Abstract
Recently, the use of click chemistry for localization of chemically modified cyanopeptides has been introduced, i.e., taking advantage of promiscuous adenylation (A) domains in non-ribosomal peptide synthesis (NRPS), allowing for the incorporation of clickable non-natural amino acids (non-AAs) into their peptide products. In this study, time-lapse experiments have been performed using pulsed feeding of three different non-AAs in order to observe the synthesis or decline of azide- or alkyne-modified microcystins (MCs) or anabaenopeptins (APs). The cyanobacteria Microcystis aeruginosa and Planktothrix agardhii were grown under maximum growth rate conditions (r = 0.35–0.6 and 0.2–0.4 (day−1), respectively) in the presence of non-AAs for 12–168 h. The decline of the azide- or alkyne-modified MC or AP was observed via pulse-feeding. In general, the increase in clickable MC/AP in peptide content reached a plateau after 24–48 h and was related to growth rate, i.e., faster-growing cells also produced more clickable MC/AP. Overall, the proportion of clickable MC/AP in the intracellular fraction correlated with the proportion observed in the dissolved fraction. Conversely, the overall linear decrease in clickable MC/AP points to a rather constant decline via dilution by growth instead of a regulated or induced release in the course of the synthesis process.
Keywords:
microcystin synthesis; anabaenopeptin synthesis; time-lapse experiments; molecule tracing; pulse-feeding; unnatural amino acids; click-chemistry; fluorescent labeling; growth rate Key Contribution:
Tracking of MC/AP synthesis by using promiscuous A domains in NRPS, allowing for the incorporation of clickable non-AAs into peptide products. During time-lapse experiments, a fast increase in clickable MC/AP content has been related to the growth rate.
1. Introduction
In addition to the visually repulsive effect of cyanobacterial blooms in freshwater and brackish water, considerable amounts of toxins are produced and released into the water, with dangerous effects for the entire food web, at the end of which humans are. Within the cyanotoxins, the microcystins (MCs) and related nodularins are globally considered the most abundant [1]. To understand cyanotoxin synthesis within cyanobacterial cells, the intracellular storage mechanism of cyanotoxins, and the factors regulating cyanotoxin occurrence outside the cell are considered of outermost importance to develop exposure scenarios that are at the basis of risk management [1].
Typically, MCs are produced inside the cells while high extracellular concentrations have been observed less frequently, i.e., after cell lysis [2]. Using individual cell culture studies under laboratory conditions, both intracellular and extracellular MCs have been measured, and the general finding was that in exponentially growing cell cultures, <10% of the total MC pool is found to be extracellular. Such experiments have been performed using a few selected strains only (i.e., Microcystis aeruginosa PCC 7806), somewhat limiting the possibility of testing variability in ecophysiological responses via comparing a larger number of genotypes or strains [3]. For example, comparing the extracellular peptide content among 56 strains of Planktothrix agardhii and P. rubescens, the percentage of extracellular peptides (from total peptide concentration) varied from 0 to 62 ± 23% (SE) for MC and 0 to 58 ± 22% for anabaenopeptin (AP) [3,4]. While the majority of the samples (77%) were found to contain less than 10% of total MC dissolved, a few strains showed a higher proportion of dissolved MC. Nevertheless, it is generally understood that MC release into the medium is primarily related to cell aging and resulting cell lysis [5], while MC production has been traditionally linked to cell growth [6,7].
Typically, those analyses are performed in parallel to strain growth under defined culture conditions, and the intra- vs. extracellular MC or other peptide concentrations are determined by fractionation, i.e., from cells filtered on glass fiber filters, while corresponding extracellular MCs are determined from the filtrate [8]. A more direct approach to monitor MC/peptide synthesis is to create a traceable MC peptide pool and to follow the fate of both intra- and extracellular MCs during time-lapse experiments. This has been attempted in the past, i.e., by supplying radioactive inorganic carbon to M. aeruginosa in cultures for 6 h and subsequent incubation in the dark [5]. The fate of this radioactive MC pool was studied by following its radioactivity in time-course experiments. Notably, no evidence for losses from the intracellular MC pool could be found either under high light or under low light conditions.
In the last years, the use of click chemistry to assist in subcellular localization of chemically modified MC/APs has been applied. In particular, visualizing the synthesis of MCs and APs directly in the cell of cyanobacteria was established by using chemo-selective reaction [9], i.e., following the principle of the copper catalyzed azide-alkyne cycloaddition (CuAAC) reaction [10]. Accordingly, using chemical-analytical methods the targeted modification of MC/AP peptides through the incorporation of an alkyne or azide side chain could be shown, i.e., for MC structure in pos. 2 in M. aeruginosa or AP structure in exocyclic pos. 1 in P. agardhii [9]. The targeted peptides then become available as CuAAC reaction sites for specific fluorophores visualized using high-resolution microscopy.
The chemo-selective labeling process requires the incorporation of amino acids carrying azide or alkyne moieties into the modified MC or AP peptide to allow its targeting. The incorporation of such non-natural amino acids (non-AAs) is possible since cyanobacteria assimilate various diverse amino acids from the media [11], and certain promiscuous adenylation (A) domains integrate the non-AAs into the growing peptide product during non-ribosomal peptide synthesis (NRPS). For the first time, those promiscuous A domains were described in M. aeruginosa some years ago [12,13]. The encoding genes have possibly evolved in order to increase the structural diversity of the resulting bioactive peptide products, i.e., for MC in M. aeruginosa [13] or for AP in P. agardhii [14]. In particular, for MC synthesis, the first A domain encoded via the mcyB gene was related to the co-production of MC molecules carrying Arg, Tyr, and Leu at pos. 2 [12,13]; while for AP synthesis, the first A domain of the apnA gene was related to Arg, Tyr, and Lys at pos. 1 of the AP molecule [14]. However, not all genotypes have evolved towards promiscuity in relevant A domains, but rather, certain phylogenetic clades have been differentiated either by producing only AP molecules with Arg at pos. 1, or with both Arg and Tyr at pos. 1, or with three or more AA at pos. 1 (e.g., Figure 6 in Entfellner et al., 2017 [15]). Consequently, if A domains of NRPS retained their substrate specificity towards a corresponding substrate, such incorporation of non-AA into the growing peptide product will not be possible. During previous studies, two cyanobacteria have been used for click-chemistry, i.e., M. aeruginosa strain Hofbauer carrying natural MC variants with Tyr and Leu at pos. 2 of the MC molecule and P. agardhii strain no371/1 carrying Arg, Tyr, and Lys at pos. 1 of the AP molecule. While no AP synthesis could be detected for M. aeruginosa strain Hofbauer, P. agardhii strain no371/1 has lost the genetic basis for MC synthesis and is considered nontoxic [4].
The CuAAC reaction with targeted peptides has been tested for its specificity through the use of genetically modified P. agardhii strain NIVA-CYA126/8, for which a specific toxin or peptide such as MC or AP has been eliminated previously [14]. In particular, P. agardhii strain NIVA-CYA126/8 wild type as well as its peptide knock-out mutants, which have been inactivated either in MC or in cyanopeptolin or in microviridin synthesis, all showed modified clickable AP and labeling using Alexa 488 [16]. In contrast, for the NIVA-CYA126/8 mutant inactivated in AP synthesis, no labeling was observed compared with the controls [16].
In this study, we applied pulse feeding of three different non-natural L-amino acids (non-AAs), i.e., 4-azidophenylalanine (Phe-Az), N-propargyloxy-carbonyl-L-lysine (Prop-Lys), or O-propargyl-L-Tyrosine (Prop-Tyr) to track and trace the intra- and extracellular dynamics of clickable MC/AP synthesis in a time series to better understand the synthesis process in a temporal manner. Using standard peptide analysis and fragmentation via LC-MSn, the build up of a traceable MC/AP pool was observed. Conversely, the decline of traceable MC/AP was followed subsequent to pulse feeding and the transfer of cells to a new medium without non-AAs. The relationships between clickable MC/AP content and resulting signal intensity from subsequent labeling using Alexa Fluor488 will be reported in a forthcoming article. Most importantly, we observed a rapid increase in clickable MC/AP content by chemical-analytical analysis (maximum after 12–24 h). Conversely, after pulsed feeding, a linear decrease in the modified peptides with time was observed (0–96 h or 0–192 h). Thus, both the build up and decline point to a rather constant production of targeted MC/AP, mostly related to growth, which is in line with the current understanding of MC or other bioactive peptide synthesis in cyanobacteria.
2. Results
2.1. Growth of Cyanobacteria in Strain Cultures During Time-Lapse Experiments Using Pulsed Feeding of Non-AAs
In general both strains, M. aeruginosa and P. agardhii, grew linearly in the presence of all three non-AAs; however, one particular non-AA (Phe-Az) was frequently found to reduce growth when compared to controls (i.e., cells grown and processed under identical conditions but without substrate) or in the presence of the other two non-AAs (Prop-Lys, Prop-Tyr), as shown in Table 1 and Table 2 (Supplementary Figures S1 and S2). Based on optical density (OD), the average growth rates varied from 0.58 ± 0.13 (SE) (day−1) during build up to 0.35 ± 0.05 during decline in the controls of M. aeruginosa. In contrast, during addition of Phe-Az, the growth decreased to 0.38 ± 0.15 and −0.04 ± 0.07, respectively. For P. agardhii, growth rates varied from 0.47 ± 0.1 to 0.22 ± 0.07 in controls during build up and decline experiments, respectively, and similarly to M. aeruginosa, the cell (filament) numbers as indicated by OD were found to be reduced in the presence of Phe-Az (Table 1). Analogously, based on dry weight (DW), reduced biomass was observed during Phe-Az addition, while Prop-Lys and Prop-Tyr did not affect the production of biomass (Table 2).

Table 1.
Mean ± SE optical density and growth rates as recorded during time-lapse signal build up and decline experiments in cyanobacteria M. aeruginosa strain Hofbauer and P. agardhii strain no371/1. Control = cells grown and processed under identical conditions but without non-AA substrate.

Table 2.
Mean ± SE dry weight and growth rates as recorded during time-lapse signal build up and decline experiments in cyanobacteria M. aeruginosa strain Hofbauer and P. agardhii strain no371/1. Control = cells grown and processed under identical conditions but without non-AA substrate.
2.2. Observation of Clickable Peptide Synthesis in Cyanobacteria Strains During Time-Lapse Build Up and Decline Experiments
As reported previously, both original MC/AP structural variants, as well as new (clickable) MC/AP structural variants, were observed in the presence of all three non-AAs, i.e., demethylated MC variants DAsp-MC-YR and D-Asp-MC-LR and methylated MC variants MC-YR and MC-LR were partly transformed into modified MC, i.e., either DAsp-MC-Phe-AzR [M + H]+ 1056.5 and MC-Phe-AzR [M + H]+ 1070.5, or DAsp-MC-Prop-LysR [M + H]+ 1078.5 and MC-Prop-LysR [M + H]+ 1092.5, or DAsp-MC-Prop-TyrR [M + H]+ 1069.5 and MC-Prop-TyrR [M + H]+ 1083.5. Analogously, for AP, the original AP structural variants AP C [M + H]+ 809.5, AP B [M + H]+ 837.5, and AP-A [M + H]+ 844.5 were transformed into clickable AP, i.e., either AP-Phe-Az [M + H]+ 843.3 or AP-Prop-Lys [M + H]+ 891.5 or AP-Prop-Tyr [M + H]+ 882.5. All of these structural variants have been identified previously using MS2 and MS3 fragmentation analysis [9,16].
In general, MC/AP peptide production was maintained in the presence of all three non-AAs (Phe-Az, Prop-Lys, Prop-Tyr), i.e., using two-way repeated measures (RM)-ANOVA, the time factor was always found to be highly significant (p < 0.001).
Except for Phe-Az in M. aeruginosa, the total MC or AP contents (intracellular MC-LR equivalents in ng/mL or AP-B equivalents in ng/mL) were constantly increasing over time (Supplementary Figures S3 and S4). This principal relationship between growth and peptide production resulted in total MC content ranging from 0.2 to 2.2 µg MC/mg DW during the time-lapse build up and 0.2–2.3 µg MC/mg DW during time-lapse decline. Analogously, the total AP content ranged from 0.15 to 0.7 µg AP/mg DW and 0.04–0.56 µg AP/mg DW during the time-lapse build up and time-lapse decline, respectively.
Nevertheless, when comparing MC or AP contents between non-AA treatments, significant differences in total intracellular peptide content vs. moderate differences in percentage of extracellular peptide were found (Table 3). When compared with controls, often the Phe-Az treatment changed in total intracellular peptide content, which was related to the lower growth rates (i.e., Table 1 and Table 2). In contrast, the percentage of total extracellular MC contents were found to be less altered in the presence of Prop-Tyr, Prop-Lys, or Phe-Az, while corresponding AP contents did not change significantly.

Table 3.
Mean ± SE total MC/AP contents (ng/mg DW), percentage extracellular peptide from total peptide and percentage of clickable MC/AP intra-and extracellular peptides recorded during time-lapse signal build up and decline experiments in cyanobacteria M. aeruginosa strain Hofbauer and P. agardhii strain no371/1. MC or AP were quantified as equivalents of MC-LR [M + H]+ 995.5 or AP-B [M + H]+ 837.5. Control = cells grown and processed under identical conditions but without non-AA substrate.
In addition, the percentages of clickable MC/AP peptides differed significantly between non-AA treatments, i.e., for MC in M. aeruginosa, the non-AA Prop-Tyr revealed the highest percentage of modified MC during both build up and decline experiments in both intracellular and extracellular fractions, i.e., MC-Prop-TyrR [M + H]+ 1069.5 and [M + H]+ 1083.5 followed by MC-Phe-AzR resulting in [M + H]+ 1056.5 and [M + H]+ 1070.5 and MC-Prop-LysR resulting in [M + H]+ 1078.5 and [M + H]+ 1092.5 (Table 3). In contrast, for AP in P. agardhii, the non-AA Prop-Lys revealed the highest percentage of modified AP, i.e., AP-Prop-Lys [M + H]+ 891.5 followed by AP-Phe-Az [M + H]+ 843.3 and AP-Prop-Tyr [M + H]+ 882.5 (Table 3).
Regarding the time-lapse build up, MC-Prop-TyrR showed a steep proportional increase until 24–48 h (>60% of total MC), followed by a plateau phase, but continued to increase until day 7 (168 h), resulting in > 80% of the total MC content. The less abundant MC-Phe-AzR and MC-Prop-LysR are already saturated after 24 h at >20% or >3% of total MC content, respectively (Figure 1, Supplementary Figure S5). Conversely, during time-lapse decline, MC-Prop-TyrR decreased linearly from >40% down to 10% of total MC, while MC-Phe-AzR and MC-Prop-LysR showed only minor, albeit significant, decreases. Analogously to M. aeruginosa, AP-Prop-Lys in P. agardhii showed a steep increase during the first 24 h, reaching a plateau phase at 60% of the total AP content (Figure 2, Supplementary Figure S6), while AP-Phe-Az and AP-Prop-Tyr saturated after 24 h at >10% or >5%, respectively. During time-lapse decline, the most abundant AP-Prop-Lys decreased linearly in proportion, while in comparison to control cells, less decrease in the percentage of µg AP-Prop-Lys per mg DW could be observed (Supplementary Figure S6).
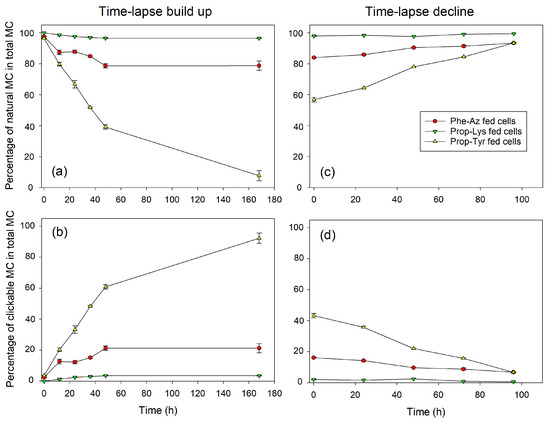
Figure 1.
Mean (±SE) proportion of natural and clickable MC in total MC (cellular fraction, composed of four MC structural variants: DAsp-MC-YR, MC-YR, DAsp-MC-LR, MC-LR) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the build up (a,b) or decline (c,d) of azide- or alkyne-modified MC in M. aeruginosa strain Hofbauer. Control cells were grown and processed under identical conditions but without non-AA substrate and could not show any clickable MC synthesis.
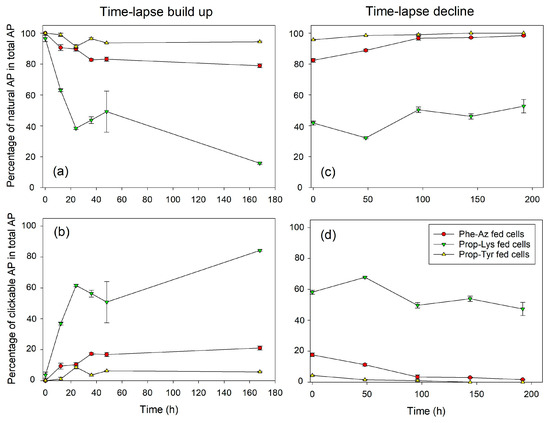
Figure 2.
Mean (±SE) proportion of natural and clickable AP in total AP (cellular fraction, composed of four AP structural variants: unknown AP, AP-C, AP-B, AP-A) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the build up (a,b) or decline (c,d) of azide- or alkyne modified AP in P. agardhii strain no371/1. Control cells were grown and processed under identical conditions but without non-AA substrate and could not show any clickable MC synthesis.
Thus, intracellular synthesis of modified MC/AP was observed, reaching a plateau rather fast (24–48 h). The efficiency of incorporation of various non-AAs into the MC/AP molecule varied between cyanobacteria strains and/or synthesis pathways. Conversely, for time-lapse decline, the proportion of modified MC/AP decreased linearly. However, clickable MC/AP peptides were removed from the cells at a slower rate than their appearance during build up.
2.3. Dissolved Fraction of Clickable and Natural MC/AP Peptides
In general, the ranking orders observed in the proportion of clickable MC/AP in the intracellular fraction, i.e., MC-Prop-TyrR > MC-Phe-AzR > MC-Prop-LysR in M. aeruginosa or AP-Prop-Lys > AP-Phe-Az > AP-Prop-Tyr in P. agardhii was the same as those found in the extracellular fraction (Table 3). The percentages of clickable and natural MC peptides in dissolved vs. intracellular fraction were compared for both time-lapse build up and decline experiments, and linear regression curves were calculated (Supplementary Figures S7 and S8). While the slope of linear curves ranged between y = 1.52x, y = 0.52x, and y = 1.19x, the coefficient of variation of regression curves varied between R2 = 0.94, 0.39, 0.8 for MC-Prop-TyrR, MC-Prop-LysR, and MC-Phe-AzR, respectively. Thus, clickable MC proportion in the medium was found to be linearly related to the respective intracellular proportion and linear relationships did not differ significantly from natural MC-LR in slope (i.e., ANCOVA, interaction between grouping and covariate p = 0.08, 0.64, 0.86 for MC-Prop-TyrR, MC-Prop-LysR, and MC-Phe-AzR, respectively). Using the equal slope model in a one-way analysis of covariance (ANCOVA), a significant difference in intercept was found when comparing the adjusted group means of MC-Prop-TyR vs. MC-LR (p < 0.001).
In contrast to MC variants in M. aeruginosa, for clickable AP variants AP-Prop-Tyr and AP-Phe-Az in P. agardhii, the slope of linear regression curves was dissimilar when comparing with regression curves for natural AP-A (p = 0.58, p = 0.15 for AP-Prop-Tyr and AP-Phe-Az, respectively), i.e., AP-Prop-Tyr (R2 = 0.48), y = 2.41x and AP-Phe-Az (R2 = 0.51), y = 0.84x. For the most abundant AP-Prop-Lys, a significantly reduced slope of the regression line between intracellular vs. extracellular fraction was found (R2 = 0.83, y = 0.91x) when compared with natural AP-A (R2 = 0.85, y = 2.13x), as recorded from the same peptide extract (ANCOVA, p = 0.008). In other words, the proportion of AP-Prop-Lys in the medium was found to be underrepresented by a factor of 2 when compared to the proportion of natural AP-A (Supplementary Figure S8). In summary, all modified MC/AP peptides could be quantified in the extracellular fraction, suggesting a more constant release procedure similar to their natural congeners.
2.4. Relationship of Clickable MC/AP Peptide Synthesis to Growth in Cyanobacteria Strains During Time-Lapse Experiments
Because of the variation in growth rate between non-AA treatments, it was important to find out whether growth or cell division rate could be related to clickable MC/AP net production rate. Thus, clickable MC or AP net production rates were calculated from intracellular MC-LR equivalents in ng/mL or AP-B equivalents in ng/mL by ln(x + 1) transformation and were plotted against the growth rate (day−1) calculated from OD (Figure 3).
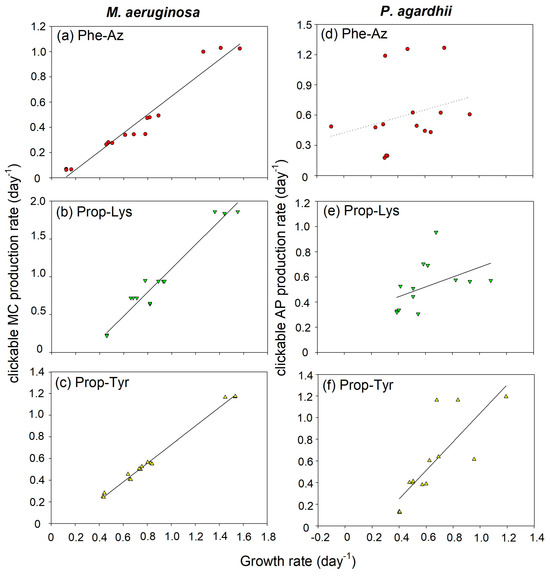
Figure 3.
Relationship between growth rate (day−1) and (a–c) the clickable MC net production rate (day−1) in M. aeruginosa strain Hofbauer (calculated from ln(x + 1) MC-LR equivalents in ng/mL) and (d–f) the clickable AP net production rate (day−1) in P. agardhii strain no371/1 (calculated from ln(x + 1) AP-B equivalents in ng/mL) during time-lapse experiments using feeding of non-natural amino acids (non-AAs) in order to observe the build up of azide- or alkyne-modified MC/AP. Details of linear regression curves are as follows: (a) MC-Phe-AzR (y = −0.08 + 0.73x, R2 = 0.95, p < 0.0001), (b) MC-Prop-LysR (y = −0.47 + 1.58x, R2 = 0.95, p < 0.0001), (c) MC-Prop-TyrR (y = −0.13 + 0.86x, R2 = 0.99, p < 0.0001), (d) AP-Phe-Az (not significant, p = 0.33), (e) AP-Prop-Lys (y = 0.29 + 0.39x, R2 = 0.21, p = 0.099), (f) AP-Prop-Tyr (y = −0.28 + 1.32x, R2 = 0.65, p = 0.0005), where y is MC/AP production rate (day−1) and x is growth rate (day−1).
Overall, the highest clickable MC/AP production rates were observed in the most rapidly growing cultures. In M. aeruginosa, for all non-AA treatments, a significant linear relationship between cell growth and the daily production of MC-Phe-AzR, MC-Prop-LysR and, MC-Prop-TyrR was found (Figure 3a–c). In P. agardhii, for AP-Phe-Az, the coefficient of determination R2 was lowest and no significant slope in regression was found (p = 0.33), while for AP-Prop-Lys and AP-Prop-Tyr, marginally or highly significant linear relationships between cell growth and the daily production were obtained (Figure 3d–f). Thus, for Phe-Az in P. agardhii, the synthesis of AP-Phe-Az was not coupled to growth, implying that cells stopped growing after the synthesis of AP-Phe-Az. Notably, for M. aeruginosa, the integration of Phe-Az into MC reduced the growth, but had less impact on the principal relationship between clickable MC synthesis rate and the (reduced) daily cellular growth. In summary, an overall influence of cell growth on clickable MC/AP peptide net production via non-AA integration was observed, implying that the principal relationship between cell growth and MC/AP production is still applicable.
2.5. Relationship of Clickable MC/AP Peptide Content Decline and Growth in Cyanobacteria Strains During Time-Lapse Experiments
During the time-lapse decline experiments, overall, a linear decrease in clickable (intracellular) MC/AP in proportion to total MC/AP was observed over time. Using growth rates calculated from OD (see above), the theoretical decline of modified MC/AP recorded at T0 was calculated and compared to the observed decline as reported above (Figure 1d and Figure 2d). If the decline in intracellular new MC/AP peptide is significantly faster than the theoretical decline through dilution by cellular growth, one may conclude on clickable MC/AP peptide breakdown or active vs. passive transport processes.
Two-Way Repeated Measures ANOVA was used to compare the observed and the theoretical estimates in the proportion of intracellular MC (Figure 4a–c) and AP (Figure 4d–f). The time factor was always found to be significant (p < 0.001) (i.e., 24–96 h for MC in M. aeruginosa). For MC-Phe-AzR, a much faster decline than predicted from the lowest cell division rate (Supplementary Figure S1) was observed, indicating potential cell lysis. In contrast, for MC-Prop-LysR and the most abundant MC-Prop-TyrR no significant difference between theoretical and observed proportions (the grouping factor) was detected (p > 0.05). Thus, even for the most abundant MC-Prop-TyrR, a linear decline during time course, mostly via cell division rate or cell growth, was concluded.
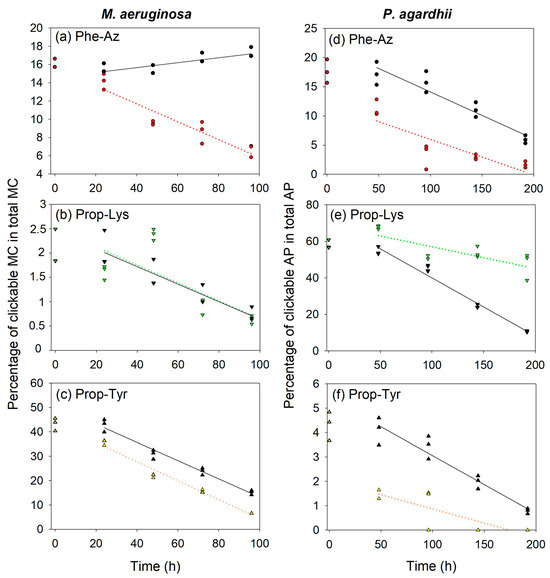
Figure 4.
Proportion of individual clickable MC in M. aeruginosa (a–c) or clickable AP in P. agardhii (d–e) in total MC/AP (cellular fraction) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the decline of (a) MC-Phe-AzR, (b) MC-Prop-LysR, (c) MC-Prop-TyrR in M. aeruginosa, or (d) AP-Phe-Az, (e) AP-Prop-Lys, (f) AP-Prop-Tyr in P. agardhii strain no371/1. Using growth rates, the theoretical decline of clickable MC/AP was calculated (black symbols, straight line) and compared to the observed decline (colored symbols, dotted line). Note that the scale at the y-axis is different, as production efficiency differs between non-AAs (Figure 1 and Figure 2).
Analogously, for clickable APs, the time factor showed a significant decrease (i.e., 48–196 h in P. agardhii). For AP-Phe-Az and AP-Prop-Tyr, the corresponding theoretical AP proportions tended to be slightly increased, which was found to be marginally significant (p = 0.02–0.03). For AP-Prop-Lys, the intracellular proportion appeared to decline less rapidly when compared with other clickable MC/AP peptides (Figure 4e). The slower decline could be related to the fact that AP-Prop-Lys in the medium was found to be underrepresented 2-fold when compared to the proportion of natural AP-A (Supplementary Figure S8). In summary, the overall linear decrease in clickable MC/AP points to a rather constant decline via dilution by growth instead of a regulated or induced release during the synthesis process.
2.6. Fate of Non-Natural Amino Acids (Non-AAs) During Build Up and Decline
During all time-lapse build up and decline experiments, the quantity of the different non-AAs in the cellular and dissolved fraction was recorded in parallel to LC-MS peptide analysis. Using the same chromatographic separation conditions, the non-AAs, Phe-Az, Prop-Lys, and Prop-Tyr eluted at 5.4 min [M + H]+ 207.5, 3.4 min [M + H]+ 229.5, and 5.2 min [M + H]+ 220.5, respectively. Typically, during the entire build up experiments, non-AAs were detected in the cellular fractions (Supplementary Figure S9). For M. aeruginosa, Prop-Tyr and Phe-Az decreased towards the end but remained available in cells, while Prop-Lys increased three-fold till the end of the experiment (168 h) (Supplementary Figure S9a). Similarly, for P. agardhii, Prop-Lys was increasing linearly by up to three-fold till the end of the experiment (168 h), while Prop-Tyr and Phe-Az were not detected.
Dissolved quantities of non-AA were, in general, one to two magnitudes higher than cellular contents and tended to decrease during build up experiments (Supplementary Figure S10). In contrast, during signal decline, dissolved non-AAs were not detected in M. aeruginosa. In P. agardhii, dissolved non-AAs occurred at T0 but were reduced to undetectable from 48 h onwards (Supplementary Figure S10). Thus, at T0, non-AAs were removed by washing through centrifugation and resuspension of the cells in BG11 medium. In contrast, during build up experiments, non-AAs remained dissolved in the medium and were available in unlimited amounts.
3. Discussion
This study is the first to track MC/AP synthesis by using promiscuous adenylation (A) domains in non-ribosomal peptide synthesis (NRPS), allowing for the incorporation of clickable non-AAs into the peptide products. While the results of visualization using the clickable amino acids as fluorescent tags will be reported in a forthcoming paper, the monitoring of modified MC/AP synthesis alone can lead to a better understanding of natural product synthesis in bloom-forming cyanobacteria. The following insights were obtained and will be discussed:
3.1. Variable Efficiency of Clickable MC/AP Production Using Different Non-AAs
In this study, a rapid increase in most clickable MC/AP peptides was observed, reaching a plateau after 24−48 h (Supplementary Figures S5 and S6). Not all non-AAs were integrated into the new modified MC/AP peptide with the same efficiency. For M. aeruginosa, MC-Prop-TyrR was produced much more efficiently than MC-Prop-LysR or MC-Phe-AzR. For P. agardhii, AP-Prop-Lys was produced most efficiently, while both AP-Prop-Tyr and AP-Phe-Az were produced in lower amounts. In general, it is well-known that cyanobacteria uptake amino acids from the media [11]. Accordingly, during build up experiments, non-AAs have been detected in the cellular fraction consistently (Supplementary Figure S9) and it is unlikely that non-AA intracellular availability caused the variable efficiency of integration into the peptide product.
Instead, it is known that A domains in NRPS are the key enzymes enabling the integration of unnatural AA into the growing peptide [17,18]. However, activation of non-AA must be compatible with the essential subsequent catalytic reactions, such as the step of peptide bond formation, i.e., condensation (C) domains are also known to exhibit moderate to high substrate specificity [19]. For example, an erroneously loaded non-AA by an A domain, which is not accepted by the responsible C domain, would prevent peptide bond formation, resulting in the build up of intermediate peptide products [20]. Thus, it is likely that for M. aeruginosa strain Hofbauer, the most efficient integration of Prop-Tyr into pos. 2 of MC-YR or MC-LR can explain its highest share in total MC pool (>80%). In a similar vein, it can be assumed that for P. agardhii strain no371/1, the most efficient integration of Prop-Lys into AP peptide product is likely related to the highest share of AP-Prop-Lys (>80%) in the total AP pool.
3.2. Effects of Non-AAs on Cell Division or Growth in Study Organisms
In this study, three different non-AAs were tested in order to better describe the influence on MC/AP production and growth. It is generally understood that non-AAs with alkyne or azide moieties react very specifically with their reaction partner, i.e., without side reactions or negative influence on native biochemical processes and growth. The specificity of the reaction is one criterion to be considered as a bio-orthogonal reaction [21]. In this study, Phe-Az addition distinctly reduced growth for M. aeruginosa as well as P. agardhii from the beginning (Table 1 and Table 2, Supplementary Figures S1 and S2). It should be noted that Phe-Az (4-azido-L-phenylalanine) is one of the most versatile non-AAs in common use, i.e., it is widely applied for protein labeling through bio-orthogonal reactions [22]. On the other hand, Phe-Az has also been described as reactive via decomposition that may cause intracellular damage by reactive nitrenes, causing structural rearrangement of proteins or even crosslinking [23]. Nevertheless, despite this inherent reactivity, a significant positive relationship between MC-Phe-AzR production and cell division rate was observed for M. aeruginosa (Figure 3a). In contrast, as previously observed [16], P. agardhii cells appeared more negatively affected, filaments were shorter and looked disintegrated and only a weak relationship between AP-Phe-Az production and cell division could be observed (Figure 3d). In general, a positive relationship between the production of MC (and other toxins) and cell division rate in individual strains has been postulated by authors Orr and Jones [6], suggesting that although MCs and other toxins (such as anatoxin a) are secondary metabolites the MC net production depends primarily on the cellular growth rate. Thus, environmental conditions influence MC production rather indirectly via the cellular growth rate. Although this significant interdependence is not understood on a cellular level, it fits to the overall consistent MC production in strain cultures observed regularly under laboratory conditions, even when severe physiological stress conditions have been investigated [24]. In summary, a high degree of interdependence between non-AA containing clickable MC/AP production and cellular growth has been observed, implying that the addition of non-AAs (even if available in excess amounts) did not overrule the tight coupling between primary and secondary metabolism during maximum growth rate conditions.
3.3. Decrease in Targeted MC/AP Content During Time-Lapse Experiment
Overall, a rather linear decrease in the proportion of clickable MC/AP was observed over time. Cells were grown as clonal strain cultures at maximum growth rate under the specified semi-continuous culture conditions, following the turbidostatic principle [25]. Thus, the influence of physiological stress or cell lysis was considered of minor importance [26]. At the onset of this study, it was hypothesized that if the decline in clickable MC/AP labels would occur faster than expected from the theoretical decline through dilution by cellular growth, one may infer intracellular processing or active/passive release processes into the medium. This was observed for MC-Phe-AzR, implying that the decline of clickable MC occurred actively/passively under conditions when cell division was very much reduced. However, the linearity of the decrease implies a constant release process instead of a regulated or induced release in the course of the synthesis pathway (Figure 4).
On the other hand, the intracellular proportion of clickable AP-Prop-Lys appeared to decline less rapidly than observed for the MC-Prop-LysR peptides (Figure 4). Accordingly, the clickable AP-Prop-Lys peptides occurred with a lower proportion in the medium when compared to its natural congener AP-A (Supplementary Figures S7 and S8). These results would imply that clickable APs were retained in the cell to some extent and not readily released into the medium as was observed for natural AP-A. Whether such a delay in the release of clickable APs into the medium can be confirmed by subcellular localization via peptide labeling remains to be proved. Preliminary results on peptide labeling using the above-mentioned chemo-selective reaction revealed an increased concentration of signal intensity near the membrane region of the cells (author’s unpublished data). If such a mechanism of intracellular compartmentalization can be confirmed via imaging analysis it would constitute an interesting observation on the intracellular enrichment of AP peptide before its release into the medium.
4. Conclusions
In summary, chemical-analytical methods quantitatively show the targeted synthesis of MC/AP peptides by incorporating an alkyne or azide side chain, which then enables visualization via chemo-selective reaction for linking fluorophores. The variable efficiency in clickable MC/AP production probably can be linked to the varying degree of promiscuity in accepting non-AA substrates by corresponding NRPS. The tight coupling between primary metabolism, i.e., cell division and growth with secondary metabolism, i.e., MC synthesis, can explain why clickable MC production is still related to growth even if the growth rate is significantly reduced (i.e., by Phe-Az). Conversely, the rather linear decrease in clickable MC/AP proportion in total MC/AP content with growth implies that dilution by growth is still the major responsible force in intra- and extracellular dynamics of clickable MC/AP synthesis.
5. Materials and Methods
5.1. Study Organisms and Growth Conditions
Precultures of clonal Microcystis aeruginosa strain Hofbauer (isolated from Neusiedlersee, Austria in 1982) and Planktothrix agardhii no371/1 (isolated from Moose Lake, Alberta, Canada in 2005) were maintained in a semi-continuous culture condition in BG11 medium [27] at 20 °C and photosynthetically active radiation of 50 µE m−2 s−1 (using fluorescent lamps Master TL-D 90 De Luxe 36W/965, Philips, Vienna, Austria) following the turbidostatic principle [3]. Under our culture conditions, M. aeruginosa strain Hofbauer grew in single cells only, while P. agardhii strain no371/1 continued to grow in long filaments (e.g., hundreds of micrometers in length). All experiments were performed in the same dedicated climate chamber under the specified conditions. During the experiments (i.e., years 2021–2022), the temperature ranged between 18.4 and 20.6 °C (mean 19.9 ± 0.1, n = 31), while irradiation varied on the shelf between 36 and 58 µE m−2 s−1 (mean 47 ± 2, n = 31). In general, precultures (40 mL culture volume in 250 mL transparent cell culture flasks, CytoOne T-75, Starlab, Vienna, Austria) were started from cultures maintained at maximum growth rate conditions until they reached an optical density (OD600nm) = 0.1 (1 cm cuvette, BioSpectrometer basic, Eppendorf AG, Vienna, Austria), then diluted to OD = 0.01 and 0.025 for M. aeruginosa and P. agardhii, respectively. As described, OD = 0.01 was equal to 662 ± 265 filaments/mL of P. agardhii strain no371/1 or 62,866 ± 29,059 cells/mL of M. aeruginosa strain Hofbauer [9]. Growth rates were calculated from OD or dry weight (DW) using the following formula: µ (day−1) = (lnODt+1 − lnODt)/Δt, where ln is the natural logarithmic function and Δt is the time in days.
The overall workflow is presented in Figure 5. For both time-lapse signal build up experiments, three technical replicates were inoculated for each time point (T0–T5) under identical conditions. At T0, treatments were supplemented once with L-isomeric non-AAs, i.e., either 4-azidophenylalanine (Phe-Az), (Carl Roth, Karlsruhe, Germany), N-propargyloxy-carbonyl-L-lysine (Prop-Lys), (Sichem, Bremen, Germany), or O-propargyl-L-Tyrosine (Prop-Tyr), (Iris Biotech GmbH, Marktredtwitz, Germany) (Figure 6), diluted in 20 mM NaOH, resulting in 0.05 mM final non-AA concentration (adding 100 µL of stock solution of 20 mM non-AA solution into 40 mL of culture volume). Control cell cultures were grown in the absence of non-AAs but supplemented with 100 µL of 20 mM NaOH only. For T0, the cells were harvested directly after adding the non-AA to the medium within 1 h. In order to observe the incorporation of the non-AA into the cyanobacterial peptides, culture flasks were harvested every 12 h for the first 48 h, with a final harvesting at 168 h.
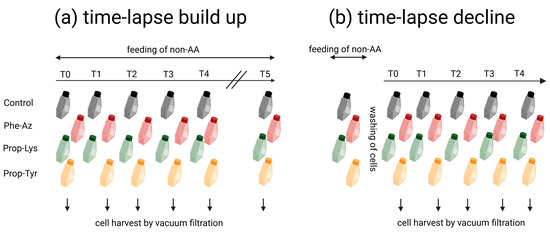
Figure 5.
Workflow of time-lapse experiments using pulsed feeding of non-AAs for real-time observation of clickable MC/AP synthesis in bloom-forming cyanobacteria (the workflow was the same for both M. aeruginosa and P. agardhii): (a) time-lapse build up experiments; (b) time-lapse decline experiments. Created with BioRender.com. Note that the labeling of clickable peptides via chemo-selective reaction with fluorophore and high-resolution microscopy and flow-cytometry analysis using Alexa Fluor488 will be reported in a follow-up article.
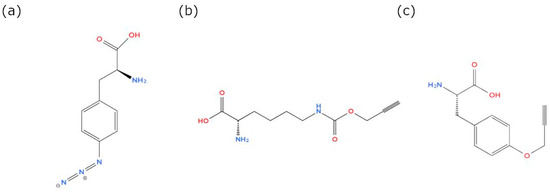
Figure 6.
Chemical structures of non-AA molecules used for clickable microcystin (MC) synthesis in M. aeruginosa and for clickable anabaenopeptin (AP) synthesis in P. agardhii: (a) 4-Azido-L-phenylalanine (Phe-Az, MW 206.20 g/mol), (b) N-Propargyl-L-Lysine (Prop-Lys, MW 228.25 g/mol), (c) O-Propargyl-L-tyrosine (Prop-Tyr, MW 219.24 g/mol).
Analogously, to observe the time-lapse decline of the peptide signal, the precultures were diluted to an OD = 0.01 and 0.05 for M. aeruginosa and P. agardhii, respectively. Three technical replicates were inoculated per strain and grown for 48 h in the presence or absence of the three non-Aas, as described above (Phe-Az, Prop-Lys, Prop-Tyr). At T0, following the 48 h incubation, cultures were centrifuged at 4000 rpm for 5 min and washed with sterile BG11 medium under sterile conditions. Cultures were centrifuged again at 4000 rpm for 5 min, resuspended in fresh non-AA free sterile BG11 medium, and transferred to fresh culture flasks. For T0, cells were harvested directly after washing and transferring into fresh BG11 medium within 1 h. Time points T1-T4 were every 24 h for M. aeruginosa and every 48 h for P. agardhii. The manipulation of cells was carried out at room temperature.
5.2. Cell Harvesting and Fixation
At T0–T4 (T5), cells from culture flasks (40 mL culture volume) were harvested via low-vacuum filtration using pre-weighed glass fiber filters (GF/C, Ø = 2.5 cm, ROTILABO® Typ CR261, Carl Roth, Karlsruhe, Germany). The filters with the collected biomass were dried using a vacuum centrifuge at RT for 4 h and dry weight was determined. Filters were stored frozen (−20 °C) until peptide extraction.
For each sample, the extracellular MC and AP peptide concentrations were determined from GF/C filtrate using standard solid phase extraction (SPE) [28]. Briefly, C18 columns (Sep-Pak® Vac tC18 cartridge, 1 cc/100 mg, 37–55 µm, Waters Corporation, Vienna, Austria) were conditioned with 3 mL of 100% methanol and equilibrated using 3 mL of BG11 medium according to the manufacturer’s instructions. Subsequently, the GF/C filtrate was allowed to flow through the column at one drop per second and the dry column was stored frozen (−20 °C) until eluting with 1 mL of 80% (v/v) methanol for HPLC-MS analysis.
5.3. Peptide Extraction and HPLC-MS Analysis
The intracellular peptide content was extracted from the dried biomass using aqueous methanol on ice, as described previously [3]. In brief, the filters carrying the algal biomass were dispersed with a pistil in the reaction tube and incubated in aqueous 50% (v/v) methanol (1.5 mL volume). The extracts were shaken on ice (30 min) and centrifuged, and the clear supernatant was transferred into new 2 mL reaction tubes, which were evaporated to dryness in a vacuum concentrator (no heating). This procedure was repeated two times to ensure efficient peptide extraction. After complete evaporation of the solvent (4.5 mL), the extract was stored frozen.
For Liquid Chromatography—Mass Spectrometry (LC-MS), the dried extracts were resuspended using 150 µL of 100% methanol. After complete resuspension of the pellet, 150 µL of MilliQ (MQ) water was added. Extracts were centrifuged and from the clear supernatants, 100 µL was injected into the HPLC-DAD (HP1100, Agilent, Vienna, Austria). Peptides were chromatographically separated using a LiChrospher® 100, octyldecyl silane (ODS), 5 µm particle size, LiChroCART® 250-4 HPLC cartridge system (Merck, Darmstadt, Germany) using a linear water/acetonitrile (0.05% trifluoroacetic acid) gradient from 80:20 to 50:50 in 45 min at a flow rate of 1 mL/min and at 30 °C oven temperature.
In order to quantify the extracellular peptide composition, the C18 columns were eluted with 1 mL of 80% (v/v) methanol, evaporated to dryness using the vacuum concentrator, and re-dissolved in 110 µL of methanol. Finally, 100 µL was injected under identical chromatographic conditions as for the intracellular peptide extract. Recovery tests using 50 ng of MC-RR [M + H]+ 1038.5, MC-YR [M + H]+ 1045.5, and MC-LR [M + H]+ 995.5 analytical standards (Simris Biologics, Berlin, Germany) revealed recoveries of 83 ± 3% (SE), 64 ± 11%, 73 ± 8% using UV DAD at 240 nm, respectively. For MS peak area (base peak chromatograms, BPC), the recovery results obtained in parallel were 48 ± 11%, 160 ± 36%, and 61 ± 13% for MC-RR, MC-YR, and MC-LR, respectively.
The HPLC system was coupled to an ESI-MS (Electrospray Ionization Mass Spectrometer) ion trap (amaZon SL Ion Trap MS, Bruker Daltonik, Bremen, Germany) and operated in positive ionization mode. Nitrogen and helium were used as sheath gas and collision gas, respectively (43 psi, 9 L/min, 250 °C), with 5 kV capillary voltage. MC and AP variants were assigned according to their retention time, protonated mass [M + H]+, and automatically recorded fragmentation patterns for MCs [29,30] or APs [31,32]. Under these conditions, the limit of detection for analytical standards MC-RR [M + H]+ 1038.5, MC-YR [M + H]+ 1045.5, and MC-LR [M + H]+ 995.5 was 10 ng per injection (Simris Biologics, Berlin, Germany). To compare the total MC/AP content, all peptides were quantified in equivalents of either AP-B [M + H]+ 837.5 or MC-LR. According to the linear regression curves y = 1 × 107x + 1 × 108 and y = 2 × 107x − 4 × 108, y was the integrated area in the base peak chromatogram (BPC), and x was ng of AP-B (1–270 ng) or MC-LR (1–180 ng) injected [9]. In order to test peptide quantification, both UV peak area (at 240 nm for MC or 210 nm for AP) and MS peak area (base peak chromatograms, BPC or extracted ion chromatograms, EIC) were compared: For MC total BPC (EIC) area vs. total UV area correlated by R2 = 0.90 (y = 6 × 10−7x) and R2 = 0.98 (y = 8 × 10−7x), signal build up, and signal decline, respectively, where y is UV240 peak area and x is BPC peak area. Similarly, for AP total BPC (EIC) area vs. total UV area correlated by R2 = 0.95 (y = 6 × 10−7x) and R2 = 0.96 (y = 4 × 10−7x) for signal build up and signal decline, respectively (Supplementary Figure S11). To quantify MC/AP modification, the BPC (EIC) peaks were manually integrated to express the individual peak area in the percentage of the total manually integrated peak area. For LC-MS the Bruker Compass DataAnalysis version 4.2 was used.
5.4. Statistical Analysis
For comparing growth data and clickable MC/AP contents, the two-way repeated measures analysis of variance (two-way RM ANOVA) was used (grouping factor: three non-AA treatments and one control; time factor: T0–T4 (T5), three replicates). The time factor was always found to be significant (p < 0.001). Assumptions on normality (Shapiro-Wilk) or equality of variance (Brown-Forsythe) were sometimes not met (p < 0.05). Since ANOVA test results are generally considered to be robust if sample sizes between treatments are equal, the statistical test results were still reported. For the pairwise multiple comparison procedure, the Bonferroni t-test (p < 0.05) was used.
For comparing growth with peptide production, cellular growth rates (calculated from OD at T0) were calculated using the formula y = (ln (x)t+1 − ln (x)t0)/Δt where y is the growth rate (day−1), x is the OD, and Δt is the time in days (t + 1 − t0). Analogously, the clickable MC or AP net production rates were calculated from intracellular MC/AP using the following formula: clickable MC/AP production rate (day−1) = (ln (x + 1)t+1 − ln (x + 1)t0)/Δt, where x is either MC-LR equivalents in ng/mL or AP-B equivalents in ng/mL. The linear regression curves (Figure 3 and Figure 4) were calculated and tested according to standard linear regression analysis.
The linear regression curves between the proportion of intracellular (independent variable) vs. extracellular (dependent) MC/AP peptides were compared in terms of slope using one-way analysis of covariance (ANCOVA) via the equality of slopes model. which tested the assumption that there is no interaction between the grouping factor (i.e., modified MC/AP vs. natural MC/AP) and the covariate (intracellular proportion of corresponding MC/AP peptide). If the equal slope model was passed, the equality of the intercepts was tested by comparing the adjusted group means via pairwise multiple comparison (Holm-Sidak, p < 0.05). All tests were performed using Sigma plot version 14.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxins16120526/s1. Supplementary Figure S1: Mean (±SE) optical density of strain cultures of (a,c) M. aeruginosa strain Hofbauer and (b,d) P. agardhii strain no371/1 during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) under maximum growth rate conditions in order to observe the build up (a,b) or decline (c,d) of azide- or alkyne-modified MC or AP peptides; Supplementary Figure S2: Mean (±SE) dry weight (mg/mL) of strain cultures of (a,c) M. aeruginosa Hofbauer and (b,d) P. agardhii no371/1 during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) under maximum growth rate conditions in order to observe the build up (a,b) or decline (c,d) of azide- or alkyne-modified MC or AP peptides; Supplementary Figure S3: Mean (±SE) cellular concentration of (a,d) total, (b,e) natural, and (c,f) clickable MC in ng of MC-LR equivalents/mL (composed of four MC structural variants: DAsp-MC-YR, MC-YR, DAsp-MC-LR, MC-LR) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the increase (c) or decline (f) in azide- or alkyne-modified MC in M. aeruginosa strain Hofbauer; Supplementary Figure S4: Mean (±SE) cellular concentration of (a,d) total, (b,e) natural, and (c,f) clickable AP in ng of AP-B equivalents/mL (composed of four AP structural variants: AP (unknown), AP-C, AP-B, AP-A) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the increase (c) or decline (f) in azide- or alkyne-modified AP in P. agardhii strain no371/1; Supplementary Figure S5: Mean (±SE) cellular content of natural and clickable MC in percentage of control in M. aeruginosa strain Hofbauer (ng MC-LR equiv./mg DW, composed of four MC structural variants: DAsp-MC-YR, MC-YR, DAsp-MC-LR, MC-LR) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the build up (a,b) or decline (c,d) of azide- or alkyne-modified MC. (Control = cells grown and processed under identical conditions but without non-AA substrate); Supplementary Figure S6: Mean (±SE) cellular proportion of natural and clickable AP in total AP in percentage of control in P. agardhii strain no371/1 (ng AP B equiv./mg DW, composed of four AP structural variants: AP unknown, AP-C, AP-B, AP-A) during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the build up (a,b) or decline (c,d) of azide- or alkyne-modified AP. (Control = cells grown and processed under identical conditions but without non-AA substrate); Supplementary Figure S7: Percentage of clickable and natural MC peptides in dissolved vs. intracellular fraction in M. aeruginosa strain Hofbauer during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the build up (open symbols) or decline (closed symbols) of azide- or alkyne-modified MC. Details of linear regression curves: (a) MC-Prop-TyrR (R2 = 0.94), y = 1.5186x; (b) MC-LR (Prop-Tyr fed cells), (R2 = 0.87), y = 0.856x; (c) MC-Prop-LysR (R2 = 0.39), y = 0.523x; (d) MC-LR (Prop-Lys fed cells), (R2 = 0.99), y = 0.9467x; (e) MC-Phe-AzR (R2 = 0.8), y = 1.1864x; (f) MC-LR (Phe-Az fed cells), (R2 = 0.91), y = 0.9185x, where y was percentage of extracellular MC in total extracellular MC and x was percentage of intracellular MC in total intracellular MC. The dotted line represents the 1:1 reference line; Supplementary Figure S8: Percentage of clickable and natural AP peptides in dissolved vs. intracellular fraction in P. agardhii strain no371/1 during time-lapse experiments using pulsed feeding of non-natural amino acids (non-AAs) in order to observe the build up (open symbols) or decline (closed symbols) of azide- or alkyne-modified AP. Details of linear regression curves: (a) AP-Prop-Tyr (R2 = 0.48), y = 2.4073x; (b) AP-A (Prop-Tyr fed cells), (R2 = 0.9), y = 1.78x; (c) AP-Prop-Lys (R2 = 0.83), y = 0.913x; (d) AP-A (Prop-Lys fed cells), (R2 = 0.85), y = 2.133x; (e) AP-Phe-Az (R2 = 0.51), y = 0.842x; (f) AP-A (Phe-Az fed cells), (R2 = 0.91), y = 2.542x, where y was percentage of extracellular AP in total extracellular AP and x was percentage of intracellular AP in total intracellular AP. The dotted line represents the 1:1 reference line; Supplementary Figure S9: Mean ± SE cellular content of non-AAs (Phe-Az, Prop-Lys, Prop-Tyr) eluting at 3.4 min (Prop-Lys), 5.2 min (Prop-Tyr), 5.4 min (Phe-Az) quantified in µg of non-AA per mg of DW in peptide extract during time-lapse experiments using pulsed feeding of non-AAs in order to observe the build up (a,c) or decline (b,d) of azide- or alkyne-modified MC/AP peptide in M. aeruginosa (a,b) and P. agardhii (c,d); Supplementary Figure S10: Mean ± SE dissolved quantity of non-AAs (Phe-Az, Prop-Lys, Prop-Tyr) eluting at 3.4 min (Prop-Lys), 5.2 min (Prop-Tyr), 5.4 min (Phe-Az) quantified in µg of non-AA per mg of DW during time-lapse experiments using pulsed feeding of non-AAs in order to observe the build up (a,c) or decline (b, d) of azide- or alkyne-modified MC/AP peptide in M. aeruginosa (a,b) and P. agardhii (c,d). Explanation for missing data in (a): No SPE measurements were performed at T1 (12 h); Supplementary Figure S11: Comparison of total MC (a) or (b) AP peptide quantification using either MS peak area (base peak chromatograms, BPC or extracted ion chromatograms, EIC) or UV peak area (at 240 nm for MC or 210 nm for AP) during all time-lapse experiments. In (b), three outliers (white triangles) were omitted from calculating the regression line for build up experiments.
Author Contributions
Conceptualization, R.K.; Methodology, R.K. and R.M.A.; Validation, R.K.; Investigation, R.K. and R.M.A.; Resources, R.K.; Writing—original draft, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Science Fund (FWF) grant number P32193.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data on growth (OD, DW) and intra- vs. extracellular MC/AP peptides have been submitted to BioStudies [33] under the Accession number S-BSST1672 (DOI 10.6019/S-BSST1672) and S-BSST1681 (DOI 10.6019/S-BSST1681) (https://www.ebi.ac.uk/biostudies, accessed on 27 November 2024).
Acknowledgments
We are most grateful to Anneliese Wiedlroither and David Schuler for their assistance during experiments. Dan Enke (Simris Biologics GmbH) in Berlin provided MC-RR, MC-YR, MC-LR analytical standards. The comments of two anonymous reviewers and the suggestions of the assistant editor improved the readability of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chorus, I.; Welker, M. (Eds.) Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021; ISBN 9781003081449. [Google Scholar]
- Fastner, J.; Humpage, A.R. 2.1 Hepatotoxic cyclic peptides—Microcystins and nodularins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Welker, M., Eds.; Taylor & Francis: Boca Raton, FL, USA; World Health Organization: Geneva, Switzerland, 2021; pp. 21–52. ISBN 9781003081449. [Google Scholar]
- Kosol, S.; Schmidt, J.; Kurmayer, R. Variation in peptide net production and growth among strains of the toxic cyanobacterium Planktothrix spp. Eur. J. Phycol. 2009, 44, 49–62. [Google Scholar] [CrossRef]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef]
- Rohrlack, T.; Hyenstrand, P. Fate of intracellular microcystins in the cyanobacterium Microcystis aeruginosa (Chroococcales, Cyanophyceae). Phycologia 2007, 46, 277–283. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Long, B.M.; Jones, G.J.; Orr, P.T. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 2001, 67, 278–283. [Google Scholar] [CrossRef]
- Wiedner, C.; Visser, P.M.; Fastner, J.; Metcalf, J.S.; Codd, G.A.; Mur, L.R. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003, 69, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Kurmayer, R.; Entfellner, E.; Weisse, T.; Offterdinger, M.; Rentmeister, A.; Deng, L. Chemically labeled toxins or bioactive peptides show a heterogeneous intracellular distribution and low spatial overlap with autofluorescence in bloom-forming cyanobacteria. Sci. Rep. 2020, 10, 2781. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Zotina, T.; Köster, O.; Jüttner, F. Photoheterotrophy and light-dependent uptake of organic and organic nitrogenous compounds by Planktothrix rubescens under low irradiance. Freshw. Biol. 2003, 48, 1859–1872. [Google Scholar] [CrossRef]
- Kurmayer, R.; Dittmann, E.; Fastner, J.; Chorus, I. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in Lake Wannsee (Berlin, Germany). Microb. Ecol. 2002, 43, 107–118. [Google Scholar] [CrossRef]
- Mikalsen, B.; Boison, G.; Skulberg, O.M.; Fastner, J.; Davies, W.; Gabrielsen, T.M.; Rudi, K.; Jakobsen, K.S. Natural variation in the microcystin synthetase operon mcyABC and impact on microcystin production in Microcystis strains. J. Bacteriol. 2003, 185, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, G.; Philmus, B.; Hemscheidt, T.; Kurmayer, R. Genetic variation of adenylation domains of the anabaenopeptin synthesis operon and evolution of substrate promiscuity. J. Bacteriol. 2011, 193, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Entfellner, E.; Frei, M.; Christiansen, G.; Deng, L.; Blom, J.; Kurmayer, R. Evolution of anabaenopeptin peptide structural variability in the cyanobacterium Planktothrix. Front. Microbiol. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Morón-Asensio, R.; Schuler, D.; Wiedlroither, A.; Offterdinger, M.; Kurmayer, R. Differential labeling of chemically modified peptides and lipids among cyanobacteria Planktothrix and Microcystis. Microorganisms 2021, 9, 1578. [Google Scholar] [CrossRef]
- Kries, H.; Wachtel, R.; Pabst, A.; Wanner, B.; Niquille, D.; Hilvert, D. Reprogramming nonribosomal peptide synthetases for “clickable” amino acids. Angew. Chem. Int. Ed. 2014, 53, 10105–10108. [Google Scholar] [CrossRef]
- Kaljunen, H.; Schiefelbein, S.H.H.; Stummer, D.; Kozak, S.; Meijers, R.; Christiansen, G.; Rentmeister, A. Structural elucidation of the bispecificity of A domains as a basis for activating non-natural amino acids. Angew. Chem. Int. Ed. 2015, 54, 8833–8836. [Google Scholar] [CrossRef] [PubMed]
- Lautru, S.; Challis, G.L. Substrate recognition by nonribosomal peptide synthetase multi-enzymes. Microbiology 2004, 150, 1629–1636. [Google Scholar] [CrossRef]
- Yeh, E.; Kohli, R.M.; Bruner, S.D.; Walsh, C.T. Type II thioesterase restores activity of a NRPS module stalled with an aminoacyl-S-enzyme that cannot be elongated. Chembiochem 2004, 5, 1290–1293. [Google Scholar] [CrossRef]
- Lang, K.; Chin, J.W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 2014, 114, 4764–4806. [Google Scholar] [CrossRef]
- Richardson, M.B.; Brown, D.B.; Vasquez, C.A.; Ziller, J.W.; Johnston, K.M.; Weiss, G.A. Synthesis and explosion hazards of 4-Azido-l-phenylalanine. J. Org. Chem. 2018, 83, 4525–4536. [Google Scholar] [CrossRef]
- Schock, M.; Bräse, S. Reactive & efficient: Organic azides as cross-linkers in Material Sciences. Molecules 2020, 25, 1009. [Google Scholar] [CrossRef] [PubMed]
- Kurmayer, R. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J. Phycol. 2011, 47, 200–207. [Google Scholar] [CrossRef]
- Kohl, J.-G.; Nicklisch, A. Ökophysiologie der Algen: Wachstum und Ressourcennutzung; Akademie-Verlag: Berlin, Germany, 1988; ISBN 9783055003158. [Google Scholar]
- Rzymski, P.; Klimaszyk, P.; Jurczak, T.; Poniedziałek, B. Oxidative stress, programmed cell death and microcystin release in Microcystis aeruginosa in response to Daphnia grazers. Front. Microbiol. 2020, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R. Isolation and purification of cyanobacteria. In Methods in Enzymology; Packer, L., Glazer, A.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 167, pp. 3–27. ISBN 978-0-12-182068-8. [Google Scholar]
- Dean, J.R. Extraction Methods for Environmental Analysis; John Wiley: Chichester, NY, USA, 1998; ISBN 0-471-98287-3. [Google Scholar]
- Fastner, J.; Erhard, M.; Döhren, H. von. Determination of oligopeptide diversity within a natural population of Microcystis spp. (cyanobacteria) by typing single colonies by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 2001, 67, 5069–5076. [Google Scholar] [CrossRef]
- Welker, M.; Fastner, J.; Erhard, M.; von Döhren, H. Applications of MALDI-TOF MS analysis in cyanotoxin research. Environ. Toxicol. 2002, 17, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Błaszczyk, A.; Meriluoto, J.; Cegłowska, M.; Mazur-Marzec, H. Structures and activity of new anabaenopeptins produced by Baltic Sea cyanobacteria. Mar. Drugs 2016, 14, 8. [Google Scholar] [CrossRef]
- Okumura, H.S.; Philmus, B.; Portmann, C.; Hemscheidt, T.K. Homotyrosine-containing cyanopeptolins 880 and 960 and anabaenopeptins 908 and 915 from Planktothrix agardhii CYA 126/8. J. Nat. Prod. 2009, 72, 172–176. [Google Scholar] [CrossRef]
- Sarkans, U.; Gostev, M.; Athar, A.; Behrangi, E.; Melnichuk, O.; Ali, A.; Minguet, J.; Rada, J.C.; Snow, C.; Tikhonov, A.; et al. The BioStudies database-one stop shop for all data supporting a life sciences study. Nucleic Acids Res. 2018, 46, D1266–D1270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).