What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review
Abstract
1. Introduction
2. Losses Due to FHB in Wheat Production
3. The Causing Agents
| Species | Geographical Incidence | Mycotoxin | |
|---|---|---|---|
| North/Center | South | ||
| F. graminearum Schwabe | +++ | +++ | DON, NIV, ZEN, AcDON, FUS |
| F. avenaceum (Fr.) Sacc. | +++ | ++ | MON, BEA, ENS |
| F. culmorum (W.G. Smith) Sacc. | +++ | ++ | DON, ZEN, ZOH, NIV |
| F. poae (Peck) Wollenw. | +++ | + | NIV, BEA, DAS, FUS, ENS |
| F. equiseti (Corda) Sacc. | +++ | + | DAS, ZEN, ZOH |
| F. tricinctum (Corda.) Sacc. | + | + | MON |
| F. cerealis (Cooke) Sacc. (F. crookwellense, Burgess, Nelson and Tous.) | + | ± | NIV, FUS, ZEN, ZOH |
| F. sporotrichioides Scherb. | + | ± | T2, HT2, T2ol, NEO |
| F. acuminatum Ellis and Everhart | ± | ± | T2, NEO |
| F. subglutinans Wollenw. and Reinking | ± | - | MON |
| F. solani (Mart.) Sacc. | ± | - | - |
| F. oxysporum Schlecht. | ± | - | - |
4. Multi Mycotoxin Contamination in Freshly Harvested and Stored Wheat
5. Multitoxin Presence, an Emerging Problem
6. Resistance to Fusarium spp.
7. What Is FHB Resistance?
8. Phenotyping, How to Evaluate Resistance
9. Breeding Aspects
- Based on the visual symptoms, the FHB resistance (disease index DI) should be evaluated.
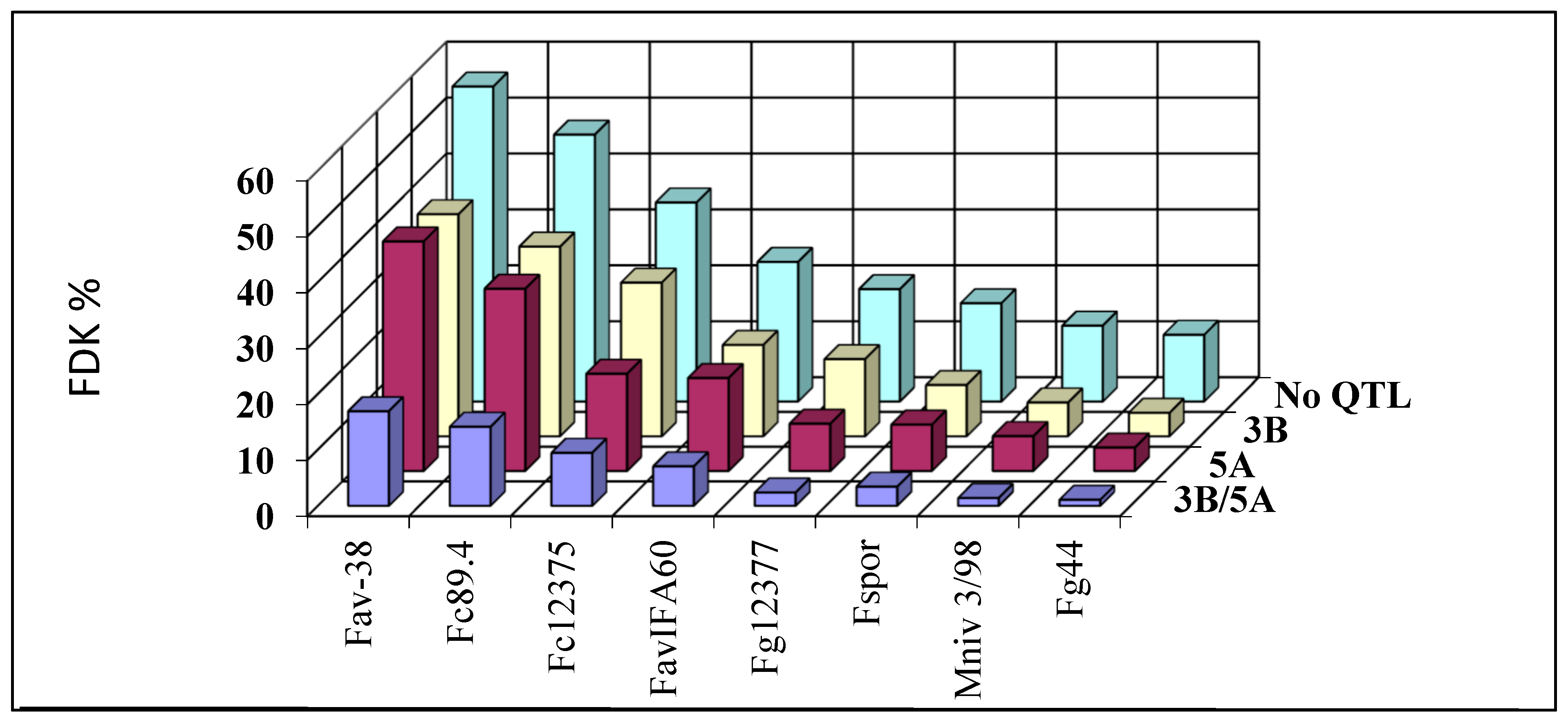
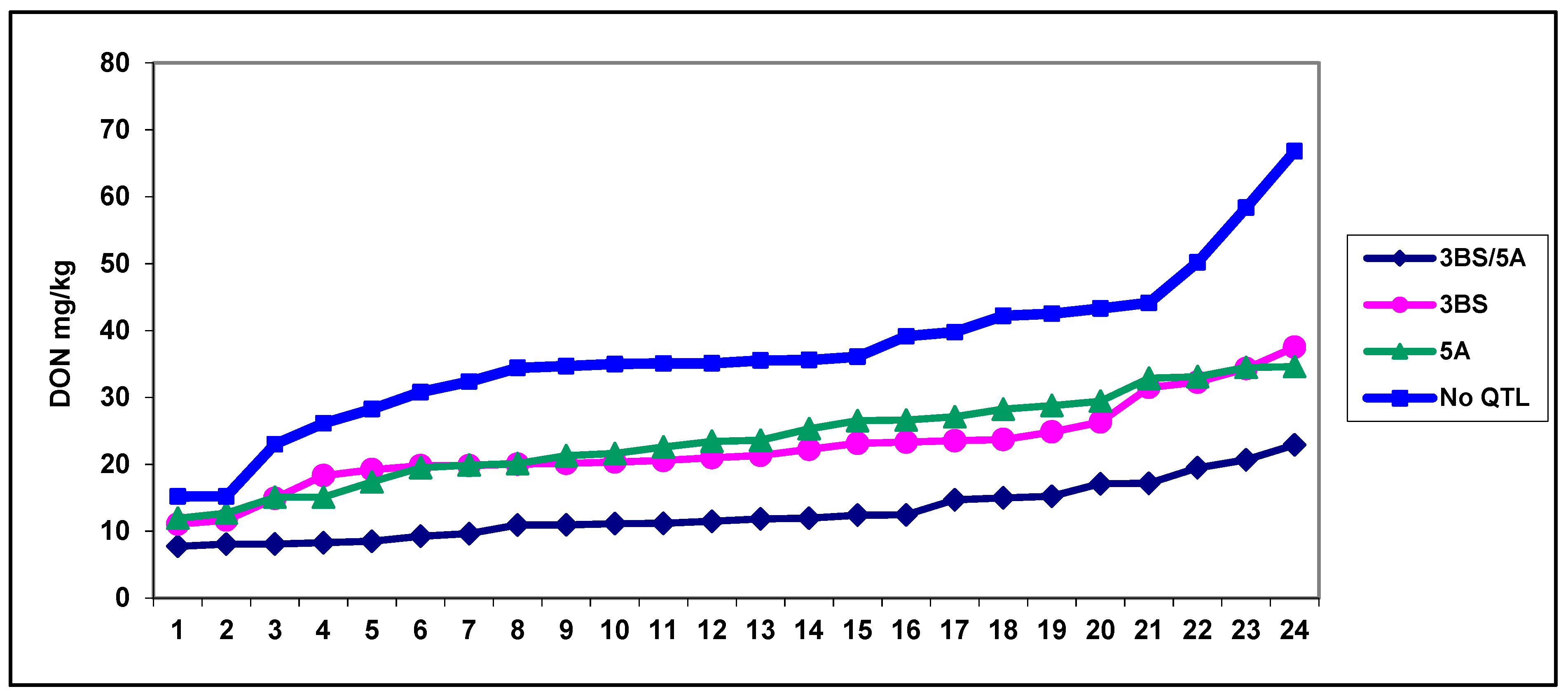
- As DI and FDK are often differentially regulated, and DON contamination correlates significantly more closely with FDK, its evaluation is important. The results were confirmed by Wu et al. [136]. They evaluated a highly sophisticated neural network methodology to evaluate FDK to improve the accuracy of genomic selection. As phenotypic and genetic correlations were lower, we achieved up to r = 0.80 or better in some tests [89], though we do not feel that this method should be changed.
- The genetic regulation of DON is often different to that of the DI and FDK, and the official limits apply to the toxins (DON, etc.), while the food safety risk cannot be evaluated without toxin measurements. There are qPCR markers to measure fungal mass in the wheat tissue for different Fusarium spp.; therefore, a multispecies approach is possible. As DON contamination for one percent FDK showed ten-fold or higher differences, a forecast of the toxin contamination on genotype level is problematic; even closeness of the correlation is medium and significant. Further research is necessary to see clearly see the situation we face.
10. Influence of Resistance to Agronomic Traits
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcDON | acetyl deoxynivalenol (3 + 5 variants) |
| AFB1 | aflatoxin B1 |
| AFB2 | aflatoxin B2 |
| AFM1 | aflatoxin M1 |
| ALT | altenuene |
| AME | alternariol methyl ether |
| AOH | alternaliol |
| aw | water activity |
| CIT | citrinin |
| DAS | Diacetoxyscirpenol |
| DH-CIT | dihidrostrinone |
| DI | disease index for FHB |
| DON | deoxynivalenol |
| ENS | enniatins |
| FAO | Food and Agriculture Organization of the United Nations |
| FB1 | fumonisin B1 |
| FB2 | fumonisin B2 |
| FDK | Fusarium damaged kernels, visual |
| FHB | Fusarium head blight |
| FUM | fumonisin |
| FUS fusarenon-XLC/MSMS | liquid chromatograph + tandem mass spectrometer |
| LC-MS/MS | High pressure liquid chromatography with mass spectrometry |
| LC-HRMS | High pressure liquid chromatography with high resolution mass spectrometry |
| MMT | million metric tons |
| NEO | neosolaniol |
| NIV | nivalenol |
| OTA | ochratoxin A |
| OTB | ochratoxin B |
| QTL | quantitative trait locus |
| T2ol | T-2 tetraol |
| ZEN | zearalenon |
| ZOH | zearaneols (a,b) |
References
- Ács, K.; Lehoczki-Krsjak, S.; Varga, M.; Kótai, C.; Ács, E.; Salgó, A.; Mesterházy, Á. Reduction of deoxynivalenol (DON) contamination by improved fungicide use in wheat. Part 3. Reduction of Fusarium head blight and influence on quality traits in cultivars with different resistance levels. Eur. J. Plant Pathol. 2018, 151, 21–38. [Google Scholar] [CrossRef]
- McMullen, M. Impacts of Fusarium head blight on the North American agricultural community: The power of one disease to catapult change. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 484–503. [Google Scholar]
- McMullen, M.; Bergstrom, G.C.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D.; Sarrocco, S.; Esteban, P.; Vicente, I.; et al. A Unified Effort to Fight an Enemy of Wheat and Barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Chen, T.-R.; Luo, Y.-J.; Zhao, P.-T.; Jia, H.-Y.; Ma, Z.-Q. Overexpression of TaJRL53 enhances the Fusarium head blight resistance in wheat. Acta Agron. Sin. 2021, 47, 19–29. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Zhao, X.; Zhang, S.; Ma, H. Exploring and applying genes to enhance the resistance to Fusarium head blight in wheat. Front. Plant Sci. 2022, 13, 1026611. [Google Scholar] [CrossRef]
- Acs, K.; Varga, M.; Szekeres, A.; Salgo, A.; Lantos, C.; Bekes, F.; Pauk, J.; Mesterhazy, A. Alteration of Carbohydrate Metabolism in Fusarium Infected Wheat Kernels Treated with Fungicides and Its Relation to Baking Technological Parameters and Deoxynivalenol Contamination. Agriculture 2023, 13, 868. [Google Scholar] [CrossRef]
- Mesterházy, Á. Types and components of resistance against Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Mesterhazy, A. Updating the Breeding Philosophy of Wheat to Fusarium Head Blight (FHB): Resistance Components, QTL Identification, and Phenotyping—A Review. Plants 2020, 9, 1702. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Szabo-Hever, A.; Lehoczki-Krsjak, S.; Varga, M.; Purnhauser, L.; Pauk, J.; Lantos, C.; Mesterhazy, A. Differential influence of QTL linked to Fusarium head blight, Fusarium-damaged kernel, deoxynivalenol contents and associated morphological traits in a Frontana-derived wheat population. Euphytica 2014, 200, 9–26. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Yao, J.; Cheng, S. Breeding for the resistance to Fusarium head blight of wheat in China. Front. Agric. Sci. Eng. 2019, 6, 251–264. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.M.; Komoróczy, R. Nature of resistance of wheat to Fusarium head blight and deoxynivalenol contamination and their consequences for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- He, X.; Singh, P.K.; Dreisigacker, S.; Singh, S.; Lillemo, M.; Duveiller, E. Dwarfing Genes Rht-B1b and Rht-D1b Are Associated with Both Type I FHB Susceptibility and Low Anther Extrusion in Two Bread Wheat Populations. PLoS ONE 2016, 11, e0162499. [Google Scholar] [CrossRef]
- Skinnes, H.; Semagn, K.; Tarkegne, Y.; Marøy, A.G.; Bjørnstad, Å. The inheritance of anther extrusion in hexaploid wheat and its relationship to Fusarium head blight resistance and deoxynivalenol content. Plant Breed. 2010, 129, 149–155. [Google Scholar] [CrossRef]
- Xu, K.; He, X.; Dreisigacker, S.; He, Z.; Singh, P.K. Anther Extrusion and Its Association with Fusarium Head Blight in CIMMYT Wheat Germplasm. Agronomy 2020, 10, 47. [Google Scholar] [CrossRef]
- Miedaner, T. Breeding wheat and rye for resistance to Fusarium diseases. Plant Breed. 1997, 116, 201–220. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Buerstmayr, M.; Schweiger, W.; Steiner, B. Breeding for resistance to head blight caused by Fusarium spp. in wheat. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2014, 9, 236–276. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Gilbert, J.; Tekauz, A. Review: Recent developments in research on fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 2000, 22, 1–8. [Google Scholar] [CrossRef]
- Mesterhazy, A. Breeding for resistance to Fusarium head blight in wheat. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA; Chichester, UK; Oxford, UK, 2014; pp. 189–208. [Google Scholar]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Snijders, C. Resistance in wheat to Fusarium infection and trichothecene formation. Toxicol. Lett. 2004, 153, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Tóth, B.; Sziebert, D. Toxintermelő gombák okozta növénybetegségek búzában és kukoricában (Wheat and maize diseases caused by toxigenic fungi and their toxins in Hungary, 2012–2017, in Hungarian). Magy. Kukoricaklub Kukorica Barométer Különszám 2019, 1–72. [Google Scholar]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Tefera, G. Microbial Function on Climate Change—A Review. Open J. Environ. Biol. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Wilson, W.W.; Nganje, W.E.; Kaitibie, S.; Leistritz, F.L. Economic impacts of Fusarium head blight in wheat and barley: 1993–2001. Agribus. Appl. Econ. Rep. 2004, 538, 53. Available online: https://www.researchgate.net/publication/23514192_Economic_impacts_of_Fusarium_head_blight_in_wheat_and_barley_1993-2001 (accessed on 15 December 2022).
- Mesterházy, Á.; Oláh, J.; Popp, J. Losses in the Grain Supply Chain: Causes and Solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef]
- Mannon, J.; Johnson, E. Fungi down on the farm. New Sci. 1985, 105, 12–16. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. Part A 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Mylona, K.; Sulyok, M.; Magan, N. Relationship between environmental factors, dry matter loss and mycotoxin levels in stored wheat and maize infected with Fusarium species. Food Addit. Contam. Part A 2012, 29, 1118–1128. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Kamani, M.H.; Fakhri, Y.; Coppa, C.F.S.C.; de Oliveira, C.A.F.; Sant’Ana, A.S. Changes in masked forms of deoxynivalenol and their co-occurrence with culmorin in cereal-based products: A systematic review and meta-analysis. Food Chem. 2019, 294, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals In northern Europe and Asia. J. Plant Pathol. 2010, 92, 7–18. [Google Scholar]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- O’Donnell, K.; McCormick, S.P.; Proctor, R.H.; Ward, T.J.; Doehring, G.; Geiser, D.M.; Alberts, J.F.; Rheeder, J.P. Toxigenic Fusarium species: Identity and mycotoxicology. Mycologia 2018, 110, 1058–1080. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Abbas, A.; Gavrilova, O.; Gagkaeva, T. Molecular Variation and Phylogeny within Fusarium avenaceum and Related Species. Diversity 2022, 14, 574. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; van der Fels-Klerx, H. Regional prediction of multi-mycotoxin contamination of wheat in Europe using machine learning. Food Res. Int. 2022, 159, 111588. [Google Scholar] [CrossRef]
- Mesterházy, Á. Fusarium species of wheat in South Hungary, 1970–1983. Cereal Res. Comm. 1984, 12, 167–170. [Google Scholar]
- Zhang, H.; van der Lee, T.; Waalwijk, C.; Chen, W.Q.; Xu, J.; Xu, J.H.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef] [PubMed]
- Booth, E. The Genus Fusarium; CMI: Kew, Surrey, UK, 1971. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell: Oxford, UK, 2005; 388p, ISBN 978-0-8138-1919-8. [Google Scholar]
- Refai, M.; Hassa, A.; Hamed, M. Monograph on the Genus Fusarium; Cairo University: Giza, Egypt, 2015; p. 275. [Google Scholar]
- Alkadri, D.; Nipoti, P.; Döll, K.; Karlovsky, P.; Prodi, A.; Pisi, A. Study of Fungal Colonization of Wheat Kernels in Syria with a Focus on Fusarium Species. Int. J. Mol. Sci. 2013, 14, 5938–5951. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur. J. Plant Path 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Xu, X.-M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blighting wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Del Ponte, E.M.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Alisaac, E.; Mahlein, A.-K. Fusarium Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Alvarenga, A.A.A.; Ouchi, J.C.M.I.; Martínez, C.C.C.; Mendes, J.M.; Colmán, A.A.; Ríos, D.F.; Arrua, P.D.; Guerreño, C.A.B.; Kohli, M.M.; Ramírez, M.L.; et al. Trichothecene Genotype Profiling of Wheat Fusarium graminearum Species Complex in Paraguay. Toxins 2022, 14, 257. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Ward, T.J.; Van Coller, G.J.; Flett, B.; Lamprecht, S.C.; O’donnell, K.; Viljoen, A. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genet. Biol. 2011, 48, 914–920. [Google Scholar] [CrossRef]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, X.; Xu, J.H.; Shi, J.R.; Lee, Y.-W.; Chen, X.Y.; Li, Y.P.; Mokoena, M.P.; Olaniran, A.O. Analysis of Fusarium graminearum Species Complex from Freshly Harvested Rice in Jiangsu Province (China). Plant Dis. 2020, 104, 2138–2143. [Google Scholar] [CrossRef]
- Amarasinghe, C.; Sharanowski, B.; Fernando, W.D. Molecular Phylogenetic Relationships, Trichothecene Chemotype Diversity and Aggressiveness of Strains in a Global Collection of Fusarium graminearum Species. Toxins 2019, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Ward, T.J.; Geiser, D.M.; Kistler, H.C.; Aoki, T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 2004, 41, 600–623. [Google Scholar] [CrossRef]
- Vaughan, M.; Backhouse, D.; Del Ponte, E. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Global warming and increasing maize cultivation demand comprehensive efforts in disease and insect resistance breeding in north-western Europe. Plant Pathol. 2021, 70, 1032–1046. [Google Scholar] [CrossRef]
- Senatore, M.T.; Prodi, A.; Tini, F.; Balmas, V.; Infantino, A.; Onofri, A.; Cappelletti, E.; Oufensou, S.; Sulyok, M.; Covarelli, L.; et al. Different diagnostic approaches for the characterization of the fungal community and Fusarium species complex composition of Italian durum wheat grain and correlation with secondary metabolite accumulation. J. Sci. Food Agric. 2023, 103, 4503–4521. [Google Scholar] [CrossRef]
- Varga, J.; Tóth, B.; Mesterhazy, A.; Teren, J.; Fazekas, B. Mycotoxigenic fungi and mycotoxins in foods and feeds in Hungary. In An Overview on Toxigenic Fungi and Mycotoxins in Europe; Logrieco, A., Visconti, A., Eds.; Kluwer Acedemic Publishers: Dordecht, The Netherlands, 2004; pp. 123–139. ISBN 1-4020-2645-5. [Google Scholar]
- Portell, X.; Verheecke-Vaessen, C.; Torrelles-Ràfales, R.; Medina, A.; Otten, W.; Magan, N.; García-Cela, E. Three-Dimensional Study of F. graminearum Colonisation of Stored Wheat: Post-Harvest Growth Patterns, Dry Matter Losses and Mycotoxin Contamination. Microorganisms 2020, 8, 1170. [Google Scholar] [CrossRef]
- Nešić, K.; Habschied, K.; Mastanjević, K. Modified Mycotoxins and Multitoxin Contamination of Food and Feed as Major Analytical Challenges. Toxins 2023, 15, 511. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Britzi, M.; Zakin, V.; Kostyukovsky, M.; Trostanetsky, A.; Quinn, E.; Sionov, E. Rapid Detection and Identification of Mycotoxigenic Fungi and Mycotoxins in Stored Wheat Grain. Toxins 2017, 9, 302. [Google Scholar] [CrossRef]
- Anonymous. GRDC: Grownotes, Grain Storage. 2017. 86p. ISBN 978-1-921779-42-8. Available online: https://grdc.com.au/grain-storage-grownotes/ (accessed on 5 May 2019).
- Anonymous. GRDC: Grownotes, Grain Storage. 2020. Available online: https://grdc.com.au/resources-and-publications/grownotes/technical-manuals/grain-storage (accessed on 5 May 2019).
- Berthiller, F.; Brera, C.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; Macdonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M.; et al. Developments in mycotoxin analysis: An update for 2015–2016. World Mycotoxin J. 2017, 10, 5–29. [Google Scholar] [CrossRef]
- Tittlemier, S.; Cramer, B.; DeRosa, M.; Lattanzio, V.; Malone, R.; Maragos, C.; Stranska, M.; Sumarah, M. Developments in mycotoxin analysis: An update for 2021–2022. World Mycotoxin J. 2023, 16, 3–24. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, W.; Zhao, X.; Cao, H.; Fan, K.; Meng, J.; Nie, D.; Wu, Y.; Han, Z. Universal screening of 200 mycotoxins and their variations in stored cereals in Shanghai, China by UHPLC-Q-TOF MS. Food Chem. 2022, 387, 132869. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Hernández-Córdoba, M.; Viñas, P. High-resolution mass spectrometry for the determination of mycotoxins in biological samples. A review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Lemming, E.W.; Montes, A.M.; Schmidt, J.; Cramer, B.; Humpf, H.-U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents—Possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Tolosa, J.; Graziani, G.; Gaspari, A.; Ritieni, A. Target analysis and retrospective screening of mycotoxins and pharmacologically active substances in milk using an ultra-high-performance liquid chromatography/high-resolution mass spectrometry approach. J. Dairy Sci. 2020, 103, 1250–1260. [Google Scholar] [CrossRef]
- Beccari, G.; Caproni, L.; Tini, F.; Uhlig, S.; Covarelli, L. Presence of Fusarium Species and Other Toxigenic Fungi in Malting Barley and Multi-Mycotoxin Analysis by Liquid Chromatography—High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 4390–4399. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef]
- Foerster, C.; Ríos-Gajardo, G.; Gómez, P.; Muñoz, K.; Cortés, S.; Maldonado, C.; Ferreccio, C. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins 2021, 13, 439. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Toscano, P.; Crisci, A.; Battilani, P.; Brera, C. Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data. Toxins 2021, 13, 695. [Google Scholar] [CrossRef]
- Schmidt, J.; Cramer, B.; Turner, P.C.; Stoltzfus, R.J.; Humphrey, J.H.; Smith, L.E.; Humpf, H.-U. Determination of Urinary Mycotoxin Biomarkers Using a Sensitive Online Solid Phase Extraction-UHPLC-MS/MS Method. Toxins 2021, 13, 418. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Osteresch, B.; Almeida, A.; Cramer, B.; Humpf, H.-U.; Viegas, C. Enniatin B and ochratoxin A in the blood serum of workers from the waste management setting. Mycotoxin Res. 2018, 34, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Valenti, I.; Tini, F.; Sevarika, M.; Agazzi, A.; Beccari, G.; Bellezza, I.; Ederli, L.; Grottelli, S.; Pasquali, M.; Romani, R.; et al. Impact of enniatin and deoxynivalenol co-occurrence on plant, microbial, insect, animal, and human systems: Current knowledge and future perspectives. Toxins 2023, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Simpson, D.R.; Wilson, A.H.; Chandler, E.; Thomset, M. Detection and differentiation of trichothecene and enniatin-producing Fusarium species on small-grain cereals. Eur. J. Plant Path 2004, 110, 503–514. [Google Scholar] [CrossRef]
- Tolosa, J.; Graziani, G.; Gaspari, A.; Chianese, D.; Ferrer, E.; Mañes, J.; Ritieni, A. Multi-Mycotoxin Analysis in Durum Wheat Pasta by Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Toxins 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nie, D.; Fan, K.; Yang, J.; Guo, W.; Meng, J.; Zhao, Z.; Han, Z. A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit. Rev. Food Sci. Nutr. 2020, 60, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- György, A.; Tóth, B.; Varga, M.; Mesterhazy, A. Methodical Considerations and Resistance Evaluation against Fusarium graminearum and F. culmorum Head Blight in Wheat. Part 3. Susceptibility Window and Resistance Expression. Microorganisms 2020, 8, 627. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Gyorgy, A.; Varga, M.; Toth, B. Methodical Considerations and Resistance Evaluation against F. graminearum and F. culmorum Head Blight in Wheat. The Influence of Mixture of Isolates on Aggressiveness and Resistance Expression. Microorganisms 2020, 8, 1036. [Google Scholar] [CrossRef]
- Toth, B.; Gyorgy, A.; Varga, M.; Mesterhazy, A. The Influence of the Dilution Rate on the Aggressiveness of Inocula and the Expression of Resistance against Fusarium Head Blight in Wheat. Plants 2020, 9, 943. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Varga, M.; György, A.; Lehoczki-Krsjak, S.; Tóth, B. The role of adapted and non-adapted resistance sources in breeding resistance of winter wheat to Fusarium head blight and deoxynivalenol contamination. World Mycotoxin J. 2018, 11, 539–557. [Google Scholar] [CrossRef]
- Mesterházy, Á. Breeding for resistance against FHB in wheat. In Mycotoxin Reduction in Grain Chains: A Practical Guide; Logrieco, A.F., Visconti, A., Eds.; Blackwell-Wiley: Ames, IA, USA; Chichester, UK; Oxford, UK, 2014; pp. 189–208. ISBN 978-0-8138-2083-5. [Google Scholar]
- van Eeuwijk, F.A.; Mesterházy, Á.; Kling, C.I.; Ruckenbauer, P.; Saur, L.; Bürstmayr, H.; Lemmens, M.; Maurin, M.; Snijders, C.H.A. Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum and F. nivale, using a multiplicative model for interaction. Theor. Appl. Genet. 1995, 90, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. Eur. J. Plant Pathol. 2002, 108, 675–684. [Google Scholar] [CrossRef]
- Miedaner, T.; Flamm, C.; Oberforster, M. The importance of Fusarium head blight resistance in the cereal breeding industry: Case studies from Germany and Austria. Plant Breed. 2024, 143, 1–15. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Kászonyi, G.; Varga, M.; Tóth, B.; Varga, J. Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. Eur. J. Plant Path 2005, 112, 267–281. [Google Scholar] [CrossRef]
- Mesterházy, A.; Lehoczki-Krsjak, S.; Varga, M.; Szabó-Hevér, Á.; Tóth, B.; Lemmens, M. Breeding for FHB Resistance via Fusarium Damaged Kernels and Deoxynivalenol Accumulation as Well as Inoculation Methods in Winter Wheat. Agric. Sci. 2015, 6, 970–1002. [Google Scholar] [CrossRef]
- Ismail, Y.; McCormick, S.; Hijri, M. A Fungal Symbiont of Plant-Roots Modulates Mycotoxin Gene Expression in the Pathogen Fusarium sambucinum. PLoS ONE 2011, 6, e17990. [Google Scholar] [CrossRef]
- Pokrzywa, P.; Cieślik, E.; Surma, M. Effect of storage conditions on the formation of type A and B trichothecenes in cereal products. J. Neurol. Sci. 2019, 26, 260–265. [Google Scholar] [CrossRef]
- Llorens, A.; Mateo, R.; Hinojo, M.J.; Logrieco, A.; Jimenez, M. Influence of the Interactions among Ecological Variables in the Characterization of Zearalenone Producing Isolates of Fusarium spp. Syst. Appl. Microbiol. 2004, 27, 253–260. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Tóth, B.; Kászonyi, G.; Bartók, T.; Varga, J.; Mesterházy, A. Common resistance of wheat to members of the Fusarium graminearum species complex and F. culmorum. Plant Breed. 2008, 127, 1–8. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol co-localizes with a major QTL for Fusarium head blight resistance in wheat. MPMI 2005, 18, 1318–1324. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Lemmens, M.; ·Hartl, L.; ·Doldi, L.; Steiner, B.; Stierschneider, M.; Ruckenbauer, P. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theor. Appl. Genet. 2002, 104, 84–91. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Steiner, B.; Hartl, L.; Griesser, M.; Angerer, N.; Lengauer, D.; Miedaner, T.; Schneider, B.; Lemmens, M. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. II. Resistance to fungal penetration and spread. Theor. Appl. Genet. 2003, 107, 503–508. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Buerstmayr, H.; Tóth, B.; Lehoczki-Krsjak, S.; Szabó-Hevér, A.; Lemmens, M. An improved strategy for breeding FHB resistant wheat must include Type I resistance. In Proceedings of the 5th Canadian Workshop on Fusarium Head Blight, Winnipeg, MB, Canada, 27–30 November 2007; pp. 51–66. [Google Scholar]
- Liu, Z.Z.; Wang, Z.Y. Improved scab resistance in China: Source of resistance and problems. In Wheat for Nontraditional, Warm Areas: A Proceedings of the International Conference, July 29-August 3, 1990, Foz Do Iguaçu, Brazil; Saunders, D.A., Ed.; CIMMYT: México-Veracruz, Mexico, 1991. [Google Scholar]
- Ye, G.; Smith, K.F. Marker-assisted gene pyramiding for inbred line development: Practical applications. Int. J. Plant Breed. 2008, 2, 11–22. [Google Scholar]
- Arthur, J.C. Wheat scab. Indiana Agricultural. Exp. Stn. Bull. 1891, 36, 129–138. [Google Scholar]
- Bai, G.-H.; Sharen, G. Variation in Fusarium graminearum and cultivar resistance in wheat scab. Plant Dis. 1996, 80, 975–979. [Google Scholar] [CrossRef]
- Yang, Z.P.; Gilbert, J.; Fedak, G.G.; Somers, D.J. Genetic characterization of QTL associated with resistance to Fusarium head blight in a doubled-haploid spring wheat population. Genome 2005, 48, 187–196. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Wagner, C.; Nosenko, T.; Omony, J.; Steiner, B.; Nussbaumer, T.; Mayer, K.F.X.; Buerstmayr, H. Fusarium head blight resistance in European winter wheat: Insights from genome-wide transcriptome analysis. BMC Genom. 2021, 22, 470. [Google Scholar] [CrossRef]
- Kang, Z.; Buchenauer, H. Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum. Physiol. Mol. Plant Path 1999, 55, 275–288. [Google Scholar] [CrossRef]
- Atanasoff, D. Fusarium-blight (scab) of wheat and other cereals. J. Agric. Res. 1920, 20, 12–40. [Google Scholar]
- Shaner, G. Epidemiology of Fusarium head blight of small grains cereals in North America. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2003; pp. 84–119. [Google Scholar]
- Strange, R.N.; Smith, H. A fungal growth stimulant in anthers which predisposes wheat to attack by Fusarium graminearum. Physiol. Plant Pathol. 1971, 1, 141–150. [Google Scholar] [CrossRef]
- Strange, R.N.; Majer, J.R.; Smith, H. The isolation and identification of choline and betaine as the two major components in anthers and wheat germ that stimulate Fusarium graminearum in vitro. Physiol. Plant Path 1974, 4, 277–290. [Google Scholar] [CrossRef]
- Strange, R.N.; Smith, H.; Majer, J.R. Choline, one of two fungal growth stimulants in anthers responsible for the susceptibility of wheat to Fusarium graminearum. Nature 1972, 238, 103–104. [Google Scholar] [CrossRef]
- Strange, R.N.; Smith, H. Partial purification, and properties of a fungal growth stimulant in anthers which predisposes wheat to attack by Fusarium graminearum. J. General Microbiol. 1976, 63, 141–150. [Google Scholar]
- Singh, L.; Anderson, J.A.; Chen, J.; Gill, B.S.; Tiwari, V.K.; Rawat, N. Development and Validation of a Perfect KASP Marker for Fusarium Head Blight Resistance Gene Fhb1 in Wheat. Plant Pathol. J. 2019, 35, 200–207. [Google Scholar] [CrossRef]
- Zwart, R.S.; Muylle, H.; Van Bockstaele, E.; Roldán-Ruiz, I. Evaluation of genetic diversity of Fusarium head blight resistance in European winter wheat. Theor. Appl. Genet. 2008, 117, 813–828. [Google Scholar] [CrossRef]
- Szabó-Hevér, A.; Lehoczki-Krsjak, S.; Tóth, B.; Purnhauser, L.; Buerstmayr, H.; Steiner, B.; Mesterházy, A. Identification and validation of fusarium head blight and Fusarium-damaged kernel QTL in a Frontana/Remus DH mapping population. Can. J. Plant Pathol. 2012, 34, 224–238. [Google Scholar] [CrossRef]
- Aviles, A.C.; Harrison, S.A.; Arceneaux, K.J.; Brown-Guidera, G.; Mason, R.E.; Baisakh, N. Identification of QTLs for Resistance to Fusarium Head Blight Using a Doubled Haploid Population Derived from Southeastern United States Soft Red Winter Wheat Varieties AGS 2060 and AGS 2035. Genes 2020, 11, 699. [Google Scholar] [CrossRef]
- Gaire, R.; Brown-Guedira, G.; Dong, Y.; Ohm, H.; Mohammadi, M. Genome-Wide Association Studies for Fusarium Head Blight Resistance and Its Trade-Off with Grain Yield in Soft Red Winter Wheat. Plant Dis. 2021, 105, 2435–2444. [Google Scholar] [CrossRef]
- Bonin, C.M.; Kolb, F.L. Resistance to Fusarium Head Blight and Kernel Damage in a Winter Wheat Recombinant Inbred Line Population. Crop Sci. 2009, 49, 1304–1312. [Google Scholar] [CrossRef]
- Hart, L.P.; Pestka, J.J.; Liu, M.T. Effect of kernel development and wet periods on production of deoxynivalenol in wheat infected with Gibberella zeae. Phytopathology 1984, 74, 1415–1418. [Google Scholar] [CrossRef]
- Draeger, R.; Gosman, N.; Steed, A.; Chandler, E.; Thomsett, M.; Srinivasachary; Schondelmaier, J.; Buerstmayr, H.; Lemmens, M.; Schmolke, M.; et al. Identification of QTLs for resistance to Fusarium head blight, DON accumulation and associated traits in the winter wheat variety Arina Theor. Appl. Genet. 2007, 115, 617–625. [Google Scholar] [CrossRef]
- Miedaner, T.; Herter, C.P.; Ebmeyer, E.; Kollers, S.; Korzun, V. Use of non-adapted quantitative trait loci for increasing Fusarium head blight resistance for breeding semi-dwarf wheat. Plant Breed. 2019, 138, 140–147. [Google Scholar] [CrossRef]
- Dweba, C.; Figlan, S.; Shimelis, H.; Motaung, T.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Surendra, A.; Cuperlovic-Culf, M. Database of resistance related metabolites in wheat Fusarium head blight disease (MWFD). Database 2017, 2017, bax076. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Pumphrey, M.O.; Liu, S.; Zhang, X.; Tiwari, V.K.; Ando, K.; Trick, H.N.; Bockus, W.W.; Akhunov, E.; Anderson, J.A.; et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxinlike domain conferring resistance to Fusarium head blight. Nat. Genet. 2016, 48, 1576–1580. [Google Scholar] [CrossRef]
- Liu, S.; Pumphrey, M.; Gill, B.; Trick, H.; Zhang, J.; Dolezel, J.; Chalhoub, B.; Anderson, J. Toward positional cloning of Fhb1, a major QTL for Fusarium head blight resistance in wheat. Cereal Res. Commun. 2008, 36, 195–201. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D.; Stępień, Ł. Relationship between Fusarium Head Blight, Kernel Damage, Concentration of Fusarium Biomass, and Fusarium Toxins in Grain of Winter Wheat Inoculated with Fusarium culmorum. Toxins 2018, 11, 2. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Varga, M.; Tóth, B.; Kótai, C.; Bartók, T.; Véha, A.; Ács, K.; Vágvölgyi, C.; Lehoczki-Krsjak, S. Reduction of deoxynivalenol (DON) contamination by improved fungicide use in wheat. Part 1. Dependence on epidemic severity and resistance level in small plot tests with artificial inoculation. Eur. J. Plant Pathol. 2018, 151, 39–55. [Google Scholar] [CrossRef]
- Lehoczki-Krsjak, S.; Szabó-Hever, Á.; Tóth, B.; Kótai, C.; Bartók, T.; Varga, M.; Farády, L.; Mesterházy, Á. Prevention of Fusarium mycotoxin contamination by breeding and fungicide application in wheat. Food Add. Cont. A 2010, 27, 616–628. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Ma, H.; Huang, L.; Ding, F.; Du, Y.; Jia, H.; Li, G.; Kong, Z.; Ran, C.; et al. Pyramiding of Fusarium Head Blight Resistance Quantitative Trait Loci, Fhb1, Fhb4, and Fhb5, in Modern Chinese Wheat Cultivars. Front. Plant Sci. 2021, 12, 694023. [Google Scholar] [CrossRef]
- Wu, J.; Ackerman, A.; Gaire, R.; Chowdhary, G.; Rutkoski, J. A neural network for phenotyping Fusarium-damaged kernels (FDKs) in wheat and its impact on genomic selection accuracy. Plant Phenome J. 2023, 6, e20065. [Google Scholar] [CrossRef]
- Larkin, D.L.; Holder, A.L.; Mason, R.E.; Moon, D.E.; Brown-Guedira, G.; Price, P.P.; Harrison, S.A.; Dong, Y. Genome-wide analysis and prediction of Fusarium head blight resistance in soft red winter wheat. Crop Sci. 2020, 60, 2882–2900. [Google Scholar] [CrossRef]
- Zhang, J.; Gill, H.S.; Brar, N.K.; Halder, J.; Ali, S.; Liu, X.; Bernardo, A.; Amand, P.S.; Bai, G.; Gill, U.S.; et al. Genomic prediction of Fusarium head blight resistance in early stages using advanced breeding lines in hard winter wheat. Crop J. 2022, 10, 1695–1704. [Google Scholar] [CrossRef]
- Mesterházy, Á. Expression of resistance to Fusarium graminearum and F. culmorum under various experimental conditions. J. Phytopathol. 1988, 133, 304–310. [Google Scholar] [CrossRef]
- Mesterházy, Á. A laboratory method to predict pathogenicity of Fusarium graminearum in field and resistance to scab. Acta Phytopath Acad. Sci. Hung. 1984, 19, 205–218. [Google Scholar]
- Mesterházy, Á. Effect of seed production area on the seedling resistance of wheat to Fusarium seedling blight. Agronomie 1985, 5, 491–497. [Google Scholar] [CrossRef]
- Takegami, S.; Sasai, K. Investigations on resistance of wheat varieties to Gibberella zeae after particular inoculation techniques. X. Experiments on improved inoculation methods involving conidiospores and hyphae. Proc. Crop. Sci. Soc. Jpn. 1970, 39, 1–6. [Google Scholar] [CrossRef][Green Version]
- Miedaner, T.; Lieberherr, B.; Gaikpa, D.S. Aggressiveness of Fusarium culmorum isolates for head blight symptoms is highly stable across four cereal crops. J. Phytopathol. 2021, 169, 387–392. [Google Scholar] [CrossRef]
- Mesterházy, Á. Breeding wheat for Fusarium head blight resistance in Europe. In Fusarium Head Blight of Wheat and Barley; Leonard, K., Bushnell, W., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 211–240. [Google Scholar]
- Mesterhazy, A. The effect of inoculation method on the expression of symptoms caused by Fusarium graminearum Schwabe on wheat in seedling stage. In Current Topics on Plant Pathology; Kiraly, Z., Ed.; Akadémiai kiadó: Budapest, Hungary, 1975; pp. 223–232. [Google Scholar]
- Brown, J.K.M. Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 2002, 5, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Giraud, T.; Zhang, N.; Begerow, D.; Cai, G.-H.; Shivas, R.G. The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Divers. 2011, 50, 121–133. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Lamper, C. Influence of cultivar resistance, epidemic severity, and Fusarium species on the efficacy of fungicide control of Fusarium head blight in wheat and deoxynivalenol (DON) contamination of grain. Plant Dis. 2003, 87, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Mesterhazy, A. Control of Fusarium head blight by fungicides. In Fusarium Head Blight of Wheat and Barley; Leonard, K., Bushnell, W., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 363–380. [Google Scholar]
- Mesterházy, Á. Chemical control of Fusarium head blight of wheat, a comprehensive view. In Mycotoxin Reduction in Grain Chains: A Practical Guide; Logrieco, A.F., Visconti, A., Eds.; Blackwell-Wiley: Ames, IA, USA; Chichester, UK; Oxford, UK, 2014; pp. 232–247. [Google Scholar]
- Mesterházy, Á.; Varga, M.; Tóth, B.; Kótai, C.; Bartók, T.; Véha, A.; Ács, K.; Vágvölgyi, C.; Lehoczki-Krsjak, S. Reduction of deoxynivalenol (DON) contamination by improved fungicide use in wheat. Part 2. Farm scale tests with different nozzle types and updating the integrated approach. Eur. J. Plant Pathol. 2018, 151, 1–20. [Google Scholar] [CrossRef]
- Nakajima, T.; Yoshida, M.; Tomimura, K. Effect of lodging on the level of mycotoxins in wheat, barley, and rice infected with the Fusarium graminearum species complex. J. Gen. Plant Pathol. 2008, 74, 289–295. [Google Scholar] [CrossRef]
- Kleber, A.; Gruber-Dorninger, C.; Platzer, A.; Payet, C.; Novak, B. Effect of Fungicide Treatment on Multi-Mycotoxin Occurrence in French Wheat during a 4-Year Period. Toxins 2023, 15, 443. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Blandino, M.; Haidukowski, M.; Pascale, M.; Plizzari, L.; Scudellari, D.; Reyneri, A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crops Res. 2012, 133, 139–149. [Google Scholar] [CrossRef]
- Shude, S.P.N.; Yobo, K.S.; Mbili, N.C. Progress in the management of Fusarium head blight of wheat: An overview. S. Afr. J. Sci. 2020, 116, 7854. [Google Scholar] [CrossRef]
- Buerstmayr, H. Rigorous phenotypic selection for Fusarium head blight resistance yields winter wheat lines combining superior FHB resistance with good agronomic traits. In Proceedings of the 72 Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Online, 22–24 November 2021; Raumberg-Gumpenstein: Raumberg, Österreich, 2022; pp. 15–16. [Google Scholar]
- Dill-Macky, R. Inoculation methods and evaluation of Fusarium head blight resistance in wheat. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 184–210. [Google Scholar]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium head blight and mycotoxins in wheat: Prevention and control strategies across the food chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Mihalache, O.A.; De Boevre, M.; Dellafiora, L.; De Saeger, S.; Moretti, A.; Pinson-Gadais, L.; Ponts, N.; Richard-Forget, F.; Susca, A.; Dall’Asta, C. The occurrence of non-regulated mycotoxins in foods: A Systematic review. Toxins 2023, 15, 583. [Google Scholar] [CrossRef] [PubMed]

| Item | MMT | % to Total Capacity | % to Harvested Yield |
|---|---|---|---|
| Total capacity | 1161 | 100 | |
| Total harvested | 774 | 67 | 100 |
| Preharvest Loss, Biot *, abiot., stresses, 33% including harvest loss 3% | 387 | 33 | - |
| Storage losses | 154 | 13 | 20 |
| Mycotoxin contamination | 78 | 7 | 10 |
| Consumer and other waste | 101 | 9 | 13 |
| Total waste | 720 | 62 | 43 |
| Total grain consumed | 441 | 38 | 57 |
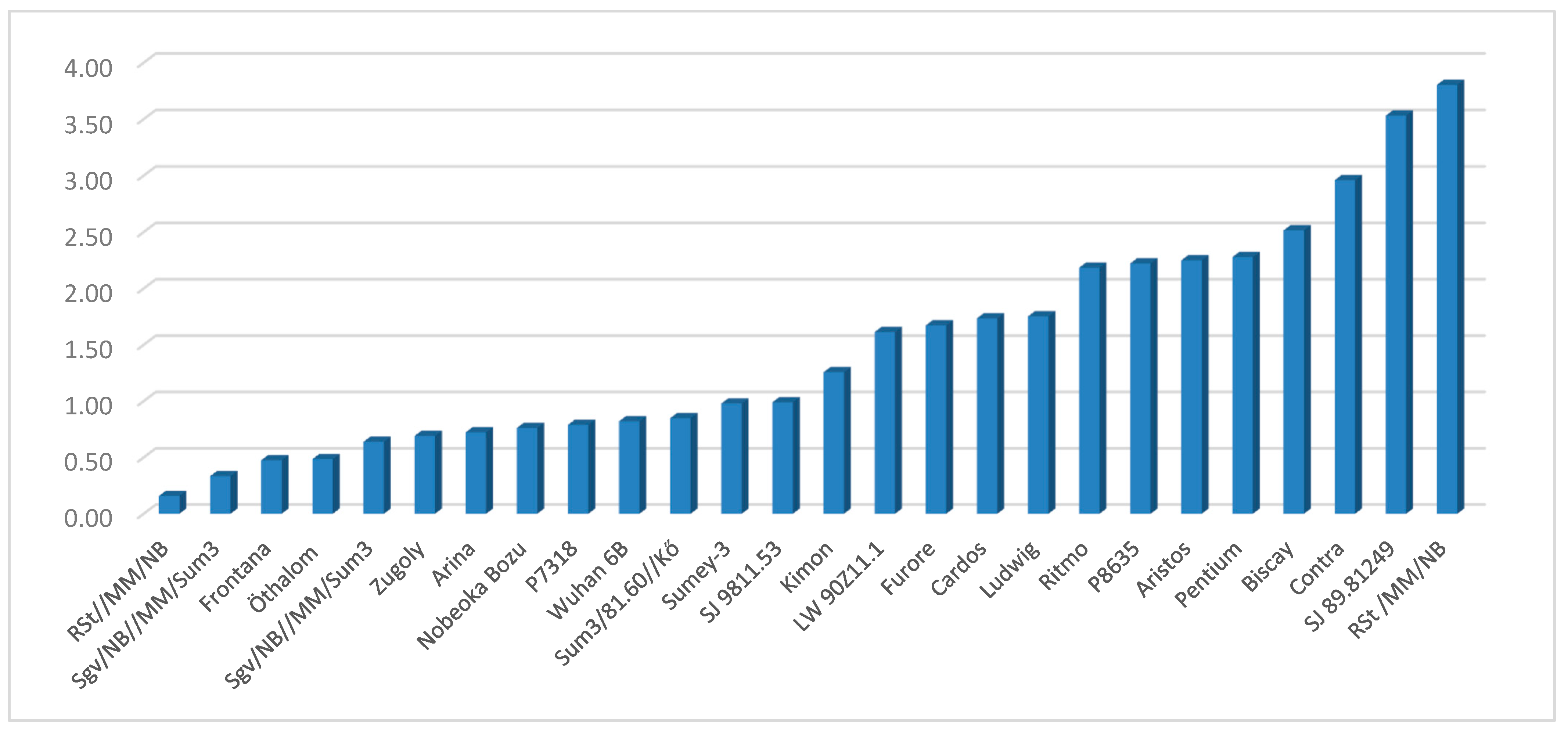
| Genotype | Fusarium species | Mean | Variance | ||||
|---|---|---|---|---|---|---|---|
| Fc | Fg | Fsamb | Fav | Fspor | |||
| Sumai-3 | 8.58 | 0.75 | 2.17 | 0.08 | 0.75 | 2.50 | 12.3 |
| Wuhan 6B | 12.00 | 1.08 | 3.17 | 0.83 | 2.17 | 3.93 | 21.6 |
| Sum3/81.60//Kő | 8.92 | 1.38 | 12.92 | 2.46 | 1.00 | 4.49 | 28.3 |
| Nobeoka Bozu | 12.17 | 6.75 | 3.00 | 1.25 | 3.33 | 5.56 | 18.7 |
| Sgv/NB//MM/Sum3 | 18.00 | 5.67 | 3.50 | 0.54 | 0.63 | 5.91 | 52.1 |
| RSt//MM/NB | 21.08 | 2.54 | 17.50 | 4.21 | 1.83 | 8.54 | 83.3 |
| Frontana | 31.25 | 6.38 | 15.83 | 0.17 | 0.46 | 10.26 | 170.8 |
| Sgv/NB//MM/Sum3 | 32.08 | 24.08 | 30.83 | 13.83 | 2.08 | 19.44 | 159.3 |
| RSt//MM/NB | 55.83 | 37.50 | 29.17 | 4.50 | 2.96 | 25.64 | 506.4 |
| Arina | 64.17 | 33.33 | 24.17 | 4.42 | 2.08 | 25.80 | 637.8 |
| Furore | 70.67 | 49.17 | 45.83 | 14.71 | 1.79 | 35.39 | 773.6 |
| P 7318 | 61.25 | 40.25 | 50.00 | 26.79 | 7.77 | 35.79 | 431.5 |
| Ludwig | 72.92 | 57.50 | 47.50 | 11.88 | 2.42 | 37.44 | 909.3 |
| Zugoly | 88.33 | 78.33 | 35.00 | 1.67 | 7.00 | 42.85 | 1591.9 |
| Kimon | 85.08 | 65.00 | 58.33 | 23.96 | 6.92 | 46.69 | 1009.2 |
| Aristos | 77.92 | 59.17 | 63.33 | 26.08 | 16.83 | 47.04 | 676.1 |
| Öthalom | 91.17 | 57.08 | 78.33 | 5.25 | 24.42 | 48.24 | 1299.2 |
| P 8635 | 85.17 | 76.67 | 61.67 | 18.58 | 19.08 | 51.19 | 1000.5 |
| Contra | 92.50 | 76.67 | 65.00 | 17.38 | 11.58 | 51.25 | 1312.0 |
| Ritmo | 87.67 | 65.00 | 76.67 | 27.33 | 23.50 | 53.74 | 847.2 |
| Carlos | 85.75 | 72.08 | 64.17 | 36.25 | 18.79 | 54.44 | 745.8 |
| LW 90.Z 11.1 | 88.42 | 68.75 | 73.33 | 52.17 | 5.75 | 55.94 | 1009.9 |
| SJ981249 | 95.83 | 69.17 | 78.33 | 33.79 | 26.63 | 58.80 | 875.5 |
| Biscay | 96.08 | 87.08 | 77.50 | 40.21 | 14.25 | 61.42 | 1195.2 |
| Pentium | 92.92 | 72.92 | 81.67 | 45.67 | 43.00 | 65.63 | 488.2 |
| SJ981153 | 98.17 | 82.50 | 90.00 | 26.75 | 47.54 | 66.66 | 929.9 |
| Mean | 62.84 | 46.03 | 45.73 | 16.95 | 11.33 | 35.56 | 645.60 |
| Fusarium spp. | Fc | Fg | Fsamb | Fav | Fspor | ||
| Fg | 0.975 | ||||||
| Ssamb | 0.930 | 0.906 | |||||
| Fav | 0.701 | 0.717 | 0.811 | ||||
| Fspor | 0.660 | 0.651 | 0.788 | 0.585 | |||
| All significant at p = 0.001. | |||||||
| Trait | Year | 1. Spray + PE * | 2. Spray + M ** | 3. Debris + M *** | Mean | LSD 5% |
|---|---|---|---|---|---|---|
| DI % | 2009 | 2.72 | 5.17 | 0.09 | 2.66 | |
| 2010 | 13.61 | 12.81 | 26.91 | 8.97 | ||
| 2011 | 15.41 | 8.61 | 6.52 | 10.18 | ||
| 2012 | 14.18 | 1.37 | 2.26 | 5.94 | ||
| Mean | 8.08 | 3.79 | 8.95 | 6.94 | 0.31 | |
| FDK% | 2009 | 2.66 | 0.81 | 0.20 | 1.22 | |
| 2010 | 16.24 | 8.91 | 7.60 | 10.92 | ||
| 2011 | 27.1 | 13.95 | 7.19 | 16.08 | ||
| 2012 | 30.22 | 4.28 | 3.15 | 12.55 | ||
| Mean | 19.06 | 6.99 | 4.54 | 10.20 | 1.66 | |
| DON ppm | 2009 | 2.12 | 0.72 | 0.40 | 1.08 | |
| 2010 | 12.82 | 5.43 | 1.56 | 6.60 | ||
| 2011 | 33.66 | 9.55 | 4.79 | 16.00 | ||
| 2012 | 9.27 | 1.62 | 1.82 | 4.24 | ||
| Mean | 14.47 | 4.33 | 2.14 | 6.98 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesterhazy, A. What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review. Toxins 2024, 16, 31. https://doi.org/10.3390/toxins16010031
Mesterhazy A. What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review. Toxins. 2024; 16(1):31. https://doi.org/10.3390/toxins16010031
Chicago/Turabian StyleMesterhazy, Akos. 2024. "What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review" Toxins 16, no. 1: 31. https://doi.org/10.3390/toxins16010031
APA StyleMesterhazy, A. (2024). What Is Fusarium Head Blight (FHB) Resistance and What Are Its Food Safety Risks in Wheat? Problems and Solutions—A Review. Toxins, 16(1), 31. https://doi.org/10.3390/toxins16010031




