as on Blood and Milk Parameters in Cows. Anim. Physiol. Nutr. 2006, 90, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.J. Microbial Metabolism in the Forestomachs and the Large Intestine of Sheep. Aust. J. Agric. Res. 1965, 16, 77–91. [Google Scholar] [CrossRef]
- Dixon, R.M.; Nolan, J.V. Studies of the Large Intestine of Sheep: 1. Fermentation and Absorption in Sections of the Large Intestine. Br. J. Nutr. 1982, 47, 289–300. [Google Scholar] [CrossRef]
- Immig, I. The Rumen and Hindgut as Source of Ruminant Methanogenesis. Environ. Monit. Assess. 1996, 42, 57–72. [Google Scholar] [CrossRef]
- Pinton, P.; Graziani, F.; Pujol, A.; Nicoletti, C.; Paris, O.; Ernouf, P.; Di Pasquale, E.; Perrier, J.; Oswald, I.P.; Maresca, M. Deoxynivalenol Inhibits the Expression by Goblet Cells of Intestinal Mucins through a PKR and MAP Kinase Dependent Repression of the Resistin-Like Molecule β. Mol. Nutr. Food Res. 2015, 59, 1076–1087. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Lucke, A.; Doupovec, B.; Zebeli, Q.; Böhm, J. A Multicomponent Mycotoxin Deactivator Modifies the Response of the Jejunal Mucosal and Cecal Bacterial Community to Deoxynivalenol Contaminated Feed and Oral Lipopolysaccharide Challenge in Chickens1. J. Anim. Sci. 2020, 98, skz377. [Google Scholar] [CrossRef] [PubMed]
- Charmley, E.; Trenholm, H.L.; Thompson, B.K.; Vudathala, D.; Nicholson, J.W.G.; Prelusky, D.B.; Charmley, L.L. Influence of Level of Deoxynivalenol in the Diet of Dairy Cows on Feed Intake, Milk Production, and Its Composition. J. Dairy. Sci. 1993, 76, 3580–3587. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Scott, P.M.; Trenholm, H.L.; Lawrence, G.A. Minimal Transmission of Zearalenone to Milk of Dairy Cows. J. Environ. 1990, 25, 87–103. [Google Scholar] [CrossRef]
- Kinoshita, A.; Keese, C.; Beineke, A.; Meyer, U.; Starke, A.; Sauerwein, H.; Dänicke, S.; Rehage, J. Effects of Fusarium Mycotoxins in Rations with Different Concentrate Proportions on Serum Haptoglobin and Hepatocellular Integrity in Lactating Dairy Cows. Anim. Physiol. Nutr. 2015, 99, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Minuti, A.; Bani, P.; Bertuzzi, T.; Cappelli, F.P.; Doupovec, B.; Faas, J.; Schatzmayr, D.; Trevisi, E. A Mycotoxin-Deactivating Feed Additive Counteracts the Adverse effects of Regular Levels of Fusarium Mycotoxins in Dairy Cows. J. Dairy Sci. 2020, 103, 11314–11331. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, C.S.; Mukherjee, A. In Vitro Inhibition of Bovine Liver Glutamate Dehydrogenase by Citrinin, a Mycotoxin. J. Antibiot. 1977, 30, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Su, D.; Tian, H.; Li, X.; Li, Y.; Ran, L.; Hu, R.; Cheng, J. Liver Metabolic Perturbations of Heat-Stressed Lactating Dairy Cows. Asian-Australas. J. Anim. Sci. 2018, 31, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-C.; Kim, M.; Jeong, J.-Y.; Oh, Y.-K.; Lee, S.-D.; Lee, Y.-K.; Ji, S.-Y.; Choi, H. Effects of Short-term Acute Heat Stress on Physiological Responses and Heat Shock Proteins of Hanwoo Steer (Korean Cattle). J. Anim. Reprod. Biotechnol. 2019, 34, 173–182. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A Review of the Impact of Mycotoxins on Dairy Cattle Health: Challenges for Food Safety and Dairy Production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Kononoff, P.J.; Heinrichs, A.J.; Lehman, H.A. The Effect of Corn Silage Particle Size on Eating Behavior, Chewing Activities, and Rumen Fermentation in Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Naumann, C.; Bassler, R. Die Chemische Untersuchung von Futtermitteln; VDLUFA Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Beauchemin, K.A.; Yang, W.Z. Effects of Physically Effective Fiber on Intake, Chewing Activity, and Ruminal Acidosis for Dairy Cows Fed Diets Based on Corn Silage. J. Dairy Sci. 2005, 88, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Kröger, I.; Humer, E.; Neubauer, V.; Kraft, N.; Ertl, P.; Zebeli, Q. Validation of a Noseband Sensor System for Monitoring Ruminating Activity in Cows under Different Feeding Regimens. Livest. Sci. 2016, 193, 118–122. [Google Scholar] [CrossRef]
- Rivera-Chacon, R.; Castillo-Lopez, E.; Ricci, S.; Petri, R.M.; Reisinger, N.; Zebeli, Q. Supplementing a Phytogenic Feed Additive Modulates the Risk of Subacute Rumen Acidosis, Rumen Fermentation and Systemic Inflammation in Cattle Fed Acidogenic Diets. Animals 2022, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Qumar, M.; Khiaosa-ard, R.; Pourazad, P.; Wetzels, S.U.; Klevenhusen, F.; Kandler, W.; Aschenbach, J.R.; Zebeli, Q. Evidence of In Vivo Absorption of Lactate and Modulation of Short Chain Fatty Acid Absorption from the Reticulo Rumen of Non-Lactating Cattle Fed High Concentrate Diets. PLoS ONE 2016, 11, e0164192. [Google Scholar] [CrossRef]
- Skidmore, A.; Brand, A.; Sniffen, C. Monitoring Milk Production: Defining Preset Targets and Execution. In Herd Health and Production Management in Dairy Practice; Wageningen Academic Publishers: Wageningen, The Netherlands, 1996; pp. 223–262. [Google Scholar]
- Wickham, H. Data Analysis. In Use R! ggplot2. Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar]


| Item | Week 0 | Week 1 | Week 2 | Week 3 | SEM 1 | p-Values 2 |
|---|---|---|---|---|---|---|
| Duration of Exposure | ||||||
| Dry matter intake, kg/day | 22.25 b | 22.64 | 23.86 a | 23.59 a | 3.59 | <0.01 |
| Ruminating time, min/day | 411.44 | 370.19 | 398.15 | 382.53 | 68.02 | 0.85 |
| Ruminating chews/min | 66.25 b | 66.98 | 66.82 | 67.77 a | 1.16 | <0.01 |
| Ruminating chews/bolus | 57.74 | 59.72 | 60.09 | 57.44 | 2.09 | 0.55 |
| Eating time, min/day | 180.79 | 201.86 | 208.44 | 210.81 | 17.38 | 0.17 |
| Drinking time *, min/da | 6.92 | 8.64 | 7.14 | 7.79 | 2.61 | 0.24 |
| Drinking gulps +, No. | 133.17 | 158.07 | 132.12 | 142.19 | 36.88 | 0.33 |
| Total chewing time, min/day | 642.55 | 666.73 | 683.31 | 671.74 | 25.29 | 0.59 |
| Chewing index, time/kg DMI | 27.47 | 29.37 | 28.14 | 27.89 | 1.39 | 0.51 |
| Item | Day 0 | Day 1 | Day 7 | Day 14 | Day 21 | SEM 1 | p-Values 2 |
|---|---|---|---|---|---|---|---|
| Duration of Exposure | |||||||
| Rumen fluid 3 | |||||||
| Total SCFA, mM | 104.69 a | 99.75 | 83.37 b | 94.96 | 94.14 | 4.46 | <0.01 |
| % of total SCFA | |||||||
| Acetate | 59.01 | 58.04 | 58.58 | 58.39 | 58.45 | 2.45 | 0.41 |
| Propionate | 24.77 | 25.22 | 25.91 | 26.19 | 26.57 | 4.85 | 0.25 |
| Butyrate | 10.58 | 11.09 | 10.21 | 10.34 | 9.82 | 0.33 | <0.05 |
| Isobutyrate | 0.89 ax | 0.90 | 0.80 y | 0.77 b | 0.78 b | 0.03 | <0.01 |
| Valerate | 1.72 x | 1.74 | 1.63 | 1.59 y | 1.60 y | 0.05 | <0.01 |
| Isovalerate | 1.28 | 1.33 | 1.30 | 1.14 | 1.19 | 0.21 | <0.05 |
| Caproate | 0.80 ax | 0.76 | 0.63 b | 0.66 y | 0.66 | 0.07 | <0.05 |
| Heptanoate | 0.12 | 0.09 | 0.09 | 0.10 | 0.10 | 0.15 | 0.73 |
| Acetate:propionate ratio | 2.49 | 2.41 | 2.35 | 2.34 | 2.31 | 0.61 | 0.32 |
| Feces 4 | |||||||
| Total SCFA, mM | 46.13 b | 48.51 | 46.52 | 51.35 | 62.07 a | 4.93 | <0.05 |
| % of total SCFA | |||||||
| Acetate | 75.28 b | 77.24 a | 76.49 | 77.20 a | 77.84 a | 0.41 | <0.01 |
| Propionate | 15.93 a | 14.55 b | 15.22 | 14.71 b | 14.48 b | 0.24 | <0.01 |
| Butyrate | 6.07 | 5.94 | 5.94 | 6.18 | 5.67 | 0.26 | 0.27 |
| Isobutyrate | 1.07 a | 0.85 b | 0.89 | 0.68 b | 0.75 b | 0.06 | <0.01 |
| Valerate | 1.10 a | 0.98 | 0.97 b | 0.88 b | 0.85 b | 0.04 | <0.01 |
| Isovalerate | 0.53 a | 0.41 | 0.46 | 0.33 b | 0.37 | 0.05 | <0.05 |
| Acetate:propionate ratio | 4.75 b | 5.34 a | 5.05 | 5.30 a | 5.40 a | 0.30 | <0.01 |
| Item | Day 0 | Day 1 | Day 7 | Day 14 | Day 21 | SEM 1 | p-Values 2 |
|---|---|---|---|---|---|---|---|
| Duration of Exposure | |||||||
| Milk yield, kg/day | 36.04 | 36.11 | 35.97 | 36.01 | 36.72 | 10.26 | 0.82 |
| Energy-corrected milk, kg/day | 35.60 | 35.34 | 34.37 | 36.35 | 36.64 | 1.43 | 0.12 |
| Milk composition | |||||||
| Fat, % | 3.99 | 3.95 | 3.70 | 4.01 | 3.94 | 1.28 | 0.16 |
| Protein, % | 3.38 | 3.31 | 3.42 | 3.54 | 3.57 | 0.46 | 0.18 |
| Lactose, % | 4.92 | 4.97 | 4.90 | 4.96 | 4.97 | 0.17 | 0.29 |
| Milk urea nitrogen, mg/dL | 23.63 b | 21.83 | 27.90 a | 22.78 | 22.77 | 7.14 | <0.01 |
| Somatic cell count *, cells/mL × 103 | 34.88 | 34.37 | 33.07 | 35.74 | 33.03 | 1.21 | 0.86 |
| Fat–protein ratio | 1.12 | 1.13 | 1.02 | 1.07 | 1.05 | 0.04 | <0.05 |
| Milk pH | 6.62 b | 6.61 | 6.57 b | 6.60 | 6.66 a | 0.14 | <0.01 |
| Diet, % DM (Unless Otherwise Stated) | |
|---|---|
| Item | TMR |
| Ingredients | |
| Grass silage | 20 |
| Corn silage | 40 |
| Energy supplement 1 | 21 |
| Protein supplement 2 | 19 |
| TMR chemical composition | |
| DM, % as fresh | 45.36 ± 0.41 |
| Crude protein | 14.9 ± 1.46 |
| Neutral detergent fiber | 39.41 ± 0.26 |
| Acid detergent fiber | 25.58 ± 0.86 |
| Starch | 28.67 ± 1.30 |
| Ether extract | 2.37 ± 0.09 |
| Non-fiber carbohydrates | 37.11 ± 2.49 |
| Ash | 6.22 ± 0.85 |
| Particle fraction (% retained) 3 | |
| Long | 9.95 |
| Medium | 56.20 |
| Short | 21.78 |
| Fine | 12.06 |
| pef 4 | 0.67 |
| pe NDF 5 > 8 mm | 26.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Chacon, R.; Hartinger, T.; Castillo-Lopez, E.; Lang, C.; Penagos-Tabares, F.; Mühleder, R.; Atif, R.M.; Faas, J.; Zebeli, Q.; Ricci, S. Duration of Zearalenone Exposure Has Implications on Health Parameters of Lactating Cows. Toxins 2024, 16, 116. https://doi.org/10.3390/toxins16030116
Rivera-Chacon R, Hartinger T, Castillo-Lopez E, Lang C, Penagos-Tabares F, Mühleder R, Atif RM, Faas J, Zebeli Q, Ricci S. Duration of Zearalenone Exposure Has Implications on Health Parameters of Lactating Cows. Toxins. 2024; 16(3):116. https://doi.org/10.3390/toxins16030116
Chicago/Turabian StyleRivera-Chacon, Raul, Thomas Hartinger, Ezequias Castillo-Lopez, Claudia Lang, Felipe Penagos-Tabares, Rita Mühleder, Rana Muhammad Atif, Johannes Faas, Qendrim Zebeli, and Sara Ricci. 2024. "Duration of Zearalenone Exposure Has Implications on Health Parameters of Lactating Cows" Toxins 16, no. 3: 116. https://doi.org/10.3390/toxins16030116
APA StyleRivera-Chacon, R., Hartinger, T., Castillo-Lopez, E., Lang, C., Penagos-Tabares, F., Mühleder, R., Atif, R. M., Faas, J., Zebeli, Q., & Ricci, S. (2024). Duration of Zearalenone Exposure Has Implications on Health Parameters of Lactating Cows. Toxins, 16(3), 116. https://doi.org/10.3390/toxins16030116





