Abstract
In recent years, more scientific data have pointed out the close connection between intestinal microbial community, nutritional habits, lifestyle, and the appearance of various affections located at certain anatomical systems. Gut dysbiosis enhances the formation and accumulation of specific metabolites with toxic potential that induce the appearance of kidney-associated illnesses. Intestinal microbes are involved in the degradation of food, drugs, or other ingested products that lead to the formation of various metabolites that end up in renal tissue. Over the last few years, the possibilities of modulating the gut microbiota for the biosynthesis of targeted compounds with bioactive properties for reducing the risk of chronic illness development were investigated. In this regard, the present narrative review provides an overview of the scientific literature across the last decade considering the relationship between bioactive compounds, pre-, pro-, and post-biotics, uremic toxicity, and kidney-associated affections, and the possibility of alleviating the accumulation and the negative effects of uremic toxins into the renal system.
Keywords:
gut microbiota; dysbiosis; uremic toxins; kidney disorders; prebiotics; probiotics; postbiotics; therapies Key Contribution:
Biotics (pre-, pro-, post-) play a crucial role in the reduction of uremic toxins accumulation as they intervene in the metabolic mechanisms triggering the formation of degraded metabolites and their accumulation in the kidney tissue.
1. Introduction: Gut Microbiota, Uremic Toxins Definition, and Literature Screening
More and more interest is paid to the prevention and reduction of metabolic and nutrition-associated affections, as the microbial community within the intestine has a fundamental role in the well-functioning of the entire human body [1]. This community is responsible for food matrix degradation, nutrient absorption, substance synthesis, energy provision, and metabolite accumulation and excretion, a fact that makes this ‘organ’ vital for the proper work of most of the physiological systems of the body. That is to say, when the symbiosis between the gut community and the host is disrupted, the risk of developing a wide range of illnesses is increasingly high [2].
There is a rising interest in nutrition and diseases associated with gut microbial transformation [3,4]. Nourishment is consumed by the numerous microbial species that co-exist in the gastrointestinal tract (GIT) and are transformed into different products that have specific roles for the body [5]. There are certain metabolites that exert particular effects that are useful for diverse microbial activities, but some of them accumulate and have a negative impact on certain tissues. Macronutrients such as proteins, lipids, sugars, fibers, and different pharmaceutical products are degraded by the gut microbes from which specific metabolites end up in the renal system triggering diverse kidney-associated affections. Consequently, the substances resulting from specific metabolic processes, but also those of an inorganic nature that circulate within the body fluids which accumulate and prejudice the renal tissue, are known as uremic toxins [6,7]. Uremic toxins may have different natures or sizes but contribute to impaired kidney functions, triggering various renal insufficiencies [8]. Moreover, uremic toxin accumulation affects multiple processes in the body; for example, they activate the immune response and trigger the inflammation process, leading to further increased risk of other physiological affections like atherosclerosis, diabetes mellitus, obesity, or cardiovascular diseases [9,10].
The accumulation of uremic toxins within the renal system may be also attributed to damaged gastrointestinal mucosa by altered microbiota that facilitates an elevated permeability [11]. Disruption of intestinal permeability can lead to the alteration of intercellular junction proteins like claudin-1, occludin, cingulin, and zonula occluden-1. These proteins play a critical role in maintaining the integrity of the intestinal barrier, which regulates the passage of substances, molecules, and ions between the gut lumen and the bloodstream [12,13,14]. In addition, the disruption of tight junction integrity can lead to increased intestinal permeability, commonly referred to as “leaky gut” [15].
Given the progressing nature of statistical data concerning the impact of uremic toxins on human health [16], it is established that a significant proportion of individuals with kidney disorders manifest an aberrant glomerular filtration rate resulting from the build-up of uremic toxins. This occurrence contributes to elevated rates of morbidity and mortality within this patient population [17]. In addition, renal impairment is tightly linked with an altered cardiovascular system as the kidney and heart share the function of maintaining the host’s health homeostasis, so the risk of developing cardiovascular pathologies by people with altered kidney functions is also high [18]. Uremic toxins, which are small molecules that are inadequately eliminated by dialysis therapies, circulate via the bloodstream and gradually accumulate in renal tissues, thereby disrupting their normal functions [10].
The negative effects of the accumulation of uremic toxins have been pointed out through the nineteenth century when the sole practice of evidencing them was through hemodialysis [18]. Since then, these toxic molecules have been the subject of much investigation as their biological activities are directly linked with the development of uremic syndrome [19]. Additionally, uremic syndrome can be defined as the outcome of modifications induced by the storage of a diversity of compounds that could not be discharged by the renal system [20]. According to the European Uremic Toxins Work Group (EUTox) database [21], over 150 uremic solutes have been identified till the present, and the list is increasing over time. The substances that are recognized as uremic toxins are firstly classified into three groups based on their physicochemical properties, as follows: (i) substances with low weight (under 500 Da) which are water-soluble (e.g., urea, creatinine), (ii) substances with medium weight that have around 500 Da (e.g., small peptides, leptins, microglobulins), and (iii) substances with high weight that are mostly protein-bound compounds (including phenols and indoles). Furthermore, uremic toxins are divided by their chemical structure, distribution in the body fluids, and by their molecular mass and volume [18].
As the prevalence of kidney illnesses based on the implications of uremic toxins has a growing trend [16,22], more attention has been paid to investigating the mechanisms involved in kidney disease progression and in finding non- or minimally invasive alternatives to treatment [23,24]. As diet and lifestyle modulation are among the most investigated non-invasive methods for diminishing kidney-associated affections, multiple scientific publications have published research which has occurred in the last decade on the mentioned topic (Figure 1). Gut microbiota modulation through the use of probiotics and prebiotics for renal illnesses also gained attention, while the impact of probiotics’ metabolites known as postbiotics in close connection with their impact on kidney disease is not sufficiently investigated yet. Based on the scientific search platforms (e.g., Web of Science Core Collection, Scopus), over 3500 publications about ‘uremic toxins’ appeared during the last decade including research articles, clinical trials, review papers, and book chapters during the last decade (Figure 1a). Moreover, among these publications over 250 papers involved associations between uremic toxins and ’gut microbiota’, over 150 papers involved ‘uremic toxins’ and ‘diet’, over 100 papers were associated with ’probiotics’, over 60 papers involved ’prebiotics’, and less than 10 papers involved associations between ‘uremic toxins’ and ’postbiotics’ according to Web of Science Core Collection and Scopus (Figure 1b).
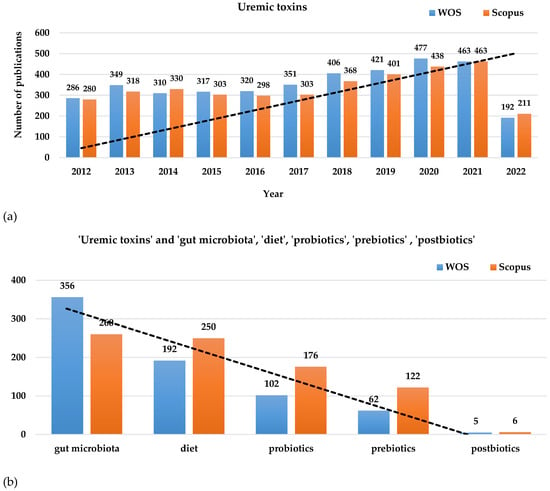
Figure 1.
Research papers distributed on Web of Science Core Collection and Scopus in the last decade (2012–2022) related to (a) ‘uremic toxins’ and the association with (b) ‘gut microbiota’, ‘diet’, ‘probiotics’, ‘prebiotics’, and ‘prebiotics’. The images show an increasing trend line for papers that include ‘uremic toxins’ and a decreasing trend line with the narrow association of uremic toxins with the mentioned terms.
As uremic toxins are actually metabolites generated from different internal processes, they intervene in a series of chronic and non-communicable diseases [25,26]. In the same context, uremic toxins are mainly produced by the microbial community within the gut through the degradation of specific macromolecules, and they further reach the host circulation by influencing other metabolic processes. In addition, if it is properly modulated, the gut community is also able to diminish the formation of metabolites with toxic potential, decreasing the risk of developing associated illnesses by the host at the same time. In this regard, the present manuscript presents the role played by gut microbiota modulation to reduce the accumulation of metabolites that exert toxicity over certain tissues such as renal tissue by the increase in the probiotic community in the GIT. Moreover, the influence of diet and the use of prebiotics for stimulating probiotic growth in association with the reduction of toxins with uremic implications are also considered. Last but not least, the metabolites biosynthesized by the probiotic strains known as postbiotics that exert positive effects over uremic toxicity are covered in the present work.
2. Gut Dysbiosis, Uremic Toxicity, and Key Kidney-Associated Diseases
Gut dysbiosis incriminates a significant number of health issues that are reflected afterward in the healthcare system, economy, and society [27]. The intestinal imbalance is also closely connected with the appearance and the progression of kidney diseases and with associated complications such as hypertension, cardiovascular affections, diabetes, and mental or cognitive impairment [14,28,29]. In addition, the gut and renal system intercommunicate through the gut–kidney axis, as the metabolites biosynthesized by the intestinal microbes reach the kidney filtration tissues [30]. Moreover, the microbial community that is found in the colon produces large quantities of metabolic waste that is excreted by the kidneys [22].
The renal system, which is composed of the kidneys, ureters, bladder, and urethra, has the main role of eliminating undesired substances from the body. Supplementarily, kidneys have an additional role in the filtration and recovery of certain compounds [31], a fact that makes them vulnerable to retaining substances with harmful potential [32]. Uremic toxins that can be both water-soluble and liposoluble, have different chemical constructions that play different biological roles, and promote deleterious effects in kidneys [18,33,34]. For instance, in Acute Kidney Injury (AKI), also known as Acute Renal Failure, uremic toxins that are generated from nitrogen metabolism—in particular, urea and creatinine—accumulate and damage the kidney tissue [35,36]. AKI is frequently diagnosed in patients with critical health conditions and appears as an unexpected episode of kidney failure that occurs within a few hours or a few days, and is a contributing factor to short- and long-term complications and elevated mortality rate [31,37]. In AKI, it was observed that the level of creatinine from serum rapidly increases (increases by 0.3 mg/dl or more in 48 h or rises to at least 1.5-fold from baseline within 7 days), while the content of urine is very much reduced. Some of the predominant uremic toxins that appear in AKI are urea, serum creatinine, hippuric acid, indoxyl sulfate, para-cresol sulfate, para-cresol glucuronide, 3-carboxy-4-methyl-5-propyl-2-furanpropionate, indole-3-acetic acid, trimethylamine N-oxide [38]. The complications induced by uremic toxin accumulation in renal tissue degenerate until the diagnosis of Chronic Kidney Disease (CKD). The progression of AKI to CKD is known as “Acute-on-Chronic Kidney Disease” (AoCKD) and occurs when an individual experiences repeated episodes of AKI or if the underlying cause of AKI persists for an extended period, leading to ongoing kidney damage and impaired kidney function over time [39]. CKD represents one of the fastest global causes of mortality, being generally characterized mainly by loss of kidney function. In this particular case, kidney dysfunction is directly proportional to the gut microbiota imbalance, as has been proved by several clinical, in vivo, or in vitro studies [27,40,41,42,43]. Unfortunately, the range of microbiota-derived toxins is increasing, and indoxyl sulfate (which is associated with vascular imbalances) and para-cresyl sulfate (which is responsible for insulin resistance and vascular disease) exemplify the complexity and significance of these compounds in contributing to various health challenges [44,45]. The associated predominant microbiota in AKI and CDK-diagnosed patients was found to be formed of Escherichia ssp., Bacteroidetes ssp., Salmonella ssp., Clostridium ssp., Ruminococcus ssp., Rothia ssp., Staphylococcus ssp., Pseudomonas ssp., Streptococcus ssp., Enterobacter ssp., Faecalibacterium ssp., and Lachnospiraceae ssp. [46,47]. Moreover, most of these pathogenic strains (especially E. coli, P. aeruginosa, and S. aureus) enter the bloodstream and spread to various organs including kidneys causing infections (through invasion and/or toxin elaboration) like urinary tract infections (UTIs), catheter-associated urinary tract infections (CAUTIs), cystitis, pyelonephritis and emphysematous pyelonephritis, glomerulonephritis, tubulointerstitial nephritis, kidney/renal abscesses, or sepsis in critical cases [48,49,50,51,52,53,54].
When the gut–kidney axis is disturbed, the entire body functionality is compromised, including the immune system. In the case of Berger’s disease (an autoimmune disease also known as IgA nephropathy), this is instantiated as an immune renal condition characterized by the deposition of Immunoglobulin A (IgA) antibodies in the glomeruli of the kidneys [55,56]. The exact mechanisms through which the gut microbiota may induce IgA nephropathy are not fully understood, and research in this area is still ongoing. Some hypotheses and associations have been proposed, such as the following: (a) dysbiosis—gut permeability: a compromised gut barrier allows bacterial components, such as lipopolysaccharides, to translocate from the gut into the bloodstream leading to endotoxemia, further triggering an immune response; this immune activation may lead to the production of IgA antibodies against these bacterial components and may contribute to the formation of immune complexes [40,57]; (b) IgA production—immune activation: the gut-associated lymphoid tissue (GALT) is rich in IgA-producing B cells; gut dysbiosis can influence the activation and proliferation of these B cells, leading to the production of more IgA antibodies; some of these IgA antibodies may be directed against gut-derived antigens or bacterial products, and in some instances, these antibodies can cross-react with antigens in the kidneys, contributing to the formation of immune complexes in the glomeruli [58]; (c) genetic factors: genetic predisposition is believed to play a role in the development of IgA nephropathy; certain genetic variations may affect the immune response to gut bacteria and their products, making some individuals more susceptible to the production of autoantibodies and the subsequent development of IgA nephropathy [59]. In IgA nephropathy, it was noticed that gut dysbiosis is induced by an increased presence of bacteria species belonging to Streptococcus ssp., Escherichia-Shigella ssp., and Eubacterium ssp., and low contents of Bifidobacterium ssp., Enterococcus ssp., Clostridium ssp., Leuconostoc ssp., Prevotella ssp., and Lactobacillus ssp. were noticed. Moreover, the metabolites produced by the mentioned strains and classified as uremic toxins like ethyl alcohol, 2,6-octadien-1-ol 3,7 dimethyl- (Z), 1-octanol, 4-methyl-phenol, phenol 4-(1,1,3,3-tetramethylbutyl), heptanoic acid 1, 1-dimethyl ethyl ester, hexyl n-valerate, heptanoic acid 1-methyl ethyl ester, benzoic acid hexadecyl ester, and phthalic acid methyl neopentyl ester were found at significantly higher levels in the feces and/or urine of progressive IgA-nephropathy-diagnosed patients [60].
End-stage Renal Disease (ESRD) is the final and most advanced stage of CKD, wherein the kidneys have significantly impaired function, necessitating dialysis as a crucial supportive measure [32]. As a result, the accumulation of uremic toxins in the bloodstream becomes a significant concern. Gut dysbiosis, gut inflammation, and heightened gut permeability are the primary contributing factors leading to the development of ESRD. These factors facilitate the movement of uremic toxins (such as indoxyl sulfate and para cresol sulfate), bacterial components, and pro-inflammatory cytokines from the gut into the bloodstream [17]. In terms of intestinal dysbiosis, in clinical studies conducted on ESRD-diagnosed patients having peritoneal dialysis, hemodialysis, or kidney transplant, significant alterations were observed among commensal microflora, as species belonging to Firmicutes and Actinobacteria were substantially decreased, while species belonging to Bacteroidetes, Proteobacteria, and Enterobacteriaceae (e.g., E. coli, P. aeruginosa) were largely increased [54,61].
3. Lifestyle, Dietary Habits, and Uremic Toxicity
It is widely known that lifestyle and dietary habits influence one’s general health status and implicitly the development of kidney-associated disorders (Figure 2). In recent studies, diet has been much investigated as the most accessible and non-invasive method of modulating gut microbiota to treat kidney diseases [23,62]. There is a thin line between our diet and the production of uremic toxins, the dietary intake being seen as the main source of substrates for uremic toxin production. Carbohydrates, lipids, proteins, minerals, and micronutrients are the precursors of protein-bound uremic toxins (PBUTSs) [63]. It seems that the everyday foods we eat, either directly or indirectly contribute to the plasma retention of uremic toxins. Furthermore, disturbances in gut microbiota composition, often referred to as dysbiosis, can lead to an imbalance in metabolite production. Dysbiosis may result from factors like dietary habits, medications, physical activity, and underlying health conditions, further impacting kidney function and disease progression [64,65].
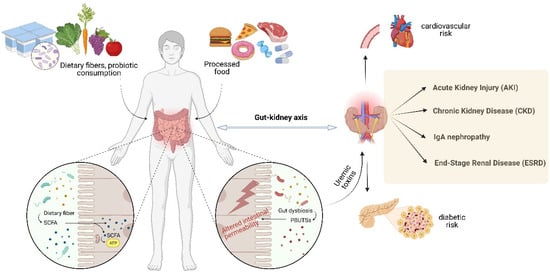
Figure 2.
The interconnection between dietary habits, general health state and the development of kidney-associated disorders (Created with BioRender.com; accessed on 22 August 2023).
Nonetheless, both vegetarian and omnivore diets have their advantages and challenges when it comes to their impact on kidney affections, such as uremia and CKD [66]. A well-balanced vegetarian diet, rich in plant-based nutrients and carefully planned to ensure adequate protein intake, can be beneficial for managing CKD and reducing the risk of uremic toxin formation. On the other hand, an omnivore diet that focuses on lean protein sources, whole grains, fruits, and vegetables while moderating the consumption of red and processed meats can also support kidney health and minimize the risk of uremia-related complications. Individual preferences, nutritional needs, and consultation with healthcare professionals are essential considerations when choosing a dietary approach for individuals with kidney disorders like AKI, CKD, or ESRD [67,68,69].
3.1. Carbohydrates
According to their degree of polymerization, carbohydrates are represented by monosaccharides, disaccharides, polyols, oligosaccharides, and polysaccharides. Reducing sugars present in this class (glucose, fructose, galactose, lactose, and maltose) are precursors of some PBUTSs. These compounds intervene in the Maillard reaction mechanism (the reaction between reducing sugars and amino acids that occurs during food treatments at high temperatures). Uremic products obtained from this reaction are fructose-lysine, 3-deoxyglucosone, glyoxal, methylglyoxal, pentosidine, Nε-carboxymethyl lysine, and Nε-carboxyethyl lysine. Fructose-lysine may be found in pasteurized milk, pasta, chocolate, cereals, and carbonated soft drinks, 3-deoxyglucosone is present in honey, and glyoxal and methylglyoxal could be found in an extended range of goods, from bread to cigarettes [70]. Moreover, carbohydrate consumption can lead to the formation of Advanced Glycation End-Products (AGEs), which are also considered uremic toxins. AGEs are a complex group of compounds formed when sugars react with proteins, lipids, or nucleic acids through a process known as glycation [33,71,72]. The accumulation of these small, water-soluble uremic toxins in the bloodstream, when the kidneys are unable to effectively clear them from the body, can contribute to oxidative stress, inflammation, and cellular damage. This, in turn, further worsens kidney function and contributes to the progression of kidney failure. Reducing sugar content in foods is recommended to prevent the generation of uremic toxins. Foods that are rich in sugars, especially when exposed to high temperatures, can also contribute to AGE formation. That is to say, other heat treatments, such as boiling and steam cooking, can be used to achieve this instead of grilling, frying, or roasting. Thus, a diet high in carbohydrates, especially refined sugars and heavily processed foods, can indirectly contribute to the accumulation of AGEs [70]. A diet low in red meat and high in whole grain fibers encourages the proliferation of saccharolytic taxa that lower levels of gut-derived uremic toxin. Furthermore, in kidney-related conditions, close attention is needed regarding carbohydrates and blood sugar control. Monitoring carbohydrate intake is crucial to ensure stable blood sugar levels and prevent complications associated with diabetes, which is a frequent comorbidity in individuals with kidney failure [73].
3.2. Lipids
Kidney-related issues can be caused by lipid-based compounds. CKD, especially in its advanced stages, can lead to dyslipidemia, which is an abnormal lipid profile characterized by elevated levels of triglycerides, low-density lipoprotein cholesterol (LDLc), and reduced levels of high-density lipoprotein cholesterol (HDLc). Dyslipidemia is further associated with an increased risk of cardiovascular disease in individuals with CKD [74]. Uremic toxins like indoxyl sulfate and para cresyl sulfate can alter the expression and function of lipid transporters and receptors in the liver and other tissues, leading to dysregulation of lipid metabolism [75]. For example, indoxyl sulfate has been shown to disrupt cholesterol homeostasis and promote lipid accumulation in macrophages, potentially contributing to atherosclerosis and cardiovascular complications. Furthermore, uremic toxins induce oxidative stress, which, in turn, leads to dyslipidemia by altering lipoproteins, increasing their atherogenicity, and contributing to the advancement of atherosclerosis [76]. Nonetheless, increased levels of uremic toxins may contribute to lipotoxicity, a condition where excessive lipids in tissues, such as the kidney and blood vessels, lead to cell dysfunction and damage [77]. Regardless of this, the intake of dietary fats can influence the accumulation and clearance of uremic toxins in individuals with kidney disease. The composition of dietary lipids, particularly the types of fats consumed, can impact the production of uremic toxins in the body. For example, diets high in unhealthy fats, such as saturated and trans fats found in processed foods and fatty meats, contribute to oxidative stress and inflammation, leading to the generation of uremic toxins [33]. When oxidative stress is present in the body, lipids, especially LDLc, can undergo oxidation. This process leads to the formation of uremic toxins derived from lipids, such as lipid hydroperoxides and oxysterols. These substances can exacerbate kidney injury and contribute to its progression [33,78]. Last but not least, adequate lipid intake, especially essential fatty acids like omega-3 polyunsaturated fatty acids found in fish and plant-based sources, may positively influence the clearance of uremic toxins. Studies have suggested that omega-3 fatty acids can improve kidney function and reduce inflammation, potentially aiding in the removal of uremic toxins [71].
3.3. Proteins
Proteins are directly implicated in the formation of uremic toxins, and their accumulation can play a significant role in kidney failure and the progression of AKI, CKD, and ESRD. As proteins are essential macronutrients that undergo metabolism in the body to provide amino acids, the building blocks necessary for various physiological processes, waste products and by-products are generated, including urea and creatinine, guanidino compounds like guanidino acetic acid, guanidino succinic acid, and guanidino butyric acid, indoxyl sulfate, para cresyl sulfate, indole-3-acetic acid, and homocysteine [79]. These waste products are typically filtered out of the blood by healthy kidneys and excreted in the urine [80,81,82]. Uremic toxins derived from protein metabolism can lead to oxidative stress, inflammation, and cellular damage in the kidneys [68,83]. The amount and quality of dietary protein intake can also influence protein-derived uremic toxin formation. Diets that are high in protein content, especially animal protein, may increase the production of specific uremic toxins. Therefore, in individuals with kidney disease, dietary protein intake may need to be moderated or adjusted based on the severity of kidney impairment [84]. In addition, in cases of advanced kidney failure, there may be an increased risk of protein-energy wasting (PEW), where the body breaks down muscle protein for energy due to inadequate nutrient intake and chronic inflammation. PEW can lead to malnutrition and further contribute to the accumulation of uremic toxins [85,86]. It was observed that anuric hemodialysis patients with a low protein/fiber index exhibited lower levels of uremic toxins like indoxyl sulfate and para cresyl sulfate in their blood. The blood concentrations of these uremic toxins were strongly influenced by the patient’s diet, showing a significant correlation with the protein/fiber index. That is to say, to investigate the potential reduction of these uremic toxins, diets with higher fiber intake should be tested as a beneficial approach to lowering their levels and minimizing the risk of undernutrition [87].
3.4. Minerals
Minerals also play a significant role in the formation and clearance of uremic toxins in the context of kidney failure and kidney diseases. Minerals like phosphate, calcium, magnesium, potassium, sodium, or trace minerals may influence both the progression of the disease and the overall health of individuals with impaired kidney function [88]. For instance, in kidney failure, the kidneys lose their ability to effectively excrete phosphate, leading to elevated levels of serum phosphate (hyperphosphatemia). High phosphate levels can bind with calcium, causing a decrease in serum-ionized calcium levels. In response, the body may release parathyroid hormone (PTH) to maintain calcium levels. In addition, prolonged elevation of PTH leads to secondary hyperparathyroidism, contributing to bone disease and vascular calcification [89,90]. CKD-diagnosed patients use phosphate binder medication to manage elevated phosphate levels. These binders work by reducing the absorption of dietary phosphate from the gastrointestinal tract, thereby helping to control phosphate levels in the blood. Phosphate binders, when taken with meals, work by binding to dietary phosphate in the gastrointestinal tract [91]. This binding reduces the amount of phosphate available for absorption into the bloodstream. There are different types of phosphate binders, including calcium-based, aluminum-based, magnesium-based, and non-calcium-, non-aluminum-based binders [91,92].
The kidneys are also responsible for maintaining a proper balance of magnesium and potassium in the blood. In kidney failure, reduced kidney function can lead to the accumulation of magnesium (hypermagnesemia) and potassium (hyperkalemia) in the bloodstream. These imbalances lead to adverse effects on the heart and nerve function, triggering cardiovascular complications [93]. On the other hand, sodium intake is usually restricted in individuals with kidney failure to manage fluid retention and blood pressure. Excessive sodium intake leads to fluid retention and edema, putting additional strain on the kidneys and exacerbating kidney damage [94,95]. Certain trace minerals, such as zinc and selenium, are essential components of antioxidant defense systems, and in kidney failure cases, imbalances in trace minerals can contribute to oxidative stress, inflammation, and cellular damage, further impairing kidney function, and overall health [96]. In addition, kidney failure can lead to anemia due to reduced production of erythropoietin, a hormone that stimulates red blood cell production. Iron deficiency is a common cause of anemia in individuals with kidney failure, so iron supplements or intravenous iron therapy may be prescribed to manage anemia in these patients [97]. Nonetheless, kidneys play a vital role in maintaining acid–base balance in the body. When renal function is compromised, acidosis (increased acidity) may develop due to reduced acid excretion. Acidosis can lead to metabolic disturbances and further contribute to kidney injury [98].
3.5. Bioactive Compounds
Bioactive compounds are naturally occurring substances found in certain foods that have specific physiological effects on the human body [99]. Some studies have suggested that certain bioactive compounds, like polyphenols found in fruits, vegetables, green tea, or turmeric, may have protective effects on kidney function [3]. These polyphenols have been shown by various studies to support kidney health and mitigate the progression of kidney injury by modulating different signaling pathways and inflammatory responses. However, while polyphenols are generally praised for their ability to neutralize harmful free radicals and reduce oxidative stress, it is important to note that some of these compounds can become detrimental when they accumulate in individuals with kidney failure [100]. Some researchers consider polyphenols as PBUTS precursors [70,101]. For instance, hippurates, which are considered uremic toxins, can be formed not only through the conversion of chlorogenic acid and (+)-catechin but also from various other phenolic compounds, such as caffeic acid, flavan-3-ol, cyanidin, and quercetin [70]. A recent cross-sectional study in Australia did not find any association between the intake of fruits and vegetables, which are the main sources of polyphenols, and plasma levels of para cresyl sulfate in hemodialysis patients. While more research is needed to establish a direct link between polyphenol intake and uremic toxicity, considering their protective role as antioxidants, it is recommended not to exclude polyphenols from the diet in CKD [70,102].
3.6. Vitamins
In individuals with kidney failure, especially those undergoing dialysis, there is an increased risk of vitamin deficiencies due to reduced dietary intake, impaired absorption, and loss during dialysis [103]. Common deficiencies may include water-soluble vitamins like B-complex vitamins (B1, B6, B12, folic acid) and fat-soluble vitamins (A, D, E, K). In the case of vitamin C (ascorbic acid), which is a water-soluble and strong antioxidant vitamin, can help neutralize free radicals and reduce oxidative stress, but in advanced kidney failure, the accumulation of oxalic acid which is a byproduct of vitamin C metabolism can contribute to the formation of kidney stones [104]. Deficiencies of B-complex vitamins, such as thiamine (B1), pyridoxine (B6), and cyanocobalamin (B12), can lead to neurological complications, including peripheral neuropathy and encephalopathy, commonly observed in individuals with advanced kidney failure [105]. Folic acid deficiency can exacerbate anemia in individuals with kidney failure, as it plays a vital role in red blood cell formation [106]. Vitamin D plays a crucial role in calcium and phosphorus metabolism and bone health. In kidney affections, impaired kidney function can lead to a decrease in the active form of vitamin D (calcitriol) production, leading to disturbances in calcium and phosphorus levels, bone disease, and secondary hyperparathyroidism [107]. In kidney diseases, disturbances in vitamin A metabolism can also occur, leading to higher levels of retinol-binding protein and alterations in vitamin A storage and transport [108].
Overall, maintaining a balanced diet that includes a variety of foods rich in macronutrients, vitamins, minerals, and antioxidants is crucial for supporting kidney health and managing uremic toxins in individuals with kidney failure. It is essential to work closely with healthcare professionals, including registered dietitians and nephrologists, to develop a personalized dietary plan that meets individual nutritional needs and helps improve overall well-being in kidney disease patients.
4. Biotics (Pre-, Pro-, Post-) and Uremic Toxicity: Prevention and Possible Therapy
The relationship between the gut, microbiota, major and/or secondary metabolites, and kidney-related conditions is highly interconnected and interdependent. This intricate network of interactions influences kidney health and can have significant implications for kidney-related conditions [109]. The interplay between the gut, microbiota, metabolites, and the kidneys is part of a bidirectional relationship known as the gut–kidney axis. Changes in the gut microbiota can influence kidney function, and kidney dysfunction can, in turn, alter the gut microbiota composition [110]. Understanding and manipulating this intricate relationship holds promise for developing new therapeutic approaches for kidney-associated affections. The strong interconnection between the gut and kidneys and other related aspects can be seen in Figure 3.
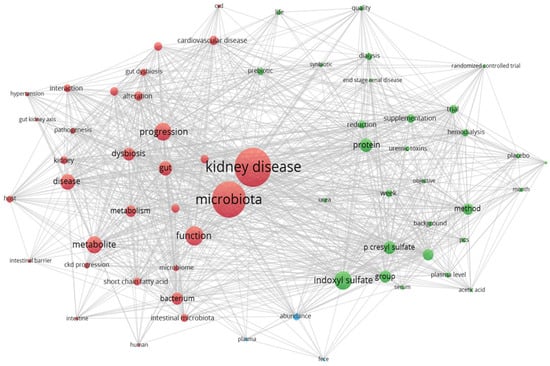
Figure 3.
The gut, microbiota, metabolites, and kidney-related conditions are closely linked and interdependent. This intricate interplay between the gut and the kidneys has a profound impact on kidney health and can significantly affect the development and progression of kidney-related conditions (VOSviewer version 1.6.17).
4.1. Prebiotics
As a general description, prebiotics are non-digestible fibers that serve as food for beneficial bacteria in the gut [14]. The quality of prebiotics can positively influence the composition and diversity of the gut microbiota. A healthy and balanced gut microbiota is crucial for the metabolism and clearance of uremic toxins; thus, beneficial gut bacteria can help reduce the production of certain uremic toxins like indoxyl sulfate and para cresyl sulfate that are produced by harmful bacteria like Clostridium or Enterobacteriaceae by metabolizing precursors in the gut [111]. Prebiotic substrates such as inulin, fructo-oligosaccharides, and β-glucans enhance the gut barrier function, reducing the translocation of bacterial components and pro-inflammatory cytokines from the gut into the bloodstream. Prebiotics stand at the basis for forming postbiotics, i.e., the extremely valuable SCFAs acetate, propionate, and butyrate. These help mitigate inflammation and oxidative stress, which are contributing factors to most kidney injuries [24,41,112,113]. Nonetheless, prebiotic substrates positively impact the gut–kidney axis, leading to improved kidney health and the clearance of uremic toxins [27,114]. Furthermore, several studies have provided evidence supporting the use of prebiotics as a nutritional therapy for managing kidney-related disorders [24,111,115]. The manipulation of the gut microbiota using prebiotics and other dietary interventions is an area of ongoing research. Modulating the gut microbiota with prebiotics and promoting the growth of beneficial bacteria may have therapeutic potential in managing kidney disorders and reducing the burden of uremic toxins.
4.2. Probiotics
Beneficial bacteria commonly recognized as probiotics can reduce the formation and accumulation of uremic toxins like indole, phenol, or para cresol by metabolizing their precursors like phenylalanine, tyrosine and tryptophan, which are amino acids found in the diet [110,116]. The probiotic strains keep the integrity of the intestinal barrier by preventing the leakage of harmful substances like uremic toxins from the gut into the bloodstream. In addition, probiotics like Bifidobacteria and lactobacilli were proven to have the ability to restore damaged colonic epithelial tight junctions and to stimulate the secretion of intestinal mucus, leading to a reduction in systemic inflammation at the same time—a process closely linked to uremic toxin accumulation and kidney-related conditions [10,117,118]. Multiple in vivo, in vitro, or clinical studies (e.g., Table 1 and Table 2) argue for the beneficial effect of probiotic strains in diminishing the symptoms and damage of kidney injuries. For example, a particular clinical study performed by Eidi and their team (2018) on 42 hemodialysis patients (32 male and 10 female) proved the efficacy of a probiotic treatment in patients undergoing hemodialysis. In this case, the probiotic strain L. rhamnosus administered for 4 weeks provided lower values for uremic toxins (p-cresol and phenol) compared with the placebo group [43]. Furthermore, recent meta-analyses focusing on the effects of biotic supplements in individuals with kidney conditions, particularly CKD and ESRD, provide robust evidence of their advantageous impact. These studies strongly endorse their ability to decrease levels of serum uremic toxins such as indoxyl sulfate, p-cresyl sulfate, blood urea nitrogen, malondialdehyde, and serum creatinine. Additionally, these supplements have shown promise in alleviating gastrointestinal symptoms and enhancing the quality of life among CKD and ESRD patients [24,119]. To sum up the main scientific outcome in this context, probiotics create an environment that is less favorable for uremic toxin accumulation.
4.3. Postbiotics
Among the essential metabolites produced by probiotic bacteria when they grow on non-fermentable fibers are bioactive compounds like peptides, organic acids, and short-chain fatty acids (SCFAs), collectively known as postbiotics [120,121]. Notably, SCFAs, such as acetate, propionate, and butyrate, have demonstrated the ability to reduce oxidation and inflammatory processes, consequently lowering the accumulation of uremic toxins in both the bloodstream and renal tissue [122]. Moreover, a six-month study conducted on senior adult cats revealed promising outcomes when they were fed a control maintenance diet supplemented with various nutrients [109]. The supplemented diet exhibited several positive effects, including increased lean-body percentage, maintained serum albumin concentrations, higher glomerular filtration rate (GFR), and decreased serum symmetric dimethylarginine (SDMA) concentrations. Additionally, the cats displayed lower plasma metabolite concentrations, indicative of reduced oxidative stress and a shift in methyl group allocation. Furthermore, the consumption of the supplemented food resulted in changes in microbial postbiotics, leading to a decrease in the plasma concentration of the uremic toxin 3-indoxyl sulfate. These observed changes have the potential to counteract the sarcopenia and chronic inflammation typically associated with aging in cats. However, further research is required to validate these health benefits and ascertain the anti-aging effects in senior adult cats [109]. Nevertheless, the use of postbiotics, particularly SCFAs, holds promise as a novel therapeutic approach for mitigating the effects of uremic toxins and supporting kidney health. Additionally, these findings suggest that tailored dietary interventions, including postbiotics and nutrient supplementation, may play a pivotal role in improving kidney function and overall health outcomes in both human and animal populations. Still, more extensive investigations are essential to fully comprehend the precise mechanisms and long-term effects of postbiotics in uremic toxin management and age-related kidney conditions.

Table 1.
Examples of major kidney-associated affections triggered by uremic toxins and pre-/pro/postbiotic treatments (pre-clinical data).
Table 1.
Examples of major kidney-associated affections triggered by uremic toxins and pre-/pro/postbiotic treatments (pre-clinical data).
| Disease | Identified Uremic Toxins | Type of Study | Biotic Treatment (Pre-/Pro-/Post-) | Main Study’s Results | Ref. |
|---|---|---|---|---|---|
| AKI | Indoxyl sulfate, p-cresol, phenol | In vivo—on male rats with cisplatin-induced AKI; In vitro—cultivated in MRS broth with indoxyl sulfate, p-cresol, and phenol for 24 and 48 h | Probiotics: Lactobacillus plantarum BCRC12251, L. paracasei BCRC12188, Streptococcus salivarius subsp. thermophilus BCRC13869 | Suppressed the accumulation of IS in the serum; reduced the level of IS after 48 h | [123] |
| Indoxyl sulfate, p-cresol sulfate | In vivo—on rats with cisplatin-induced AKI; In vitro—on Caco-2 cell line | Probiotic: Lactobacillus salivarius BP121 | Reduced renal inflammation and oxidative stress; reduced intestinal permeability | [124] | |
| CKD | Indoxyl sulfate, p-cresol sulfate, phenyl-acetyl-glutamine, and trimethylamine N-oxide | In vivo—on adenine-induced CKD in rodent models | Probiotic: Bifidobacterium animalis A6 | Decreased abundance of Eggerthella lenta and Fusobacterium nucleatum and reduced levels of toxins and the severity of the disease | [42] |
| Hippuric acid, 3-carboxy-4-methyl-5-propyl-2-furan propionate, indole-3-acetic acid, indoxyl sulfate, p-cresol sulfate, para-cresyl glucuronide, trimethylamine N-oxide | In vivo—on adenine-induced CKD in rats | Probiotics: Bacillus subtilis TO-A, Enterococcus faecium T-110, and Clostridium butyricum TO-A | Decrease in intestinal pH by increasing SCFA production that further suppressed the production of uremic toxins | [125] | |
| Indole, p-cresol, p-cresyl sulfate | In vitro—on fecal batches collected from healthy and CKD subjects | Probiotic: Bifidobacterium animalis BLC1, Lacticaseibacillus casei LC4P1; Prebiotic: inulin, fructooligosaccharides, quercetin, resveratrol, and proanthocyanidins | On fecal batches collected from CKD—modified the viable cell densities of some cultivable bacterial patterns, and increased the concentration of acetic acid and decane, while reducing the concentration of nonanoic acid, dimethyl trisulfide, and indoxyl sulfate | [41] | |
| IgA neprop-athy | In vivo—on IGA-induced mouse model | Probiotic: Bifidobacterium longum and Lactobacillus bulgaricus | Alleviation in gut dysbiosis associated with induction of IgA nephropathy | [126] |

Table 2.
Examples of major kidney-associated affections triggered by uremic toxins and pre-/pro/postbiotic treatments (clinical data).
Table 2.
Examples of major kidney-associated affections triggered by uremic toxins and pre-/pro/postbiotic treatments (clinical data).
| Disease | Identified Uremic Toxins | Type of Study | Biotic Treatment (Pre-/Pro-/Post-) | Main Study’s Results | Ref. |
|---|---|---|---|---|---|
| CKD | Indoxyl sulfate, p-cresol sulfate | In vivo study—on stage 3–4 CKD patients; 6-week, double-blind, placebo-controlled, parallel-arm, randomized controlled trial | Prebiotic: high-amylose maize-resistant starch type 2 (RS-2) | Reduction in indoxyl sulfate and p-cresol sulfate, reduction in key markers of inflammation | [111] |
| Indoxyl sulfate, p-cresyl sulfate | In vivo study—on 37 predialysis adult participants with CKD; 6 weeks, randomized, double-blind, placebo-controlled, crossover trial | Synbiotic therapy; prebiotic: high-molecular-weight inulin (inulin high-performance), fructo-oligosaccharides, and galacto-oligosaccharides (GOSs); probiotic: nine different strains across the Lactobacillus, Bifidobacteria, and Streptococcus genera | A low reduction of serum indoxyl sulfate, and a significant reduction of serum p-cresyl sulfate; alteration in stool microbiome, particularly with enrichment of Bifidobacterium and depletion of Ruminococcaceae | [127] | |
| p-cresyl sulfate, p-cresyl glucuronide, indoxyl sulfate, trimethy- lamine N-oxide, phenylacetylglutamine | In vivo study—on 40 participants with CKD not yet on dialysis; 4 weeks, randomized, placebo-controlled, double-blind, cross-over study design. | Prebiotic: arabinoxylan oligosaccharides (AXOS) and maltodextrin | No significant effect of AXOS on serum p-cresyl sulfate, p-cre- syl glucuronide, indoxyl sulfate, and phenylacetylglutamine. A small, albeit significant, decreasing effect on serum trimethylamine N-oxide was observed. No effect of AXOS on 24 h urinary excretion of p-cresyl sulfate, p-cresyl glucuronide, indoxyl sulfate, and phenylacetylglutamine, nor on trimethylamine N-oxide | [128] | |
| p-cresol | In vivo study—on 13 individuals with CKD; 12-week, single-blind study | Prebiotic: sucrose, pea hull fiber, inulin | Plasma p-cresol decreased from 7.25 ± 1.74 mg/L during week 1 to 5.82 ± 1.72 mg/L during week 12 | [129] | |
| IgA nephropathy | Clinical study—on 35 IgA nephropathy-diagnosed patients and 25 healthy controls; 8 weeks, randomized controlled trial | Probiotic: Bifidobacterium longum and Lactobacillus bulgaricus | Both probiotics and their SCFA metabolites could attenuate the clinicopathological manifestations of IgAN by inhibiting the NLRP3/ASC/Caspase 1 signaling pathway | [126] | |
| ESRD | Urea, hippuric acid, phenyl-acetyl-glutamine | Clinical study—on a cohort of 12 ESRD patients and 12 healthy controls; randomized controlled trial | Probiotic: Bifidobacterium animalis A6 | Decreased abundance of Eggerthella lenta and Fusobacterium nucleatum and reduced levels of toxins and the severity of the disease | [42] |
| Indoxyl sulfate and para cresol sulfate | Pilot study—on 20 patients on maintenance hemodialysis on systemic inflammation; 12 weeks, single-center non-randomized pilot study | Postbiotic: sodium propionate (SP) | Reduction in pro-inflammatory parameters and oxidative stress and improved insulin resistance and iron metabolism. SP effectively lowered uremic toxins indoxyl and para cresol sulfate | [130] |
Research in this field is ongoing, and ongoing studies continue to shed light on the complex interactions between the gut, microbiota, metabolites, and kidney health. As the understanding of this interconnection deepens, targeted interventions involving dietary modifications, prebiotics, probiotics, and other treatments may emerge to support kidney health and manage kidney-related conditions more effectively. However, it is important to acknowledge the limitations inherent in this line of investigation. Studies often exhibit heterogeneity in design, sample sizes, and outcomes, making direct comparisons challenging. Small sample sizes, short follow-up periods, and variability in pre-/pro-/postbiotic compositions can hinder the generalizability of findings. Additionally, the lack of standardized protocols and understanding of mechanisms, along with potential confounding factors and publication bias, further complicate the interpretation of results. Nonetheless, a holistic approach that addresses the gut kidney axis may pave the way for improved outcomes and better management of kidney-associated affections.
5. Conclusions and Future Perspectives
In conclusion, the emerging field of research on the interplay between gut microbiota, dietary habits, metabolites, uremic toxins, and kidney disorders has revealed significant insights into potential therapeutic strategies for managing kidney health and related conditions. The gut microbiota, consisting of a diverse community of microorganisms, plays a crucial role in the metabolism of dietary components and endogenous compounds. This intricate network of interactions influences kidney health and can have profound implications for the accumulation and elimination of uremic toxins, which are associated with kidney dysfunction. The consumption of a balanced diet, rich in prebiotics, probiotics, and dietary fibers, has been shown to promote the growth of beneficial gut bacteria and foster the production of beneficial metabolites known as postbiotics like SCFAs. SCFAs, such as acetate, propionate, and butyrate, exhibit anti-inflammatory and antioxidant properties that can indirectly reduce uremic toxin formation and accumulation. However, the specific effects of these interventions may vary among individuals due to differences in gut microbiota composition, diet, and health status. Personalized approaches to diet and supplementation, guided by healthcare professionals, are crucial for optimizing the benefits of these interventions in managing kidney disorders. Future perspectives in this area of research are promising. As technology and knowledge advance, further studies can explore the intricate mechanisms underlying the gut–kidney axis and the impact of dietary habits on gut microbiota. Unraveling the specific interactions between gut metabolites, uremic toxins, and kidney function will pave the way for more targeted therapies and nutritional interventions. Furthermore, translational research is essential to bring these findings from preclinical and animal models to clinical trials in human populations. Long-term studies evaluating the effects of dietary modifications, gut microbiota modulation, and postbiotic supplementation in individuals with kidney disorders will provide valuable data for evidence-based therapeutic recommendations.
In conclusion, the relationship between gut microbiota, dietary habits, metabolites, uremic toxins, and kidney disorders is a multifaceted and dynamic area of research. By harnessing the power of the gut microbiota and its metabolites through targeted dietary approaches, we may be able to make strides in preventing, managing, and potentially even reversing kidney-related conditions, ultimately improving the quality of life for affected individuals.
Author Contributions
L.M.: conceptualization, software, writing—original draft preparation; M.M.: original draft preparation; C.-R.P.: writing—review and editing; A.-M.R.: visualization, conceptualization; D.-C.V.: writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Research, Development and Innovation, developed with the support of UEFISCDI (Project No. 14 PFE-2022-2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Vodnar, D.C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Knauf, F.; Brewer, J.R.; Flavell, R.A. Immunity, microbiota and kidney disease. Nat. Rev. Nephrol. 2019, 15, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Massry, S.G. Chapter 6—Uremic toxins: An integrated overview of classification and pathobiology. In Nutritional Management of Renal Disease, 4th ed.; Kopple, J.D., Massry, S.G., Kalantar-Zadeh, K., Fouque, D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 77–89. [Google Scholar]
- Maroz, N.; Simman, R. Wound Healing in Patients With Impaired Kidney Function. J. Am. Coll. Clin. Wound Spec. 2013, 5, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, J.I.; Weir, M.R. Chapter 31—Hematologic and Infectious Complications of Chronic Kidney Disease. In Chronic Renal Disease, 2nd ed.; Kimmel, P.L., Rosenberg, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 477–502. [Google Scholar]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxidative Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Mitch, W.E. Chapter 90—Chronic Kidney Disease: Pathophysiology and the Influence of Dietary Protein. In Seldin and Giebisch’s The Kidney, 5th ed.; Alpern, R.J., Moe, O.W., Caplan, M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3021–3072. [Google Scholar]
- Bhat, M.I.; Sowmya, K.; Kapila, S.; Kapila, R. Escherichia coli K12: An evolving opportunistic commensal gut microbe distorts barrier integrity in human intestinal cells. Microb. Pathog. 2019, 133, 103545. [Google Scholar] [CrossRef]
- Bhat, M.I.; Sowmya, K.; Kapila, S.; Kapila, R. Potential Probiotic Lactobacillus rhamnosus (MTCC-5897) Inhibits Escherichia coli Impaired Intestinal Barrier Function by Modulating the Host Tight Junction Gene Response. Probiotics Antimicrob. Proteins 2020, 12, 1149–1160. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef] [PubMed]
- NIDDK. National Institute on Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease (accessed on 1 July 2022).
- El Chamieh, C.; Liabeuf, S.; Massy, Z. Uremic Toxins and Cardiovascular Risk in Chronic Kidney Disease: What Have We Learned Recently beyond the Past Findings? Toxins 2022, 14, 280. [Google Scholar] [CrossRef]
- Falconi, C.A.; Junho, C.V.d.C.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Remuzzi, G.; Noris, M. Hemolytic uremic syndrome. Semin. Immunopathol. 2014, 36, 399–420. [Google Scholar]
- Loirat, C.; Saland, J.; Bitzan, M. Management of hemolytic uremic syndrome. La Presse Médicale 2012, 41, e115–e135. [Google Scholar] [CrossRef]
- EUTox. European Uremic Toxins Work Group. Available online: https://www.uremic-toxins.org/ (accessed on 6 July 2022).
- Chu, C.D.; Powe, N.R.; McCulloch, C.E.; Crews, D.C.; Han, Y.; Bragg-Gresham, J.L.; Saran, R.; Koyama, A.; Burrows, N.R.; Tuot, D.S.; et al. Trends in Chronic Kidney Disease Care in the US by Race and Ethnicity, 2012–2019. JAMA Netw. Open 2021, 4, e2127014. [Google Scholar] [CrossRef] [PubMed]
- Favero, C.; Ortiz, A.; Sanchez-Niño, M.D. Probiotics for kidney disease. Clin. Kidney J. 2022, 15, 1981–1986. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, J.; Qin, Y.; Wang, Y.; Zhang, Y.; Sun, S. Probiotics, Prebiotics, and Synbiotics Improve Uremic, Inflammatory, and Gastrointestinal Symptoms in End-Stage Renal Disease With Dialysis: A Network Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 850425. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Massy, Z.A.; Chesnaye, N.C.; Larabi, I.A.; Dekker, F.W.; Evans, M.; Caskey, F.J.; Torino, C.; Porto, G.; Szymczak, M.; Drechsler, C.; et al. The relationship between uremic toxins and symptoms in older men and women with advanced chronic kidney disease. Clin. Kidney J. 2022, 15, 798–807. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, T.; Dong, S.; Jiang, H.; Zhang, J.; Raza, H.K.; Lei, G. Association between gut dysbiosis and chronic kidney disease: A narrative review of the literature. J. Int. Med. Res. 2021, 49, 03000605211053276. [Google Scholar] [CrossRef]
- Vodnar, D.-C.; Mitrea, L.; Teleky, B.-E.; Szabo, K.; Călinoiu, L.-F.; Nemeş, S.-A.; Martău, G.-A. Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: A Real Challenge for Human Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 575559. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Nemeş, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Nouri, Z.; Zhang, X.-Y.; Khakisahneh, S.; Degen, A.A.; Wang, D.-H. The microbiota-gut-kidney axis mediates host osmoregulation in a small desert mammal. npj Biofilms Microbiomes 2022, 8, 16. [Google Scholar] [CrossRef]
- Siew, E.D.; Abdel-Kader, K.; Perkins, A.M.; Greevy, R.A.; Parr, S.K.; Horner, J.; Vincz, A.J.; Denton, J.; Wilson, O.D.; Hung, A.M.; et al. Timing of Recovery from Moderate to Severe AKI and the Risk for Future Loss of Kidney Function. Am. J. Kidney Dis. 2020, 75, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Chaudhary, S.; Williams, A.W.; Dillon, J.J.; Norby, S.M.; Gregoire, J.R.; Albright, R.C.; McCarthy, J.T.; Thorsteinsdottir, B.; Rule, A.D. Predictors of Outpatient Kidney Function Recovery Among Patients Who Initiate Hemodialysis in the Hospital. Am. J. Kidney Dis. 2015, 65, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Florens, N.; Calzada, C.; Lyasko, E.; Juillard, L.; Soulage, C.O. Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins 2016, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Ma, S.; Liu, T.; Tian, H.; Zhu, Q.; Wang, W.; Li, Y.; Ding, F. Increasing the removal of protein-bound uremic toxins by liposome-supported hemodialysis. Artif. Organs 2019, 43, 490–503. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Shao, X.; Tian, L.; Xu, W.; Zhang, Z.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. Diagnostic Value of Urinary Kidney Injury Molecule 1 for Acute Kidney Injury: A Meta-Analysis. PLoS ONE 2014, 9, e84131. [Google Scholar] [CrossRef]
- André, C.; Bennis, Y.; Titeca-Beauport, D.; Caillard, P.; Cluet, Y.; Kamel, S.; Choukroun, G.; Maizel, J.; Liabeuf, S.; Bodeau, S. Two rapid, accurate liquid chromatography tandem mass spectrometry methods for the quantification of seven uremic toxins: An application for describing their accumulation kinetic profile in a context of acute kidney injury. J. Chromatogr. B 2020, 1152, 122234. [Google Scholar] [CrossRef]
- Acosta-Ochoa, I.; Bustamante-Munguira, J.; Mendiluce-Herrero, A.; Bustamante-Bustamante, J.; Coca-Rojo, A. Impact on Outcomes across KDIGO-2012 AKI Criteria According to Baseline Renal Function. J. Clin. Med. 2019, 8, 1323. [Google Scholar] [CrossRef]
- Matei, C.M.; Andrei, S.; Buza, V.; Cernea, M.; Dumitraș, D.; Neagu, D.; Rafa, H.; Popovici, C.P.; Pop, R.; Catinean, A.; et al. Evaluation Of Endotoxemia, Soluble Cd14 And Il-1β In Dogs With Intestinal Dysbiosis That Were Treated With Probiotics: A Prospective Study. Farmacia 2021, 69, 1153–1158. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Lenucci, M.S.; Fontana, S.; Forgia, F.M.; Minervini, F.; Scarano, A.; Santino, A.; Dalfino, G.; Gesualdo, L.; et al. In Vitro Selection of Probiotics, Prebiotics, and Antioxidants to Develop an Innovative Synbiotic (NatuREN G) and Testing Its Effect in Reducing Uremic Toxins in Fecal Batches from CKD Patients. Microorganisms 2021, 9, 1316. [Google Scholar] [CrossRef] [PubMed]
- Xifan, W.; Songtao, Y.; Shenghui, L.; Liang, Z.; Yanling, H.; Junjie, Q.; Lian, Z.; Chengying, Z.; Weijing, B.; Li, Z.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131. [Google Scholar] [CrossRef]
- Eidi, F.; Poor-reza Gholi, F.; Ostadrahimi, A.; Dalili, N.; Samadian, F.; Barzegari, A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: A double blind randomized clinical trial. Clin. Nutr. ESPEN 2018, 28, 158–164. [Google Scholar] [CrossRef]
- Popkov, V.A.; Zharikova, A.A.; Demchenko, E.A.; Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Gut Microbiota as a Source of Uremic Toxins. Int. J. Mol. Sci. 2022, 23, 483. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, E.; Caldiroli, L.; Callegari, M.L.; Miragoli, F.; Zanoni, F.; Armelloni, S.; Rizzo, V.; Messa, P.; Vettoretti, S. Association of Sarcopenia and Gut Microbiota Composition in Older Patients with Advanced Chronic Kidney Disease, Investigation of the Interactions with Uremic Toxins, Inflammation and Oxidative Stress. Toxins 2021, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Saranya, G.R.; Viswanathan, P. Gut microbiota dysbiosis in AKI to CKD transition. Biomed. Pharmacother. 2023, 161, 114447. [Google Scholar] [CrossRef]
- Chou, Y.-T.; Kan, W.-C.; Shiao, C.-C. Acute Kidney Injury and Gut Dysbiosis: A Narrative Review Focus on Pathophysiology and Treatment. Int. J. Mol. Sci. 2022, 23, 3658. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, G.; Cappelli, L.; Cinelli, P.; Cuffaro, R.; Manca, B.; Nicchi, S.; Tondi, S.; Vezzani, G.; Viviani, V.; Delany, I.; et al. Strategies to Tackle Antimicrobial Resistance: The Example of Escherichia coli and Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 4943. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Ulett, G.C. Evaluation of hematogenous spread and ascending infection in the pathogenesis of acute pyelonephritis due to group B streptococcus in mice. Microb. Pathog. 2020, 138, 103796. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Mitrea, L.; Calinoiu, L.F.; Szabo, K.; Stefanescu, B.E. 11—Removal of bacteria, viruses, andother microbial entities by means of nanoparticles. In Advanced Nanostructures for Environmental Health; Lucian, B., Zsolt, P., Klara, H., Monica, B., Trombaco, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 465–491. [Google Scholar]
- Takayasu, M.; Hirayama, K.; Shimohata, H.; Kobayashi, M.; Koyama, A. Staphylococcus aureus infection-related glomerulonephritis with dominant IgA deposition. Int. J. Mol. Sci. 2022, 23, 7482. [Google Scholar] [CrossRef] [PubMed]
- Okunaga, I.; Makino, S.-i.; Honda, D.; Tatsumoto, N.; Aizawa, M.; Oda, T.; Asanuma, K. IgA-dominant infection-related glomerulonephritis with NAPlr-positive tubulointerstitial nephritis. CEN Case Rep. 2023, 2023, 1–6. [Google Scholar] [CrossRef]
- Poh, C.W.M.; Seah, X.F.V.; Chong, C.Y.; Ganesan, I.; Maiwald, M.; Nadua, K.; Kam, K.-Q.; Tan, N.W.H. Salmonella Renal Abscess in an Immunocompetent Child: Case Report and Literature Review. Glob. Pediatr. Health 2021, 8, 2333794X211022263. [Google Scholar] [CrossRef]
- Järvisalo, M.J.; Hellman, T.; Uusalo, P. Mortality and associated risk factors in patients with blood culture positive sepsis and acute kidney injury requiring continuous renal replacement therapy—A retrospective study. PLoS ONE 2021, 16, e0249561. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, G.; Stasi, A.; Franzin, R.; Fiorentino, M.; Cimmarusti, M.T.; Deleonardis, A.; Palieri, R.; Pontrelli, P.; Gesualdo, L. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives. Toxins 2023, 15, 115. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Berthelot, L. Role of gut-kidney axis in renal diseases and IgA nephropathy. Curr. Opin. Gastroenterol. 2021, 37, 565–571. [Google Scholar] [CrossRef]
- Han, S.; Shang, L.; Lu, Y.; Wang, Y. Gut Microbiome Characteristics in IgA Nephropathy: Qualitative and Quantitative Analysis from Observational Studies. Front. Cell. Infect. Microbiol. 2022, 12, 577. [Google Scholar] [CrossRef]
- Barratt, J.; Rovin, B.H.; Cattran, D.; Floege, J.; Lafayette, R.; Tesar, V.; Trimarchi, H.; Zhang, H. Why Target the Gut to Treat IgA Nephropathy? Kidney Int. Rep. 2020, 5, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsukaguchi, H.; Higasa, K.; Kawata, N.; Inui, K.; Linh, T.N.T.; Quynh, T.T.H.; Yoshihiko, I.; Koiwa, F.; Yoshimura, A. Positive renal familial history in IgA nephropathy is associated with worse renal outcomes: A single-center longitudinal study. BMC Nephrol. 2021, 22, 230. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Montemurno, E.; Piccolo, M.; Vannini, L.; Lauriero, G.; Maranzano, V.; Gozzi, G.; Serrazanetti, D.; Dalfino, G.; Gobbetti, M.; et al. Microbiota and Metabolome Associated with Immunoglobulin A Nephropathy (IgAN). PLoS ONE 2014, 9, e99006. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Salgado, J.; Vehaskari, V.M.; Stewart, T.; Ferris, M.; Zhang, Q.; Wang, G.; Blanchard, E.E.; Taylor, C.M.; Kallash, M.; Greenbaum, L.A.; et al. Intestinal microbiota in pediatric patients with end stage renal disease: A Midwest Pediatric Nephrology Consortium study. Microbiome 2016, 4, 50. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Nemes, S.A.; Teleky, B.E.; Calinoiu, L.F.; Mitrea, L.; Martau, G.A.; Szabo, K.; Mihai, M.; Vodnar, D.C.; Crișan, G. Microencapsulation and Bioaccessibility of Phenolic Compounds of Vaccinium Leaf Extracts. Antioxidants 2022, 11, 674. [Google Scholar] [CrossRef]
- Jansen, J.; Jankowski, J.; Gajjala, P.R.; Wetzels, J.F.; Masereeuw, R. Disposition and clinical implications of protein-bound uremic toxins. Clin. Sci. 2017, 131, 1631–1647. [Google Scholar] [CrossRef]
- Yang, H.-L.; Feng, P.; Xu, Y.; Hou, Y.-Y.; Ojo, O.; Wang, X.-H. The Role of Dietary Fiber Supplementation in Regulating Uremic Toxins in Patients With Chronic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2021, 31, 438–447. [Google Scholar] [CrossRef]
- Matei, C.M.; Popovici, C.P.; Neagu, D.; Buza, V.; Szakacs, A.R.; Stefanut, L.C. Hematological Aspects on Dogs with Apparent Dysbiosis after Bacillus Subtilis, Bacillus Licheniformis and Pediococcus Acidilactici Probiotic Administration- Pilot Study. Bull. UASVM Vet. Med. 2020, 77, 2. [Google Scholar] [CrossRef]
- Świątek, Ł.; Jeske, J.; Miedziaszczyk, M.; Idasiak-Piechocka, I. The impact of a vegetarian diet on chronic kidney disease (CKD) progression—A systematic review. BMC Nephrol. 2023, 24, 168. [Google Scholar] [CrossRef]
- Valim, A.; Carpes, L.S.; Nicoletto, B.B. Effect of vegetarian diets on renal function in patients with chronic kidney disease under non-dialysis treatment: A scoping review. J. Bras. Nefrol. 2022, 44, 395–402. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The impact of CKD on uremic toxins and gut microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.; Fouque, D.; Amaral, A.C.F.; Mafra, D. Trimethylamine N-Oxide From Gut Microbiota in Chronic Kidney Disease Patients: Focus on Diet. J. Ren. Nutr. 2015, 25, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; Farré, R.; Evenepoel, P.; Overbeek, S.A.; Meijers, B. Food-Derived Uremic Toxins in Chronic Kidney Disease. Toxins 2023, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Jackson, M.I.; Yerramilli, M.; Jewell, D.E. Soluble Fiber and Omega-3 Fatty Acids Reduce Levels of Advanced Glycation End Products and Uremic Toxins in Senior Dogs by Modulating the Gut Microbiome. J. Food Sci. Nutr. Res. 2020, 3, 18–33. [Google Scholar] [CrossRef]
- Nemes, S.A.; Călinoiu, L.F.; Dulf, F.V.; Fărcas, A.C.; Vodnar, D.C. Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach. Antioxid. 2022, 11, 2159. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.; Titan, S.; Vencio, S. Interactions between kidney disease and diabetes: Dangerous liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef]
- Theofilis, P.; Vordoni, A.; Koukoulaki, M.; Vlachopanos, G.; Kalaitzidis, R.G. Dyslipidemia in Chronic Kidney Disease: Contemporary Concepts and Future Therapeutic Perspectives. Am. J. Nephrol. 2021, 52, 693–701. [Google Scholar] [CrossRef]
- Hobson, S.; de Loor, H.; Kublickiene, K.; Beige, J.; Evenepoel, P.; Stenvinkel, P.; Ebert, T. Lipid Profile Is Negatively Associated with Uremic Toxins in Patients with Kidney Failure-A Tri-National Cohort. Toxins 2022, 14, 412. [Google Scholar] [CrossRef]
- Ceja-Galicia, Z.A.; Aranda-Rivera, A.K.; Amador-Martínez, I.; Aparicio-Trejo, O.E.; Tapia, E.; Trujillo, J.; Ramírez, V.; Pedraza-Chaverri, J. The Development of Dyslipidemia in Chronic Kidney Disease and Associated Cardiovascular Damage, and the Protective Effects of Curcuminoids. Foods 2023, 12, 921. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.-P.; Dounousi, E. Chronic Kidney Disease and Disproportionally Increased Cardiovascular Damage: Does Oxidative Stress Explain the Burden? Oxidative Med. Cell. Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef] [PubMed]
- Rapp, N.; Evenepoel, P.; Stenvinkel, P.; Schurgers, L. Uremic Toxins and Vascular Calcification–Missing the Forest for All the Trees. Toxins 2020, 12, 624. [Google Scholar] [CrossRef]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Di Iorio, B.R.; Vacca, M.; Cosola, C.; Marzocco, S.; di Bari, I.; Calabrese, F.M.; Ciarcia, R.; De Angelis, M.; Gesualdo, L. Ketoanalogs’ Effects on Intestinal Microbiota Modulation and Uremic Toxins Serum Levels in Chronic Kidney Disease (Medika2 Study). J. Clin. Med. 2021, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Cosola, C.; di Bari, I.; Magnani, S.; Galleggiante, V.; Scandiffio, L.; Dalfino, G.; Netti, G.S.; Atti, M.; Corciulo, R. Efficacy of divinylbenzenic resin in removing indoxyl sulfate and p-cresol sulfate in hemodialysis patients: Results from an in vitro study and an in vivo pilot trial (xuanro4-Nature 3.2). Toxins 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Borges, N.A.; Barros, A.F.; Nakao, L.S.; Dolenga, C.J.; Fouque, D.; Mafra, D. Protein-Bound Uremic Toxins from Gut Microbiota and Inflammatory Markers in Chronic Kidney Disease. J. Ren. Nutr. 2016, 26, 396–400. [Google Scholar] [CrossRef]
- Graboski, A.L.; Redinbo, M.R. Gut-Derived Protein-Bound Uremic Toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef]
- Martín-del-Campo, F.; Avesani, C.M.; Stenvinkel, P.; Lindholm, B.; Cueto-Manzano, A.M.; Cortés-Sanabria, L. Gut microbiota disturbances and protein-energy wasting in chronic kidney disease: A narrative review. J. Nephrol. 2023, 36, 873–883. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Singh, B.S.; Khor, B.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef]
- Ebersolt, M.; Santana Machado, T.; Mallmann, C.; Mc-Kay, N.; Dou, L.; Bouchouareb, D.; Brunet, P.; Burtey, S.; Sallée, M. Protein/Fiber Index Modulates Uremic Toxin Concentrations in Hemodialysis Patients. Toxins 2022, 14, 589. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukagawa, M. Uremic Toxicity and Bone in CKD. J. Nephrol. 2017, 30, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Cobo, G.; Dai, L.; Lindholm, B.; Stenvinkel, P. Role of Uremic Toxins in Early Vascular Ageing and Calcification. Toxins 2021, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Lin, S.-H. Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins. Toxins 2020, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Au, K.; Francis, R.S.; Mudge, D.W.; Johnson, D.W.; Pillans, P.I. Phosphate binders in patients with chronic kidney disease. Aust. Prescr. 2017, 40, 10–14. [Google Scholar] [CrossRef]
- Bover Sanjuán, J.; Navarro-González, J.F.; Arenas, M.D.; Torregrosa, J.-V.; Tamargo Menéndez, J.; de Francisco, A.L.M.; González-Parra, E.; Lloret Cora, M.J.; Sánchez Álvarez, J.E.; Martín-Malo, A.; et al. Pharmacological interactions of phosphate binders. Nefrología (Engl. Ed.) 2018, 38, 573–578. [Google Scholar] [CrossRef]
- van Ham, W.B.; Cornelissen, C.M.; van Veen, T.A.B. Uremic toxins in chronic kidney disease highlight a fundamental gap in understanding their detrimental effects on cardiac electrophysiology and arrhythmogenesis. Acta Physiol. 2022, 236, e13888. [Google Scholar] [CrossRef]
- Chmielewski, M.; Heimbürger, O.; Stenvinkel, P.; Lindholm, B. Uremic toxicity. In Nutritional Management of Renal Disease; Elsevier Inc.: New York, NY, USA, 2013; pp. 49–77. [Google Scholar]
- Twardowski, Z.J.; Misra, M. A need for a paradigm shift in focus: From Kt/Vurea to appropriate removal of sodium (the ignored uremic toxin). Hemodial. Int. 2018, 22, S29–S64. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative Stress in ESRD Patients on Dialysis and the Risk of Cardiovascular Diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Hamza, E.; Metzinger, L.; Metzinger-Le Meuth, V. Uremic Toxins Affect Erythropoiesis during the Course of Chronic Kidney Disease: A Review. Cells 2020, 9, 2039. [Google Scholar] [CrossRef]
- Mansoor, S.; De Klerk, L.; Lineen, J.; Fahad, M.; Ali, I.; Coffey, B.; Mulry, M.A.; Saadat, S.; Kelly, S.; Adenan, M.H.; et al. Lentiform fork sign in a uremic patient with a high anion gap metabolic acidosis with seizures: A case report from North West of Ireland. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 101. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Salarolli, R.T.; Alvarenga, L.; Cardozo, L.F.M.F.; Teixeira, K.T.R.; de SG Moreira, L.; Lima, J.D.; Rodrigues, S.D.; Nakao, L.S.; Fouque, D.; Mafra, D. Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int. Urol. Nephrol. 2021, 53, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Zheng, H.; Bird, L.; Borst, A.; Fuller, A.; Lambert, K. Associations among plant-based diet quality, uremic toxins, and gut microbiota profile in adults undergoing hemodialysis therapy. J. Ren. Nutr. 2021, 31, 177–188. [Google Scholar] [CrossRef]
- Jankowska, M. Chapter 27—Vitamins in chronic kidney disease. In Molecular Nutrition; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 561–582. [Google Scholar]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Calderon-Ospina, C.A.; Nava-Mesa, M.O.; Paez-Hurtado, A.M. Update on Safety Profiles of Vitamins B1, B6, and B12: A Narrative Review. Ther. Clin. Risk Manag. 2020, 16, 1275–1288. [Google Scholar] [CrossRef]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef]
- Auguste, B.L.; Avila-Casado, C.; Bargman, J.M. Use of vitamin D drops leading to kidney failure in a 54-year-old man. Can. Med. Assoc. J. 2019, 191, E390–E394. [Google Scholar] [CrossRef]
- Chazot, C.; Steiber, A.L.; Kopple, J.D. Chapter 26—Vitamin metabolism and requirements in chronic kidney disease and kidney failure. In Nutritional Management of Renal Disease, 4th ed.; Kopple, J.D., Massry, S.G., Kalantar-Zadeh, K., Fouque, D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 413–465. [Google Scholar]
- Hall, J.A.; Jackson, M.I.; Farace, G.; Yerramilli, M.; Jewell, D.E. Influence of Dietary Ingredients on Lean Body Percent, Uremic Toxin Concentrations, and Kidney Function in Senior-Adult Cats. Metabolites 2019, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Beker, B.M.; Colombo, I.; Gonzalez-Torres, H.; Musso, C.G. Decreasing microbiota-derived uremic toxins to improve CKD outcomes. Clin. Kidney J. 2022, 15, 2214–2219. [Google Scholar] [CrossRef]
- Headley, S.A.; Chapman, D.J.; Germain, M.J.; Evans, E.E.; Hutchinson, J.; Madsen, K.L.; Ikizler, T.A.; Miele, E.M.; Kirton, K.; O’Neill, E.; et al. The effects of 16-weeks of prebiotic supplementation and aerobic exercise training on inflammatory markers, oxidative stress, uremic toxins, and the microbiota in pre-dialysis kidney patients: A randomized controlled trial-protocol paper. BMC Nephrol. 2020, 21, 517. [Google Scholar] [CrossRef]
- Pei, M.; Wei, L.; Hu, S.; Yang, B.; Si, J.; Yang, H.; Zhai, J. Probiotics, prebiotics and synbiotics for chronic kidney disease: Protocol for a systematic review and meta-analysis. BMJ Open 2018, 8, e020863. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Mazzaferro, S.; Muscaritoli, M.; Mastroluca, D.; Testorio, M.; Perrotta, A.; Esposito, Y.; Carta, M.; Campagna, L.; Di Grado, M.; et al. Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.I.; Armani, R.G.; Canziani, M.E.F.; Dalboni, M.A.; Dolenga, C.J.R.; Nakao, L.S.; Campbell, K.L.; Cuppari, L. Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: A randomized controlled trial. Nephrol. Dial. Transplant. 2018, 34, 1876–1884. [Google Scholar] [CrossRef]
- Jerez-Morales, A.; Merino, J.S.; Díaz-Castillo, S.T.; Smith, C.T.; Fuentealba, J.; Bernasconi, H.; Echeverría, G.; García-Cancino, A. The Administration of the Synbiotic Lactobacillus bulgaricus 6c3 Strain, Inulin and Fructooligosaccharide Decreases the Concentrations of Indoxyl Sulfate and Kidney Damage in a Rat Model. Toxins 2021, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Linnane, A.W.; Gobe, G.C. From the Gastrointestinal Tract (GIT) to the Kidneys: Live Bacterial Cultures (Probiotics) Mediating Reductions of Uremic Toxin Levels via Free Radical Signaling. Toxins 2013, 5, 2042–2057. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Effect of Synbiotic Therapy on Gut-Derived Uremic Toxins and the Intestinal Microbiome in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Stuivenberg, G.A.; Chmiel, J.A.; Akouris, P.P.; Burton, J.P.; Reid, G. Probiotic Bifidobacteria Mitigate the Deleterious Effects of para-Cresol in a Drosophila melanogaster Toxicity Model. mSphere 2022, 7, e00446-22. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, J.; Yang, H.; Wang, D.; Zhang, Y.; Yang, Y.; Xing, G.; Kon, V. Biotic Supplements in Patients With Chronic Kidney Disease: Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2022, 32, 10–21. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Lopes, R.d.C.S.O.; Theodoro, J.M.V.; da Silva, B.P.; Queiroz, V.A.V.; de Castro Moreira, M.E.; Mantovani, H.C.; Hermsdorff, H.H.; Martino, H.S.D. Synbiotic meal decreases uremic toxins in hemodialysis individuals: A placebo-controlled trial. Food Res. Int. 2019, 116, 241–248. [Google Scholar] [CrossRef]
- Deng, M.; Li, X.; Li, W.; Gong, J.; Zhang, X.; Ge, S.; Zhao, L. Short-Chain Fatty Acids Alleviate Hepatocyte Apoptosis Induced by Gut-Derived Protein-Bound Uremic Toxins. Front. Nutr. 2021, 8, 756730. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Lu, J.-R.; Chen, B.-J.; Wu, C.; Chen, Y.-P.; Chen, M.-J. Selection of uremic toxin-reducing probiotics in vitro and in vivo. J. Funct. Foods 2014, 7, 407–415. [Google Scholar] [CrossRef]
- Lee, T.H.; Park, D.; Kim, Y.J.; Lee, I.; Kim, S.; Oh, C.T.; Kim, J.Y.; Yang, J.; Jo, S.K. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 2020, 45, 1130–1140. [Google Scholar] [CrossRef]
- Inatomi, T.; Honma, M. Ameliorating effect of probiotics in a rat model of chronic kidney disease. PLoS ONE 2023, 18, e0281745. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Dong, L.; Jiang, Z.; Tan, L.; Luo, X.; Pei, G.; Qin, A.; Zhong, Z.; Liu, X.; Tang, Y.; et al. Probiotics ameliorate IgA nephropathy by improving gut dysbiosis and blunting NLRP3 signaling. J. Transl. Med. 2022, 20, 382. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Delcour, J.A.; Courtin, C.M.; Kuypers, D.; Augustijns, P.; Verbeke, K.; Meijers, B. The Influence of Prebiotic Arabinoxylan Oligosaccharides on Microbiota Derived Uremic Retention Solutes in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0153893. [Google Scholar] [CrossRef]
- Salmean, Y.A.; Segal, M.S.; Palii, S.P.; Dahl, W.J. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J. Ren. Nutr. 2015, 25, 316–320. [Google Scholar] [CrossRef]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).