Renal Embolization-Induced Uremic Swine Model for Assessment of Next-Generation Implantable Hemodialyzers
Abstract
:1. Introduction
2. Results
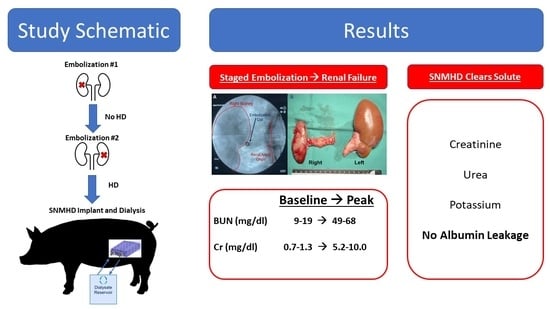
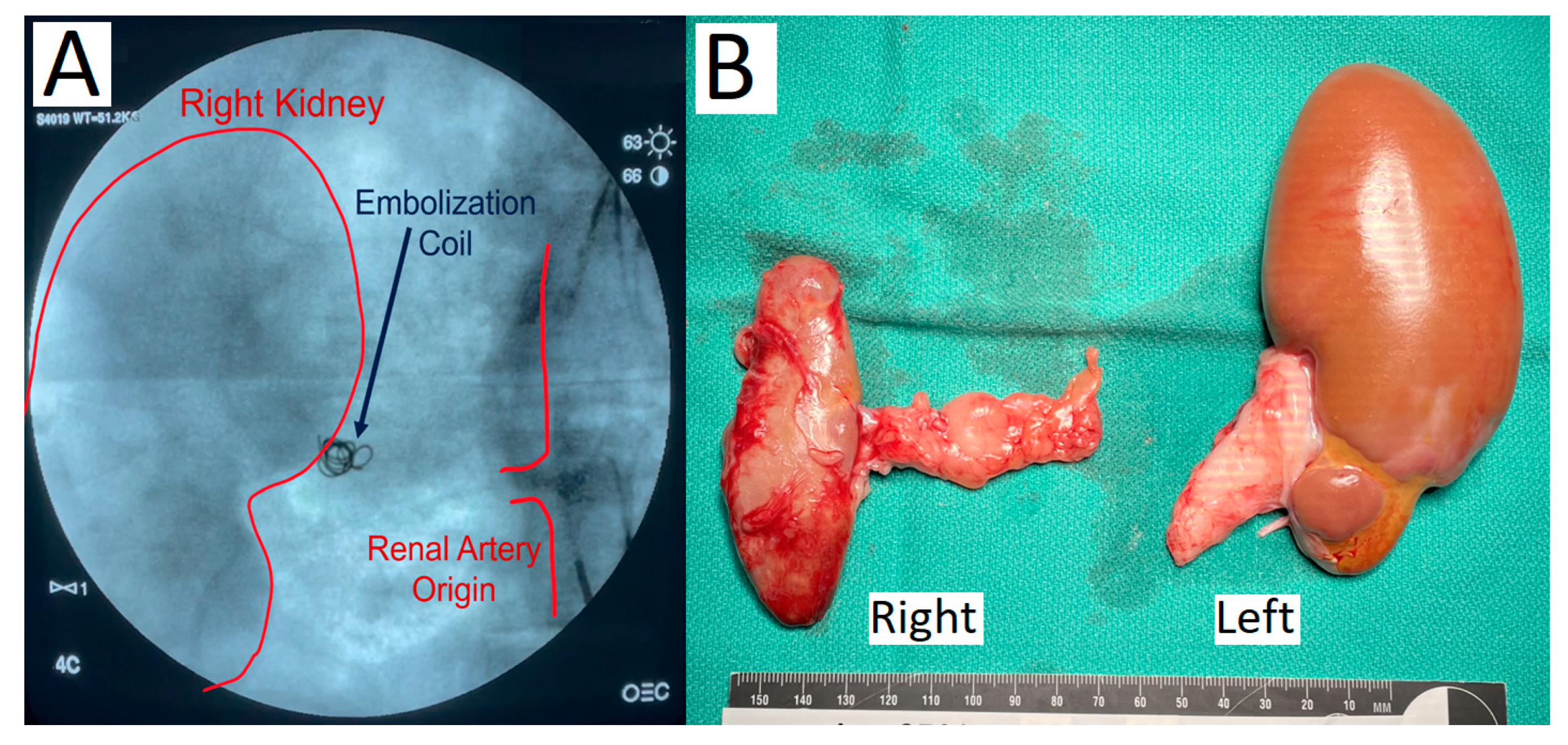
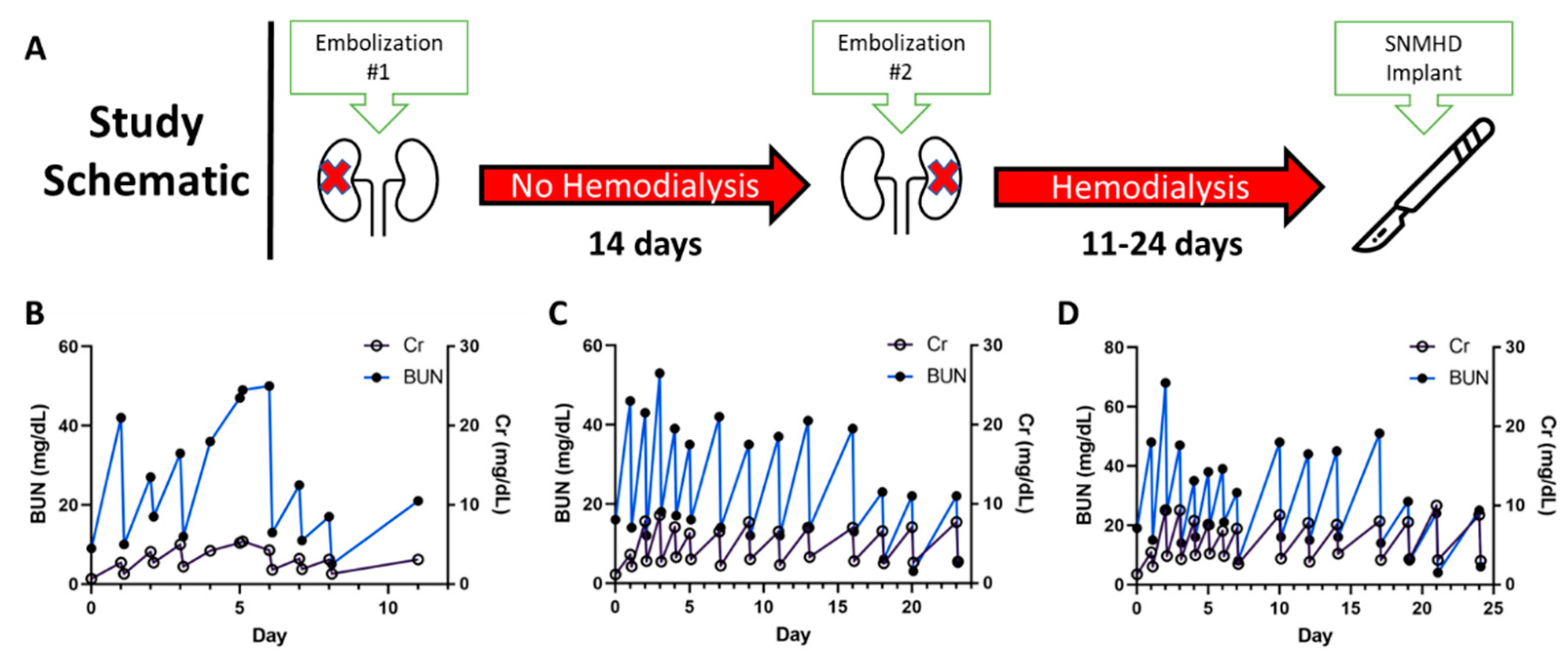
2.1. Staged Bilateral Renal Artery Embolization Induces a Stable Renal Failure State
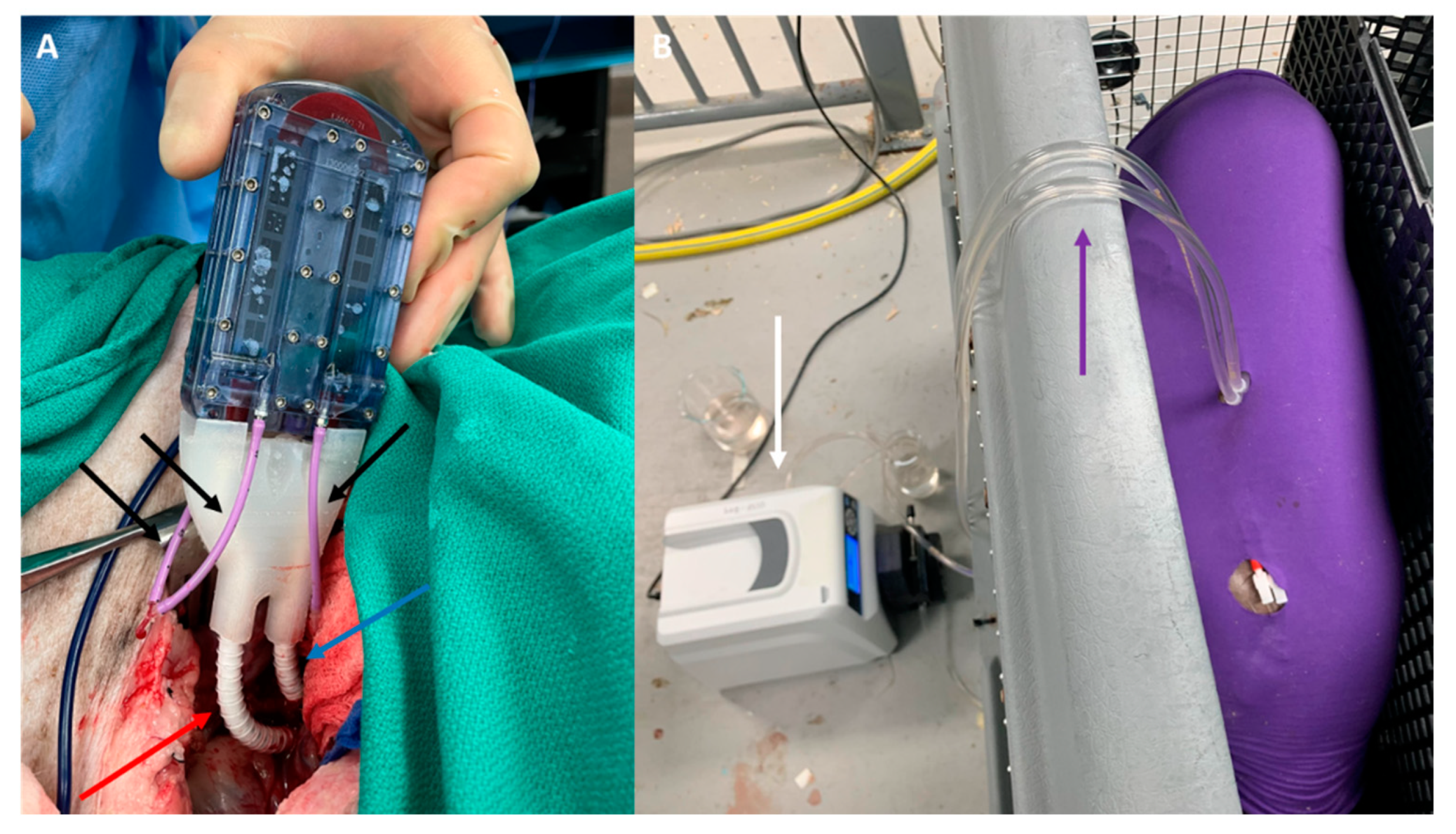
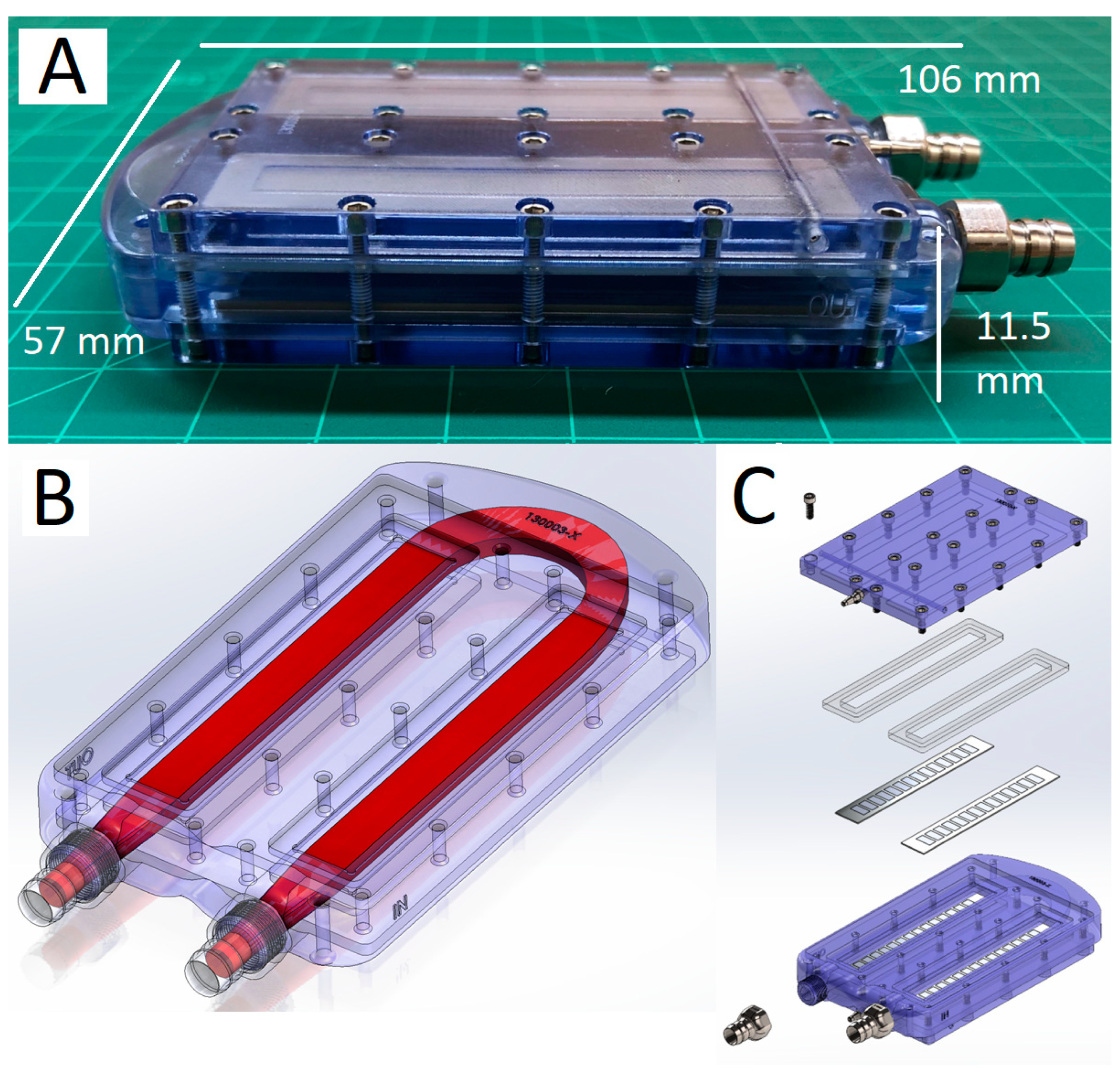
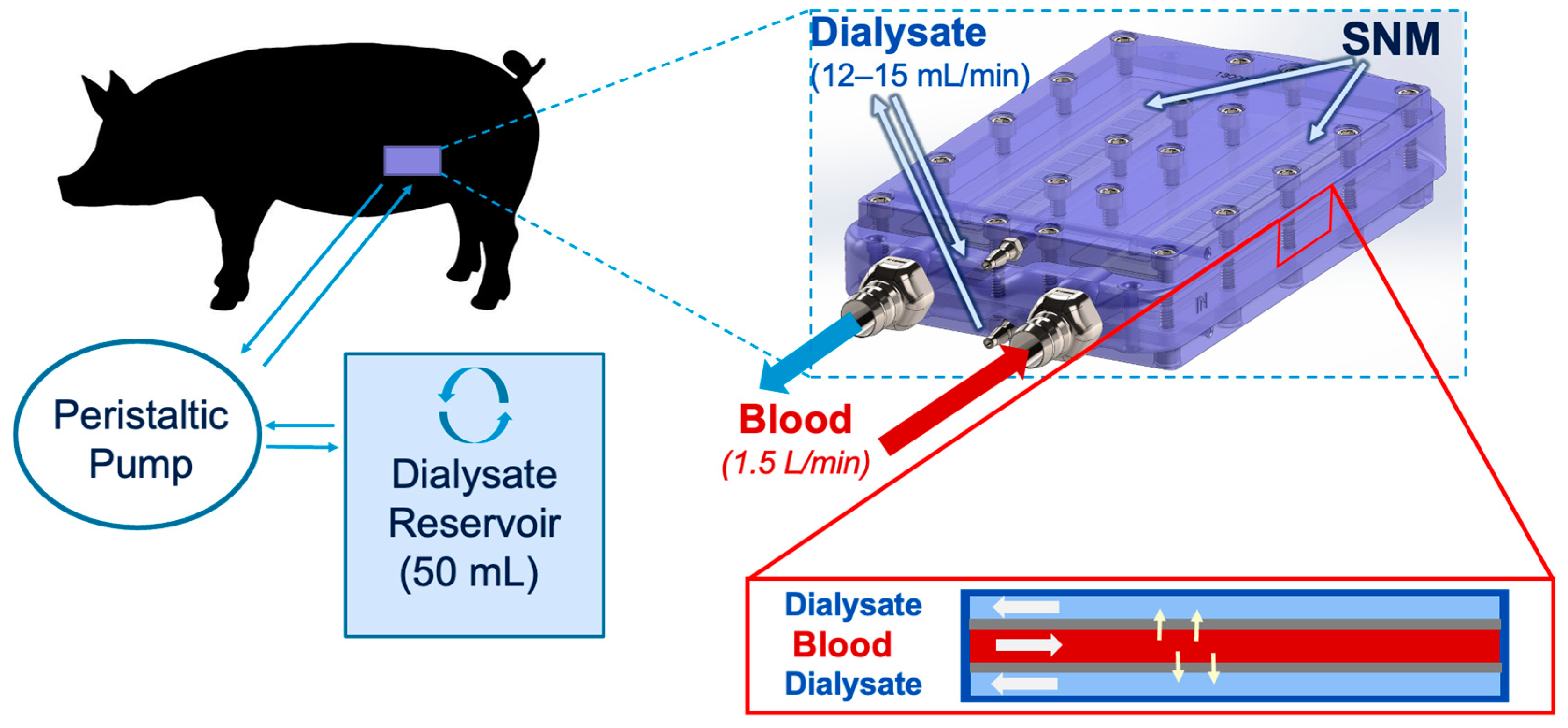
2.2. SNMHD Retroperitoneal Implantation and Patency
2.3. SNMHD Effectively Clears Potassium, Creatinine, BUN While Preventing Albumin Loss
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Silicon Nanopore Membrane Fabrication
5.2. Construction of Silicon Nanopore Membrane Hemodialyzer
5.3. Swine Renal Artery Embolization and Hemodialysis
5.4. SNMHD Implantation Procedure
5.5. Post-Procedure Animal and Device Dialysis and Solute Measurement
5.6. Explant and Retrieval of the SNM Hemodialyzer
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lentine, K.L.; Smith, J.M.; Hart, A.; Miller, J.; Skeans, M.A.; Larkin, L.; Robinson, A.; Gauntt, K.; Israni, A.K.; Hirose, R.; et al. OPTN/SRTR 2020 Annual Data Report: Kidney. Am. J. Transplant. 2022, 22, 21–136. [Google Scholar] [CrossRef]
- NIDDK; USRDS. Annual Data Report. Available online: https://adr.usrds.org/ (accessed on 10 January 2023).
- Tamura, M.K.; Yaffe, K. Dementia and Cognitive Impairment in ESRD: Diagnostic and Therapeutic Strategies. Kidney Int. 2011, 79, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Leavey, S.F.; Weitzel, W.F. Endocrine Abnormalities in Chronic Renal Failure. Endocrinol. Metab. Clin. N. Am. 2002, 31, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of Kidney Function with Anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Tappe, K.; Turkelson, C.; Doggett, D.; Coates, V. Disability under Social Security for Patients with ESRD: An Evidence-Based Review. Disabil. Rehabil. 2001, 23, 177–185. [Google Scholar] [CrossRef]
- Lacson, E.; Xu, J.; Suri, R.S.; Nesrallah, G.; Lindsay, R.; Garg, A.X.; Lester, K.; Ofsthun, N.; Lazarus, M.; Hakim, R.M. Survival with Three-Times Weekly In-Center Nocturnal versus Conventional Hemodialysis. J. Am. Soc. Nephrol. 2012, 23, 687–695. [Google Scholar] [CrossRef]
- Gura, V.; Rivara, M.B.; Bieber, S.; Munshi, R.; Smith, N.C.; Linke, L.; Kundzins, J.; Beizai, M.; Ezon, C.; Kessler, L.; et al. A Wearable Artificial Kidney for Patients with End-Stage Renal Disease. JCI Insight 2016, 1, e86397. [Google Scholar] [CrossRef]
- Gura, V.; Macy, A.S.; Beizai, M.; Ezon, C.; Golper, T.A. Technical Breakthroughs in the Wearable Artificial Kidney (WAK). Clin. J. Am. Soc. Nephrol. 2009, 4, 1441–1448. [Google Scholar] [CrossRef]
- Miller, J.J.; Carter, J.A.; Hill, K.; DesOrmeaux, J.-P.S.; Carter, R.N.; Gaborski, T.R.; Roussie, J.A.; McGrath, J.L.; Johnson, D.G. Free Standing, Large-Area Silicon Nitride Membranes for High Toxin Clearance in Blood Surrogate for Small-Format Hemodialysis. Membranes 2020, 10, 119. [Google Scholar] [CrossRef]
- Nesbitt, H. American Society of Nephrology|Kidney Week—Abstract Details. 2021. Available online: https://www.asn-online.org/education/kidneyweek/2021/program-abstract.aspx?controlId=3604321 (accessed on 10 January 2023).
- Kensinger, C.; Karp, S.; Kant, R.; Chui, B.W.; Goldman, K.; Yeager, T.; Gould, E.R.; Buck, A.; Laneve, D.C.; Groszek, J.J.; et al. First Implantation of Silicon Nanopore Membrane Hemofilters. ASAIO J. 2016, 62, 491–495. [Google Scholar] [CrossRef]
- van Gelder, M.K.; de Vries, J.C.; Simonis, F.; Monninkhof, A.S.; Hazenbrink, D.H.M.; Ligabue, G.; Giovanella, S.; Joles, J.A.; Verhaar, M.C.; Bajo Rubio, M.A.; et al. Evaluation of a System for Sorbent-assisted Peritoneal Dialysis in a Uremic Pig Model. Physiol. Rep. 2020, 8, e14593. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Stegmayr, B.G.; Ash, S.R.; Kuchinka, J.; Wieringa, F.P.; Fissell, W.H.; Roy, S. Wearable and Implantable Artificial Kidney Devices for End-Stage Kidney Disease Treatment—Current Status and Review. Artif. Organs 2023, 47, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Chevtchik, N.V.; Fedecostante, M.; Jansen, J.; Mihajlovic, M.; Wilmer, M.; Rüth, M.; Masereeuw, R.; Stamatialis, D. Upscaling of a Living Membrane for Bioartificial Kidney Device. Eur. J. Pharmacol. 2016, 790, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Buffington, D.A.; MacKay, S.M.; Funke, A.J.; Weitzel, W.F. Replacement of Renal Function in Uremic Animals with a Tissue-Engineered Kidney. Nat. Biotechnol. 1999, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, P.; Ip, T.K.; Panol, G.; Galletti, P.M. The Bioartificial Kidney: Progress towards an Ultrafiltration Device with Renal Epithelial Cells Processing. Life Support Syst. 1987, 5, 159–168. [Google Scholar]
- Tumlin, J.; Wali, R.; Williams, W.; Murray, P.; Tolwani, A.J.; Vinnikova, A.K.; Szerlip, H.M.; Ye, J.; Paganini, E.P.; Dworkin, L.; et al. Efficacy and Safety of Renal Tubule Cell Therapy for Acute Renal Failure. J. Am. Soc. Nephrol. 2008, 19, 1034–1040. [Google Scholar] [CrossRef]
- Lee, D.B.N.; Roberts, M. A Peritoneal-Based Automated Wearable Artificial Kidney. Clin. Exp. Nephrol. 2008, 12, 171–180. [Google Scholar] [CrossRef]
- Gerritsen, K. WEAKID—Clinical Validation of Miniature Wearable Dialysis Machine—H2020. Impact 2018, 2018, 55–57. [Google Scholar] [CrossRef]
- Bujok, J.; Walski, T.; Czerski, A.; Gałecka, K.; Grzeszczuk-Kuć, K.; Zawadzki, W.; Witkiewicz, W.; Komorowska, M. Sheep Model of Haemodialysis Treatment. Lab. Anim. 2018, 52, 176–185. [Google Scholar] [CrossRef]
- de Vries, J.C.; van Gelder, M.K.; Monninkhof, A.S.; Ahmed, S.; Hazenbrink, D.H.M.; Nguyen, T.Q.; de Kort, G.A.P.; Vonken, E.-J.P.A.; Vaessen, K.R.D.; Joles, J.A.; et al. A Uremic Pig Model for Peritoneal Dialysis. Toxins 2022, 14, 635. [Google Scholar] [CrossRef]
- van Gelder, M.K.; de Vries, J.C.; Ahmed, S.; Monninkhof, A.S.; de Kort, G.A.P.; Vonken, E.-J.P.A.; Hazenbrink, D.H.M.; Vaessen, K.R.D.; Nguyen, T.Q.; Verhaar, M.C.; et al. A Uremic Goat Model Created by Subtotal Renal Artery Embolization and Gentamicin. Biology 2021, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Gordon, J.D.; Fu, A.A.; Glockner, J.F.; Chade, A.R.; Mandrekar, J.; Lerman, L.; Mukhopadhyay, D. The Porcine Remnant Kidney Model of Chronic Renal Insufficiency. J. Surg. Res. 2006, 135, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-F.; Li, H.; Bai, G.; Zhang, Q.-Z.; Gao, X.; Liu, T.; Wang, H.-B. Establishment of Renal Failure Models by Laparoscopy in Bama Pigs Which Underwent Partial Nephrectomy and Radical Contralateral Nephrectomy. J. Vet. Res. 2019, 63, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Bechara, G.R.; Damasceno-Ferreira, J.A.; Abreu, L.A.D.S.; Costa, W.S.; Sampaio, F.J.B.; Pereira-Sampaio, M.A.; Souza, D.B.D. Glomerular Loss after Arteriovenous and Arterial Clamping for Renal Warm Ischemia in a Swine Model. Acta Cir. Bras. 2016, 31, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Damasceno-Ferreira, J.A.; Abreu, L.A.S.; Bechara, G.R.; Costa, W.S.; Pereira-Sampaio, M.A.; Sampaio, F.J.B.; De Souza, D.B. Mannitol Reduces Nephron Loss after Warm Renal Ischemia in a Porcine Model. BMC Urol. 2018, 18, 16. [Google Scholar] [CrossRef]
- Baldwin, D.D.; Maynes, L.J.; Berger, K.A.; Desai, P.J.; Zuppan, C.W.; Zimmerman, G.J.; Winkielman, A.M.; Sterling, T.H.; Tsai, C.K.; Ruckle, H.C. Laparoscopic Warm Renal Ischemia in the Solitary Porcine Kidney Model. Urology 2004, 64, 592–597. [Google Scholar] [CrossRef]
- Orvieto, M.A.; Tolhurst, S.R.; Chuang, M.S.; Lyon, M.B.; Ritch, C.R.; Rapp, D.E.; Shalhav, A.L. Defining Maximal Renal Tolerance to Warm Ischemia in Porcine Laparoscopic and Open Surgery Model. Urology 2005, 66, 1111–1115. [Google Scholar] [CrossRef]
- Huang, J.; Bayliss, G.; Zhuang, S. Porcine Models of Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2021, 320, F1030–F1044. [Google Scholar] [CrossRef]
- Cui, J.; Tang, L.; Hong, Q.; Lin, S.; Sun, X.; Cai, G.; Bai, X.-Y.; Chen, X. N-Acetylcysteine Ameliorates Gentamicin-Induced Nephrotoxicity by Enhancing Autophagy and Reducing Oxidative Damage in Miniature Pigs. Shock 2019, 52, 622–630. [Google Scholar] [CrossRef]
- Robbins, M.E.; Bywaters, T.B.; Jaenke, R.S.; Hopewell, J.W.; Matheson, L.M.; Tothill, P.; Whitehouse, E. Long-Term Studies of Cisplatin-Induced Reductions in Porcine Renal Functional Reserve. Cancer Chemother. Pharmacol. 1992, 29, 309–315. [Google Scholar] [CrossRef]
- Kim, S.; Feinberg, B.; Kant, R.; Chui, B.; Goldman, K.; Park, J.; Moses, W.; Blaha, C.; Iqbal, Z.; Chow, C.; et al. Diffusive Silicon Nanopore Membranes for Hemodialysis Applications. PLoS ONE 2016, 11, e0159526. [Google Scholar] [CrossRef]
- Fissell, W.H.; Dubnisheva, A.; Eldridge, A.N.; Fleischman, A.J.; Zydney, A.L.; Roy, S. High-Performance Silicon Nanopore Hemofiltration Membranes. J. Membr. Sci. 2009, 326, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kanani, D.M.; Fissell, W.H.; Roy, S.; Dubnisheva, A.; Fleischman, A.; Zydney, A.L. Permeability—Selectivity Analysis for Ultrafiltration: Effect of Pore Geometry. J. Membr. Sci. 2010, 349, 405. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.A.; Hansford, D.J.; Kulinsky, L.; Nashat, A.H.; Rasi, G.; Tu, J.; Wang, Y.; Zhang, M.; Ferrari, M. Nanopore Technology for Biomedical Applications. Biomed. Microdevices 1999, 2, 11–40. [Google Scholar] [CrossRef]
- Song, S.; Yeung, R.; Park, J.; Posselt, A.M.; Desai, T.A.; Tang, Q.; Roy, S. Glucose-Stimulated Insulin Response of Silicon Nanopore-Immunoprotected Islets under Convective Transport. ACS Biomater. Sci. Eng. 2017, 3, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.G.; Khire, T.S.; Lyubarskaya, Y.L.; Smith, K.J.P.; DesOrmeaux, J.-P.S.; Taylor, J.G.; Gaborski, T.R.; Shestopalov, A.A.; Striemer, C.C.; McGrath, J.L. Ultrathin Silicon Membranes for Wearable Dialysis. Adv. Chronic Kidney Dis. 2013, 20, 508–515. [Google Scholar] [CrossRef]
- Hill, K.; Walker, S.N.; Salminen, A.; Chung, H.L.; Li, X.; Ezzat, B.; Miller, J.J.; DesOrmeaux, J.-P.S.; Zhang, J.; Hayden, A.; et al. Second Generation Nanoporous Silicon Nitride Membranes for High Toxin Clearance and Small Format Hemodialysis. Adv. Healthc. Mater. 2020, 9, e1900750. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Wang, X.; Wang, Z.; Jin, W.; Huang, J.; Wang, Y. Atomic Layer Deposition of Alumina on Porous Polytetrafluoroethylene Membranes for Enhanced Hydrophilicity and Separation Performances. J. Membr. Sci. 2012, 415–416, 435–443. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Kong, L.; Zhang, J.; Zuo, W. Static Adsorption of Protein-Polysaccharide Hybrids on Hydrophilic Modified Membranes Based on Atomic Layer Deposition: Anti-Fouling Performance and Mechanism Insight. J. Membr. Sci. 2018, 548, 470–480. [Google Scholar] [CrossRef]
- Iqbal, Z.; Kim, S.; Moyer, J.; Moses, W.; Abada, E.; Wright, N.; Kim, E.J.; Park, J.; Fissell, W.H.; Vartanian, S.; et al. In Vitro and in Vivo Hemocompatibility Assessment of Ultrathin Sulfobetaine Polymer Coatings for Silicon-Based Implants. J. Biomater. Appl. 2019, 34, 297–312. [Google Scholar] [CrossRef]
- Moyer, J.; Wilson, M.; Santandreu, A.; Chen, C.; Hu, D.; Kerdok, A.; Porock, E.; Wright, N.; Ly, J.; Blaha, C.; et al. Endovascular Nephrectomy in Swine for Evaluation of Implantable Devices for Renal Replacement Therapy. In Proceedings of the American Society of Nephrology Kidney Week 2022, Orlando, FL, USA, 3–6 November 2022. [Google Scholar]
- Morya, R.; Kumar, K.; Kumar, P. Anatomical and Physiological Similarities of Kidney in Different Experimental Animals Used for Basic Studies. J. Clin. Exp. Nephrol. 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Gómez, F.A.; Ballesteros, L.E.; Estupiñan, H.Y. Morphological Characterization of the Renal Arteries in the Pig: Comparative Analysis with the Human. Int. J. Morphol. 2017, 35, 319–324. [Google Scholar] [CrossRef]
- Packialakshmi, B.; Stewart, I.J.; Burmeister, D.M.; Chung, K.K.; Zhou, X. Large Animal Models for Translational Research in Acute Kidney Injury. Ren. Fail. 2020, 42, 1042–1058. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, L.; Croubels, S.; Paepe, P.D.; Wallis, S.C.; Pandey, S.; Roberts, J.A.; Lipman, J.; Cock, P.D.; Devreese, M. Conventional Pig as Animal Model for Human Renal Drug Excretion Processes: Unravelling the Porcine Renal Function by Use of a Cocktail of Exogenous Markers. Front. Pharmacol. 2020, 11, 883. [Google Scholar] [CrossRef]
- Sharma, S.; Johnson, R.W.; Desai, T.A. Evaluation of the Stability of Nonfouling Ultrathin Poly(Ethylene Glycol) Films for Silicon-Based Microdevices. Langmuir 2004, 20, 348–356. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, L.-C.; Yang, K.-C.; Chen, M.-J.; Lin, H.-C.; Han, Y.-Y. Enhancement of the Anticoagulant Capacity of Polyvinyl Chloride Tubing for Cardiopulmonary Bypass Circuit Using Aluminum Oxide Nanoscale Coating Applied through Atomic Layer Deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 527–534. [Google Scholar] [CrossRef]
- Chui, B.W.; Wright, N.J.; Ly, J.; Maginnis, D.A.; Haniff, T.M.; Blaha, C.; Fissell, W.H.; Roy, S. A Scalable, Hierarchical Rib Design for Larger-Area, Higher-Porosity Nanoporous Membranes for the Implantable Bio-Artificial Kidney. J. Microelectromech. Syst. 2020, 29, 762–768. [Google Scholar] [CrossRef]
- AVMA Guidelines for the Euthanasia of Animals. Available online: https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals (accessed on 18 February 2023).

| Current Work | de Vries et al. | Misra et al. | |
|---|---|---|---|
| Animal | Female Yucatan minipig (age: 4–14 months, weight: 43–84 kg) | Female Yorkshire pig (age: 4 months, weight ~40 kg) | Male domestic pig (age: 7–8 months old, weight: 35 ± 1 kg) |
| Premedication | Antiplatelets: 3 days prior to device implant: Aspirin 81 mg daily, clopidogrel 75 mg daily Antibiotics: Trimethoprim/sulfamethoxazole 15 mg/kg daily starting day of device implant and continued for duration of study | Antibiotics: Amoxicillin/clavulanic acid 10 mg/kg × 1 pre-operatively, then twice daily after PD catheter insertion | None described |
| Anesthesia and Post-Operative Analgesia | Premedication: Telazol + atropine General anesthetic: Isoflurane +/− ketamine Intraoperative analgesia: Butorphanol + buprenorphine Postoperative analgesia: Buprenorphine IM/IV + NSAID per veterinary discretion | Premedication: Ketamine + atropine General anesthetic: Propofol Intraoperative analgesia: Remifentanil Postoperative analgesia: Meloxicam ×1 day, buphrenophine patch ×6 days | General anesthesia for embolization and additional procedures. Other details not provided. |
| Embolization Procedure | Dual staged embolization: Complete right renal artery embolization with PVA particles and microcoils; 14-day recovery; partial left inferior pole renal artery embolization | Single stage sub-total renal artery embolization (one kidney completely embolized, other partially) with PVA particles or microspheres | Single stage sub-total renal artery embolization (one kidney completely embolized, the other partially) with PVA particles and microcoils |
| Surgical Technique for Device Implantation | Implantation of hemodialyzer into left retroperitoneum with vascular connections to iliac vessels and dialysis catheters tunneled externally | Insertion of peritoneal dialysis catheter inserted via standard subcutaneous tunneling method | No device implant |
| Grade of Kidney Dysfunction | Peak creatinine: 8.0 ± 2.4 mg/dL Peak BUN: 57.0 ± 9.6 mg/dL | Peak creatinine: 10.5 ± 5.3 mg/dL Peak BUN: 46.7 ± 14.8 mg/dL | Peak creatinine: 5.7 ± 2.8 Peak BUN: 56.5 ± 31.7 mg/dL |
| Complications | None | Pulmonary hemorrhage in two animals after embolization with PVA; no further complications after change to embolization with microspheres | Five animals died in pilot group: one from hyperkalemia, one euthanized for hindlimb paralysis, and three following general anesthesia for blood draws. Three animals died in the definitive group: one immediately after embolization, and two euthanized for hindlimb paralysis. |
| Maximum Duration of Study | 31 days | 285 days | 84 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyer, J.; Wilson, M.W.; Sorrentino, T.A.; Santandreu, A.; Chen, C.; Hu, D.; Kerdok, A.; Porock, E.; Wright, N.; Ly, J.; et al. Renal Embolization-Induced Uremic Swine Model for Assessment of Next-Generation Implantable Hemodialyzers. Toxins 2023, 15, 547. https://doi.org/10.3390/toxins15090547
Moyer J, Wilson MW, Sorrentino TA, Santandreu A, Chen C, Hu D, Kerdok A, Porock E, Wright N, Ly J, et al. Renal Embolization-Induced Uremic Swine Model for Assessment of Next-Generation Implantable Hemodialyzers. Toxins. 2023; 15(9):547. https://doi.org/10.3390/toxins15090547
Chicago/Turabian StyleMoyer, Jarrett, Mark W. Wilson, Thomas A. Sorrentino, Ana Santandreu, Caressa Chen, Dean Hu, Amy Kerdok, Edward Porock, Nathan Wright, Jimmy Ly, and et al. 2023. "Renal Embolization-Induced Uremic Swine Model for Assessment of Next-Generation Implantable Hemodialyzers" Toxins 15, no. 9: 547. https://doi.org/10.3390/toxins15090547
APA StyleMoyer, J., Wilson, M. W., Sorrentino, T. A., Santandreu, A., Chen, C., Hu, D., Kerdok, A., Porock, E., Wright, N., Ly, J., Blaha, C., Frassetto, L. A., Fissell, W. H., Vartanian, S. M., & Roy, S. (2023). Renal Embolization-Induced Uremic Swine Model for Assessment of Next-Generation Implantable Hemodialyzers. Toxins, 15(9), 547. https://doi.org/10.3390/toxins15090547