Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond
Abstract
1. Introduction
2. Impact of Hemodiafiltration on Biomarkers
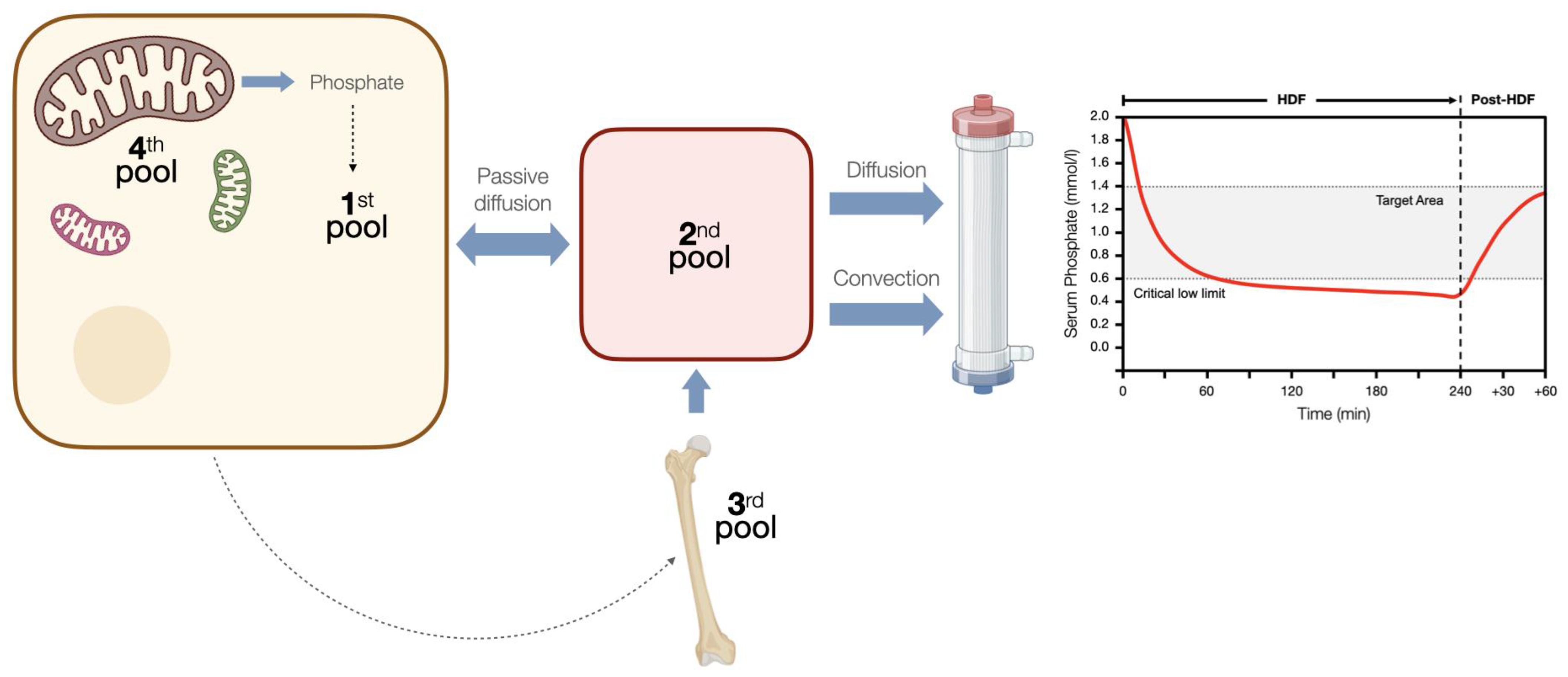
2.1. Phosphatemia
2.2. Oxidative Stress and Inflammation
3. Impact of Hemodiafiltration on Clinical Outcomes
3.1. Anemia
3.2. Immune Response and Infections
3.3. Nutritional Effects
3.4. Effects on Cardiovascular System
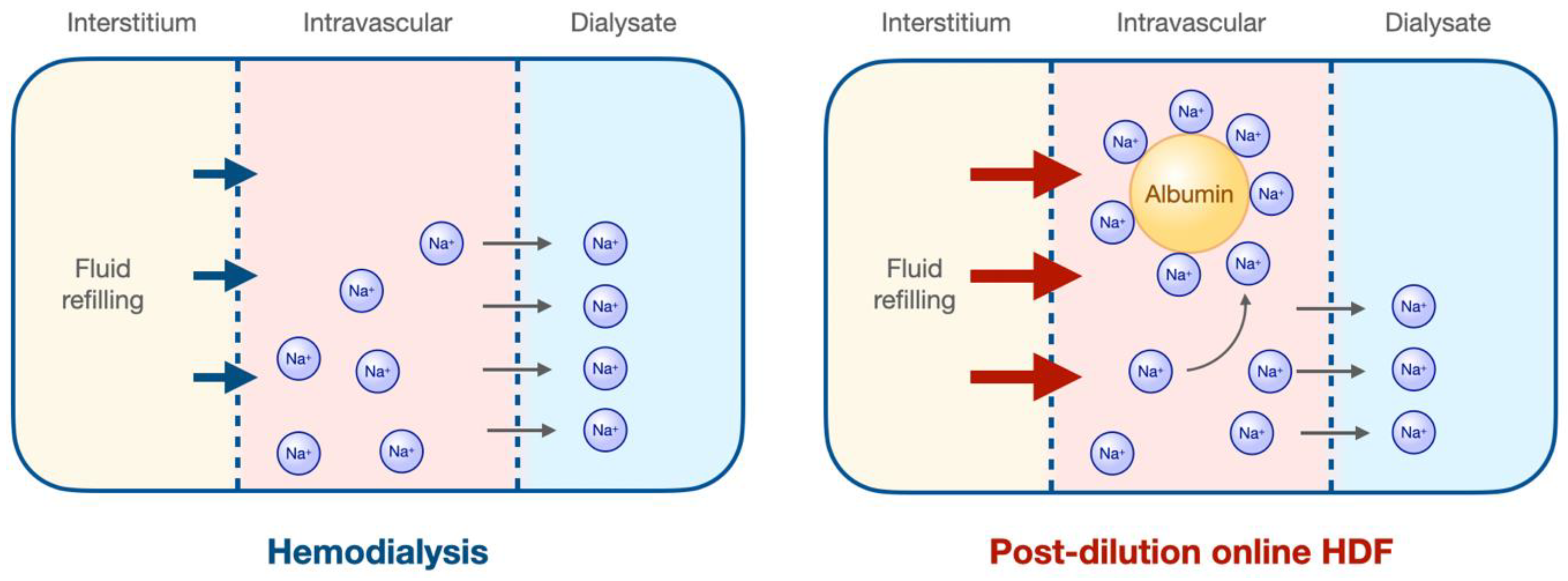
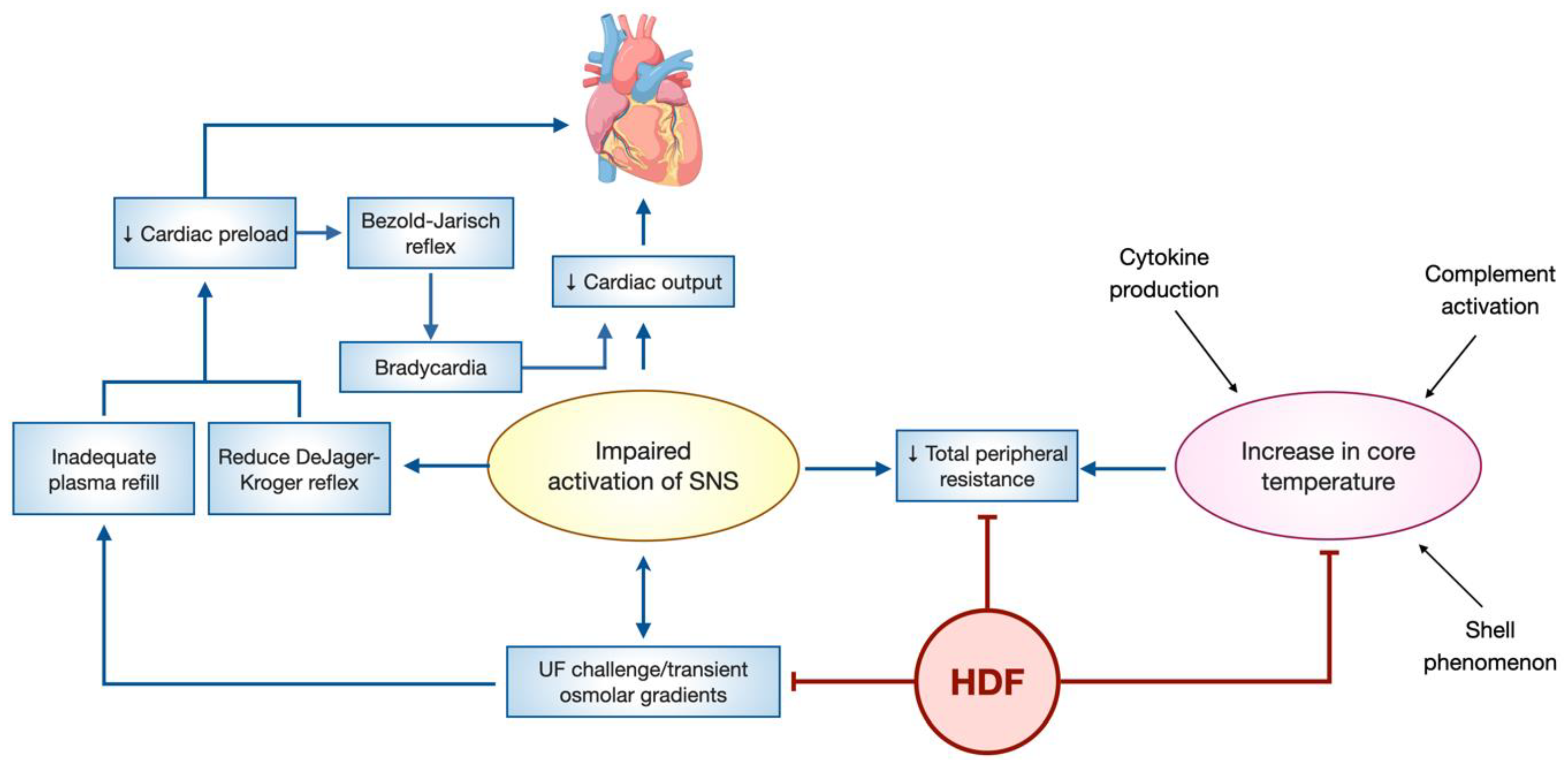
3.5. Hemodynamic Stability
3.6. Amyloidosis and Joint Pain
3.7. Neurological Symptoms
3.8. Patient-Reported Outcomes
4. Impact of Hemodiafiltration on Long-Term Outcomes
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Himmelfarb, J.; Ikizler, T.A. Hemodialysis. N. Engl. J. Med. 2010, 363, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; Kim, S.J.; McArthur, E.; Garg, A.X.; McCallum, M.K.; Knoll, G.A. Mortality in Incident Maintenance Dialysis Patients Versus Incident Solid Organ Cancer Patients: A Population-Based Cohort. Am. J. Kidney Dis. 2019, 73, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Gao, D.; Neri, M.; Ronco, C. Solute Transport in Hemodialysis: Advances and Limitations of Current Membrane Technology. Contrib. Nephrol. 2017, 191, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Clark, W.R. Haemodialysis Membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, J.E.; Ward, R.A.; Canaud, B.; Blankestijn, P.J.; Bots, M.; Covic, A.; Davenport, A.; Grooteman, M.; Gura, V.; Hegbrant, J.; et al. Online Haemodiafiltration: Definition, Dose Quantification and Safety Revisited. Nephrol. Dial. Transplant. 2013, 28, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Leber, H.; Wizemann, V.; Goubeaud, G.; Rawer, P.; Schütterle, G. Hemodiafiltration: A New Alternative to Hemofiltration and Conventional Hemodialysis. Artif. Organs 1978, 2, 150–153. [Google Scholar] [CrossRef]
- Canaud, B.; Nguyen, Q.V.; Argiles, A.; Polito, C.; Polascheoo, H.D.; Mion, C. Hemodiafiltration Using Dialysate as Substitution Fluid. Artif. Organs 1987, 11, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Lornoy, W.; Becaus, I.; Billiouw, J.M.; Sierens, L.; Malderen, P.V.; D’Haenens, P. On-line Haemodiafiltration. Remarkable Removal of Β2-microglobulin. Long-Term Clinical Observations. Nephrol. Dial. Transplant. 2000, 15, 49–54. [Google Scholar] [CrossRef]
- Guedes, M.; Vernooij, R.W.M.; Davenport, A.; Kuhlmann, M.K.; Aregger, F.; Pecoits-Filho, R. Clinical Performance, Intermediate and Long-Term Outcomes of High-Volume Hemodiafiltration in Patients with Kidney Failure. Semin. Dial. 2022, 35, 420–426. [Google Scholar] [CrossRef]
- Tong, A.; Manns, B.; Wang, A.Y.M.; Hemmelgarn, B.; Wheeler, D.C.; Gill, J.; Tugwell, P.; Pecoits-Filho, R.; Crowe, S.; Harris, T.; et al. Implementing Core Outcomes in Kidney Disease: Report of the Standardized Outcomes in Nephrology (SONG) Implementation Workshop. Kidney Int. 2018, 94, 1053–1068. [Google Scholar] [CrossRef]
- Farrington, K.; Davenport, A. The ESHOL Study: Hemodiafiltration Improves Survival—But How? Kidney Int. 2013, 83, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F. Fifteen Years of Experience with On-Line Hemodiafiltration. Contrib. Nephrol. 2011, 175, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Bragg-Gresham, J.L.; Marshall, M.R.; Desmeules, S.; Gillespie, B.W.; Depner, T.; Klassen, P.; Port, F.K. Mortality Risk for Patients Receiving Hemodiafiltration versus Hemodialysis: European Results from the DOPPS. Kidney Int. 2006, 69, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Grooteman, M.P.C.; van den Dorpel, M.A.; Bots, M.L.; Penne, E.L.; van der Weerd, N.C.; Mazairac, A.H.A.; den Hoedt, C.H.; van der Tweel, I.; Lévesque, R.; Nubé, M.J.; et al. Effect of Online Hemodiafiltration on All-Cause Mortality and Cardiovascular Outcomes. J. Am. Soc. Nephrol. 2012, 23, 1087–1096. [Google Scholar] [CrossRef]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and Cardiovascular Events in Online Haemodiafiltration (OL-HDF) Compared with High-Flux Dialysis: Results from the Turkish OL-HDF Study. Nephrol. Dial. Transplant. 2013, 28, 192–202. [Google Scholar] [CrossRef]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macià, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-Efficiency Postdilution Online Hemodiafiltration Reduces All-Cause Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2013, 24, 487–497. [Google Scholar] [CrossRef]
- Morena, M.; Jaussent, A.; Chalabi, L.; Leray-Moragues, H.; Chenine, L.; Debure, A.; Thibaudin, D.; Azzouz, L.; Patrier, L.; Maurice, F.; et al. Treatment Tolerance and Patient-Reported Outcomes Favor Online Hemodiafiltration Compared to High-Flux Hemodialysis in the Elderly. Kidney Int. 2017, 91, 1495–1509. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.C.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and Mortality in End-Stage Kidney Disease Patients: A Pooled Individual Participant Data Analysis from Four Randomized Controlled Trials. Nephrol. Dial. Transplant. 2016, 31, 978–984. [Google Scholar] [CrossRef]
- Fülöp, T.; Tapolyai, M.B.; Zsom, L.; Molnar, M.Z.; Salim, S.A.; Újhelyi, L.; Becs, G.; Balla, J.; Hamrahian, M. Successful Practice Transitioning Between Hemodialysis and Hemodiafiltration in Outpatient Units: Ten Key Issues for Physicians to Remember. Artif. Organs 2018, 42, 925–932. [Google Scholar] [CrossRef]
- Canaud, B.; Köhler, K.; Sichart, J.-M.; Möller, S. Global Prevalent Use, Trends and Practices in Haemodiafiltration. Nephrol. Dial. Transplant. 2019, 35, 398–407. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef]
- Eknoyan, G.; Beck, G.J.; Cheung, A.K.; Daugirdas, J.T.; Greene, T.; Kusek, J.W.; Allon, M.; Bailey, J.; Delmez, J.A.; Depner, T.A.; et al. Effect of Dialysis Dose and Membrane Flux in Maintenance Hemodialysis. N. Engl. J. Med. 2002, 347, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Masakane, I.; Sakurai, K. Current Approaches to Middle Molecule Removal: Room for Innovation. Nephrol. Dial. Transplant. 2018, 33, iii12–iii21. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Creput, C.; Bouzernidj, M.; Rodriguez, A.; Chalabi, L.; Seigneuric, B.; Lauret, C.; Bargnoux, A.-S.; Dupuy, A.-M.; Cristol, J.-P. Randomised Trial on Clinical Performances and Biocompatibility of Four High-Flux Hemodialyzers in Two Mode Treatments: Hemodialysis vs Post Dilution Hemodiafiltration. Sci. Rep. 2019, 9, 18265. [Google Scholar] [CrossRef] [PubMed]
- Leypoldt, J.K.; Storr, M.; Agar, B.U.; Boschetti-de-Fierro, A.; Bernardo, A.A.; Kirsch, A.H.; Rosenkranz, A.R.; Krieter, D.H.; Krause, B. Intradialytic Kinetics of Middle Molecules during Hemodialysis and Hemodiafiltration. Nephrol. Dial. Transplant. 2018, 34, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Roumelioti, M.-E.; Trietley, G.; Nolin, T.D.; Ng, Y.-H.; Xu, Z.; Alaini, A.; Figueroa, R.; Unruh, M.L.; Argyropoulos, C.P. Beta-2 Microglobulin Clearance in High-Flux Dialysis and Convective Dialysis Modalities: A Meta-Analysis of Published Studies. Nephrol. Dial. Transplant. 2018, 33, 542. [Google Scholar] [CrossRef]
- Lima, J.D.; Guedes, M.; Rodrigues, S.D.; Flórido, A.C.S.; Moreno-Amaral, A.N.; Barra, A.B.; Canziani, M.E.; Cuvello-Neto, A.; Poli-de-Figueiredo, C.E.; Pecoits-Filho, R.; et al. High-Volume Hemodiafiltration Decreases the Pre-Dialysis Concentrations of Indoxyl Sulfate and p-Cresyl Sulfate Compared to Hemodialysis: A Post-Hoc Analysis from the HDFit Randomized Controlled Trial. J. Nephrol. 2022, 35, 1449–1456. [Google Scholar] [CrossRef]
- Movilli, E.; Camerini, C.; Gaggia, P.; Poiatti, P.; Pola, A.; Viola, B.F.; Zubani, R.; Jeannin, G.; Cancarini, G. Effect of Post-Dilutional on-Line Haemodiafiltration on Serum Calcium, Phosphate and Parathyroid Hormone Concentrations in Uraemic Patients. Nephrol. Dial. Transplant. 2011, 26, 4032–4037. [Google Scholar] [CrossRef][Green Version]
- Penne, E.L.; van der Weerd, N.C.; van den Dorpel, M.A.; Grooteman, M.P.C.; Lévesque, R.; Nubé, M.J.; Bots, M.L.; Blankestijn, P.J.; ter Wee, P.M.; Investigators, C. Short-Term Effects of Online Hemodiafiltration on Phosphate Control: A Result from the Randomized Controlled Convective Transport Study (CONTRAST). Am. J. Kidney Dis. 2010, 55, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Comparison of Measured vs Kinetic-Model Predicted Phosphate Removal during Hemodialysis and Hemodiafiltration. Nephrol. Dial. Transplant. 2022, 37, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.; Hillion, D.; Dongradi, G. Phosphate Kinetics in Dialysis Patients. Nephrol. Dial. Transplant. 1991, 6 (Suppl. 2), 108–113. [Google Scholar]
- Kuhlmann, M.K. Phosphate Elimination in Modalities of Hemodialysis and Peritoneal Dialysis. Blood Purif. 2010, 29, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Bellizzi, V.; Cioffi, M.; Iodice, C.; Giannattasio, P.; Andreucci, M.; Terracciano, V.; Iorio, B.R.D.; Conte, G.; Nicola, L.D. Postdialytic Rebound of Serum Phosphorus: Pathogenetic and Clinical Insights. J. Am. Soc. Nephrol. 2002, 13, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Spalding, E.M.; Chamney, P.W.; Farrington, K. Phosphate Kinetics during Hemodialysis: Evidence for Biphasic Regulation. Kidney Int. 2002, 61, 655–667. [Google Scholar] [CrossRef]
- Chazot, G.; Lemoine, S.; Kocevar, G.; Kalbacher, E.; Sappey-Marinier, D.; Rouvière, O.; Juillard, L. Intracellular Phosphate and ATP Depletion Measured by Magnetic Resonance Spectroscopy in Patients Receiving Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2021, 32, 229–237. [Google Scholar] [CrossRef]
- Vaithilingam, I.; Polkinghorne, K.R.; Atkins, R.C.; Kerr, P.G. Time and Exercise Improve Phosphate Removal in Hemodialysis Patients. Am. J. Kidney Dis. 2004, 43, 85–89. [Google Scholar] [CrossRef]
- Eloot, S.; Biesen, W.V.; Dhondt, A.; de Wynkele, H.V.; Glorieux, G.; Verdonck, P.; Vanholder, R. Impact of Hemodialysis Duration on the Removal of Uremic Retention Solutes. Kidney Int. 2008, 73, 765–770. [Google Scholar] [CrossRef]
- Cornelis, T.; van der Sande, F.M.; Eloot, S.; Cardinaels, E.; Bekers, O.; Damoiseaux, J.; Leunissen, K.M.; Kooman, J.P. Acute Hemodynamic Response and Uremic Toxin Removal in Conventional and Extended Hemodialysis and Hemodiafiltration: A Randomized Crossover Study. Am. J. Kidney Dis. 2014, 64, 247–256. [Google Scholar] [CrossRef]
- Locatelli, F.; Canaud, B.; Eckardt, K.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative Stress in End-Stage Renal Disease: An Emerging Threat to Patient Outcome. Nephrol. Dial. Transplant. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3494867. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Hemodialysis: Causative Mechanisms, Clinical Implications, and Possible Therapeutic Interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Santoro, A.; Mancini, E. Is Hemodiafiltration the Technical Solution to Chronic Inflammation Affecting Hemodialysis Patients? Kidney Int. 2014, 86, 235–237. [Google Scholar] [CrossRef]
- Cohen, S.D.; Phillips, T.M.; Khetpal, P.; Kimmel, P.L. Cytokine Patterns and Survival in Haemodialysis Patients. Nephrol. Dial. Transplant. 2010, 25, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Tripepi, G.; Mallamaci, F. Dissecting Inflammation in ESRD: Do Cytokines and C-Reactive Protein Have a Complementary Prognostic Value for Mortality in Dialysis Patients? J. Am. Soc. Nephrol. 2006, 17, S169–S173. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.; Chieti, A.; Pontrelli, P.; Rascio, F.; Castellano, G.; Stallone, G.; Infante, B.; Gesualdo, L.; Grandaliano, G.; Pertosa, G. On-Line Hemodiafiltration Modulates Atherosclerosis Signaling in Peripheral Lymphomonocytes of Hemodialysis Patients. J. Nephrol. 2021, 34, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Dellepiane, S.; Fonsato, V.; Medica, D.; Marengo, M.; Migliori, M.; Quercia, A.D.; Pitino, A.; Formica, M.; Panichi, V.; et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating MiR-223 Expression in Plasma Extracellular Vesicles. J. Immunol. 2019, 202, 2372–2383. [Google Scholar] [CrossRef]
- Calò, L.A.; Naso, A.; Carraro, G.; Wratten, M.L.; Pagnin, E.; Bertipaglia, L.; Rebeschini, M.; Davis, P.A.; Piccoli, A.; Cascone, C. Effect of Haemodiafiltration with Online Regeneration of Ultrafiltrate on Oxidative Stress in Dialysis Patients. Nephrol. Dial. Transplant. 2007, 22, 1413–1419. [Google Scholar] [CrossRef]
- González-Diez, B.; Cavia, M.; Torres, G.; Abaigar, P.; Muñiz, P. Effect of a Hemodiafiltration Session with On-Line Regeneration of the Ultrafiltrate on Oxidative Stress. Blood Purif. 2008, 26, 505–510. [Google Scholar] [CrossRef]
- Filiopoulos, V.; Hadjiyannakos, D.; Metaxaki, P.; Sideris, V.; Takouli, L.; Anogiati, A.; Vlassopoulos, D. Inflammation and Oxidative Stress in Patients on Hemodiafiltration. Am. J. Nephrol. 2008, 28, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rizza, G.M.; Paoletti, S.; Bigazzi, R.; Aloisi, M.; Barsotti, G.; Rindi, P.; Donati, G.; Antonelli, A.; Panicucci, E.; et al. Chronic Inflammation and Mortality in Haemodialysis: Effect of Different Renal Replacement Therapies. Results from the RISCAVID Study. Nephrol. Dial. Transplant. 2008, 23, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Merino, A.; Nogueras, S.; Carretero, D.; Berdud, I.; Ramírez, R.; Tetta, C.; Rodríguez, M.; Martín-Malo, A.; Aljama, P. On-Line Hemodiafiltration Reduces the Proinflammatory CD14+CD16+ Monocyte-Derived Dendritic Cells: A Prospective, Crossover Study. J. Am Soc. Nephrol. 2006, 17, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Rama, I.; Llaudó, I.; Fontova, P.; Cerezo, G.; Soto, C.; Javierre, C.; Hueso, M.; Montero, N.; Martínez-Castelao, A.; Torras, J.; et al. Online Haemodiafiltration Improves Inflammatory State in Dialysis Patients: A Longitudinal Study. PLoS ONE 2016, 11, e0164969. [Google Scholar] [CrossRef]
- Panichi, V.; Paoletti, S.; Consani, C. Inflammatory Pattern in Hemodiafiltration. Contrib. Nephrol. 2008, 161, 185–190. [Google Scholar] [CrossRef]
- Vanholder, R.; Smet, R.D.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; Deyn, P.P.D.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Maduell, F.; del Pozo, C.; Garcia, H.; Sanchez, L.; Hdez-Jaras, J.; Albero, M.D.; Calvo, C.; Torregrosa, I.; Navarro, V. Change from Conventional Haemodiafiltration to On-Line Haemodiafiltration. Nephrol. Dial. Transplant. 1999, 14, 1202–1207. [Google Scholar] [CrossRef][Green Version]
- Bonforte, G.; Grillo, P.; Zerbi, S.; Surian, M. Improvement of Anemia in Hemodialysis Patients Treated by Hemodiafiltration with High-Volume On-Line-Prepared Substitution Fluid. Blood Purif. 2002, 20, 357–363. [Google Scholar] [CrossRef]
- Marcelli, D.; Bayh, I.; Merello, J.I.; Ponce, P.; Heaton, A.; Kircelli, F.; Chazot, C.; Benedetto, A.D.; Marelli, C.; Ladanyi, E.; et al. Dynamics of the Erythropoiesis Stimulating Agent Resistance Index in Incident Hemodiafiltration and High-Flux Hemodialysis Patients. Kidney Int. 2016, 90, 192–202. [Google Scholar] [CrossRef]
- Stefánsson, B.V.; Abramson, M.; Nilsson, U.; Haraldsson, B. Hemodiafiltration Improves Plasma 25-Hepcidin Levels: A Prospective, Randomized, Blinded, Cross-Over Study Comparing Hemodialysis and Hemodiafiltration. Nephron. Extra 2012, 2, 55–65. [Google Scholar] [CrossRef]
- Vaslaki, L.; Major, L.; Berta, K.; Karatson, A.; Misz, M.; Pethoe, F.; Ladanyi, E.; Fodor, B.; Stein, G.; Pischetsrieder, M.; et al. On-Line Haemodiafiltration versus Haemodialysis: Stable Haematocrit with Less Erythropoietin and Improvement of Other Relevant Blood Parameters. Blood Purif. 2006, 24, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, L.A.; Comelli, M.; Ruggiero, P.; Feliciani, A.; Manfrini, V.; Cozzi, G.; Castellano, A.; Pezzotta, M.; Gatti, G.; Arazzi, M.; et al. Mixed Hemodiafiltration Reduces Erythropoiesis Stimulating Agents Requirement in Dialysis Patients: A Prospective Randomized Study. J. Nephrol. 2020, 33, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, R.; Marcelli, D.; Cardinal, H.; Caron, M.-L.; Grooteman, M.P.C.; Bots, M.L.; Blankestijn, P.J.; Nubé, M.J.; Grassmann, A.; Canaud, B.; et al. Cost-Effectiveness Analysis of High-Efficiency Hemodiafiltration Versus Low-Flux Hemodialysis Based on the Canadian Arm of the CONTRAST Study. Appl. Health Econ. Health Policy 2015, 13, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of Immune Dysfunction in End-Stage Renal Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef]
- Haag-Weber, M.; Cohen, G.; Hörl, W.H. Clinical Significance of Granulocyte-Inhibiting Proteins. Nephrol. Dial. Transplant. 2000, 15, 15–16. [Google Scholar] [CrossRef][Green Version]
- Ward, R.A.; Schmidt, B.D.; Hullin, J.; Hillebrand, G.D.F.; Samtleben, W. A Comparison of On-Line Hemodiafiltration and High-Flux Hemodialysis: A Prospective Clinical Study. J. Am. Soc. Nephrol. 2000, 11, 2344–2350. [Google Scholar] [CrossRef]
- McIntyre, C.W.; Harrison, L.E.A.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.-C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating Endotoxemia: A Novel Factor in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef]
- Nongnuch, A.; Ngampongpan, W.; Srichatrapimuk, S.; Wongsa, A.; Thongpraphai, S.; Boonarkart, C.; Sanmeema, N.; Chittaganpitch, M.; Auewarakul, P.; Tassaneetrithep, B.; et al. Immune Response to Influenza Vaccination in ESRD Patients Undergoing Hemodialysis vs. Hemodiafiltration. PLoS ONE 2020, 15, e0227719. [Google Scholar] [CrossRef]
- Chuva, T.; Santos, T.; Gonçalves, F.; Costa, L.; Alves, E.; Neves, I.; Paiva, A.; Carvalho, B.; Sousa, T.; Ramalheiro, A.; et al. Humoral Immunity against COVID-19 Six Months after the Pfizer BNT162b2 Vaccine in Hemodialysis Patients: Data from Five Dialysis Units. Is There a Protective Role for Hemodiafiltration in the COVID-19 Pandemic? J. Nephrol. 2022, 35, 1543–1545. [Google Scholar] [CrossRef]
- Lioulios, G.; Fylaktou, A.; Asouchidou, D.; Xochelli, A.; Nikolaidou, V.; Stai, S.; Christodoulou, M.; Giamalis, P.; Tsouchnikas, I.; Papagianni, A.; et al. Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination against SARS-CoV-2 in Dialysis Patients. Ann. Lab. Med. 2023, 43, 451–460. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing Outcome Predictability of Markers of Malnutrition–Inflammation Complex Syndrome in Haemodialysis Patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Merabet, E.; Dagogo-Jack, S.; Coyne, D.W.; Klein, S.; Santiago, J.V.; Hmiel, S.P.; Landt, M. Increased Plasma Leptin Concentration in End-Stage Renal Disease. J. Clin. Endocrinol. Metab. 1997, 82, 847–850. [Google Scholar] [CrossRef]
- Mandolfo, S.; Borlandelli, S.; Imbasciati, E. Leptin and Beta2-Microglobulin Kinetics with Three Different Dialysis Modalities. Int. J. Artif. Organs 2006, 29, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Vizcaíno, B.; Molina, M.D.; Beltrán, S.; González-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Ávila, A.I.; Górriz, J.L.; et al. The Effect of High-Volume Online Haemodiafiltration on Nutritional Status and Body Composition: The ProtEin Stores PrEservaTion (PESET) Study. Nephrol. Dial. Transplant. 2018, 33, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Seikaly, M.G.; Salhab, N.; Warady, B.A.; Stablein, D. Use of RhGH in Children with Chronic Kidney Disease: Lessons from NAPRTCS. Pediatr. Nephrol. 2007, 22, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.; Terzic, J.; Menouer, S.; Dheu, C.; Seuge, L.; Zalosczic, A. Daily on Line Haemodiafiltration Promotes Catch-up Growth in Children on Chronic Dialysis. Nephrol. Dial. Transplant. 2010, 25, 867–873. [Google Scholar] [CrossRef]
- Shroff, R.; Bayazit, A.; Stefanidis, C.J.; Askiti, V.; Azukaitis, K.; Canpolat, N.; Agbas, A.; Anarat, A.; Aoun, B.; Bakkaloglu, S.; et al. Effect of Haemodiafiltration vs Conventional Haemodialysis on Growth and Cardiovascular Outcomes in Children—The HDF, Heart and Height (3H) Study. BMC Nephrol. 2018, 19, 199. [Google Scholar] [CrossRef]
- Zan, F.; Schmitt, C.P.; Shroff, R. Hemodiafiltration in the Pediatric Population. Semin. Dial. 2022, 35, 427–430. [Google Scholar] [CrossRef]
- Vanholder, R.; Smet, R.D.; Lameire, N. Protein-Bound Uremic Solutes: The Forgotten Toxins. Kidney Int. 2001, 59, S266–S270. [Google Scholar] [CrossRef]
- Krieter, D.H.; Canaud, B. High Permeability of Dialysis Membranes: What Is the Limit of Albumin Loss? Nephrol. Dial. Transplant. 2003, 18, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Quiroga, B.; Abad, S.; Aragoncillo, I.; Arroyo, D.; Panizo, N.; López-Gómez, J.M. Albumin Leakage in Online Hemodiafiltration, More Convective Transport, More Losses? Ther. Apher. Dial. 2014, 19, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Birmelé, B.; François, M.; Prat, L.; Halimi, J.-M. Factors Associated with Albumin Loss in Post-Dilution Hemodiafiltration and Nutritional Consequences. Int. J. Artif. Organs 2015, 38, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Macías, N.; Vega, A.; Abad, S.; Santos, A.; Cedeño, S.; Linares, T.; García-Prieto, A.M.; Aragoncillo, I.; Yuste, C.; López-Gómez, J.M. Is High-Volume Online Hemodiafiltration Associated with Malnutrition? Ther. Apher. Dial. 2017, 21, 361–369. [Google Scholar] [CrossRef]
- Salame, C.; Eaton, S.; Grimble, G.; Davenport, A. Protein Losses and Urea Nitrogen Underestimate Total Nitrogen Losses in Peritoneal Dialysis and Hemodialysis Patients. J. Renal. Nutr. 2018, 28, 317–323. [Google Scholar] [CrossRef]
- Potier, J.; Queffeulou, G.; Bouet, J. Are All Dialyzers Compatible with the Convective Volumes Suggested for Postdilution Online Hemodiafiltration? Int. J. Artif. Organs 2016, 39, 460–470. [Google Scholar] [CrossRef]
- Cuvelier, C.; Tintillier, M.; Migali, G.; Ende, C.V.; Pochet, J.-M. Albumin Losses during Hemodiafiltration: All Dialyzers Are Not Created Equal—A Case Report. BMC Nephrol. 2019, 20, 392. [Google Scholar] [CrossRef]
- Murtas, S.; Aquilani, R.; Deiana, M.L.; Iadarola, P.; Secci, R.; Cadeddu, M.; Salis, S.; Serpi, D.; Bolasco, P. Differences in Amino Acid Loss between High-Efficiency Hemodialysis and Postdilution and Predilution Hemodiafiltration Using High Convection Volume Exchange—A New Metabolic Scenario? A Pilot Study. J. Renal. Nutr. 2019, 29, 126–135. [Google Scholar] [CrossRef]
- Cheung, Y.; Wong, S.J.; Ho, M.H.K. Relationship between Carotid Intima-Media Thickness and Arterial Stiffness in Children after Kawasaki Disease. Arch. Dis. Child 2007, 92, 43. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; Sánchez, M.L.; Alvarez, F.L.; Nieto, C.S.; Mäkitie, A.A.; Olsen, K.D.; Ferlito, A. Evaluation of Intima-Media Thickness and Arterial Stiffness as Early Ultrasound Biomarkers of Carotid Artery Atherosclerosis. Cardiol. Ther. 2022, 11, 231–247. [Google Scholar] [CrossRef]
- Bellien, J.; Fréguin-Bouilland, C.; Joannidès, R.; Hanoy, M.; Rémy-Jouet, I.; Monteil, C.; Iacob, M.; Martin, L.; Renet, S.; Vendeville, C.; et al. High-Efficiency on-Line Haemodiafiltration Improves Conduit Artery Endothelial Function Compared with High-Flux Haemodialysis in End-Stage Renal Disease Patients. Nephrol. Dial. Transplant. 2014, 29, 414–422. [Google Scholar] [CrossRef]
- Buchanan, C.; Mohammed, A.; Cox, E.; Köhler, K.; Canaud, B.; Taal, M.W.; Selby, N.M.; Francis, S.; McIntyre, C.W. Intradialytic Cardiac Magnetic Resonance Imaging to Assess Cardiovascular Responses in a Short-Term Trial of Hemodiafiltration and Hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Power, A.; Charitaki, E.; Davenport, A. Haemodialysis and Haemodiafiltration Lead to Similar Changes in Vascular Stiffness during Treatment. Int. J. Artif. Organs 2016, 39, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.; Gaudino, M.; Naik, A.; Rahouma, M.; Robinson, N.B.; Ruan, Y.; Demetres, M.; Bossola, M. Comparison of the Effects of Hemodialysis and Hemodiafiltration on Left Ventricular Hypertrophy in End-Stage Renal Disease Patients: A Systematic Review and Meta-analysis. Semin. Dial. 2020, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Huang, C.-C.; Chang, C.-T.; Wu, M.-S.; Hung, C.-C.; Chien, C.-C.; Yang, C.-W. Clinical improvement by increased frequency of on-line hemodialfiltration. Renal. Fail. 2001, 23, 193–206. [Google Scholar] [CrossRef]
- Locatelli, F.; Altieri, P.; Andrulli, S.; Bolasco, P.; Sau, G.; Pedrini, L.A.; Basile, C.; David, S.; Feriani, M.; Montagna, G.; et al. Hemofiltration and Hemodiafiltration Reduce Intradialytic Hypotension in ESRD. J. Am. Soc. Nephrol. 2010, 21, 1798–1807. [Google Scholar] [CrossRef]
- Chazot, C.; Deleuze, S.; Fadel, B.; Hebibi, H.; Jean, G.; Levannier, M.; Puyoo, O.; Attaf, D.; Stuard, S.; Canaud, B. Is High-Volume Post-Dilution Haemodiafiltration Associated with Risk of Fluid Volume Imbalance? A National Multicentre Cross-Sectional Cohort Study. Nephrol. Dial. Transplant. 2019, 34, 2089–2095. [Google Scholar] [CrossRef]
- Kong, J.; Davies, M.; Mount, P. The Importance of Residual Kidney Function in Haemodialysis Patients: Residual Kidney Function in Haemodialysis Patients. Nephrology 2018, 23, 1073–1080. [Google Scholar] [CrossRef]
- Jansen, M.A.M.; Hart, A.A.M.; Korevaar, J.C.; Dekker, F.W.; Boeschoten, E.W.; Krediet, R.T.; Group, N.S. Predictors of the Rate of Decline of Residual Renal Function in Incident Dialysis Patients. Kidney Int. 2002, 62, 1046–1053. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M.; Fischer, R. Ultrapure Dialysis Fluid Slows Loss of Residual Renal Function in New Dialysis Patients. Nephrol. Dial. Transplant. 2002, 17, 1814–1818. [Google Scholar] [CrossRef]
- Mckane, W.; Chandna, S.M.; Tattersall, J.E.; Greenwood, R.N.; Farrington, K. Identical Decline of Residual Renal Function in High-Flux Biocompatible Hemodialysis and CAPD. Kidney Int. 2002, 61, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, T.; Koutoku, N. Preservation of Residual Renal Function with HDF. Contrib. Nephrol. 2010, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Grooteman, M.; Nubé, M. Reappraisal of Hemodiafiltration for Managing Uremic Complications. Clin. J. Am. Soc. Nephrol. 2021, 16, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.E.; Xue, H.; Lynch, K.E.; Curhan, G.C.; Brunelli, S.M. Association of Mortality Risk with Various Definitions of Intradialytic Hypotension. J. Am. Soc. Nephrol. 2015, 26, 724–734. [Google Scholar] [CrossRef]
- Cedeño, S.; Vega, A.; Macías, N.; Sánchez, L.; Abad, S.; López-Gómez, J.M.; Luño, J. Intradialytic Hypotension Definitions with Mortality Prediction Capacity in a Cohort of Haemodialysis Patients. Nefrol. Engl. Ed. 2020, 40, 402–412. [Google Scholar] [CrossRef]
- Gul, A.; Miskulin, D.; Harford, A.; Zager, P. Intradialytic Hypotension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Ertuglu, L.A.; Afsar, B.; Ozdogan, E.; Siriopol, D.; Covic, A.; Basile, C.; Ortiz, A. An Update Review of Intradialytic Hypotension: Concept, Risk Factors, Clinical Implications and Management. Clin. Kidney J. 2020, 13, sfaa078. [Google Scholar] [CrossRef]
- Hamrahian, S.M.; Vilayet, S.; Herberth, J.; Fülöp, T. Prevention of Intradialytic Hypotension in Hemodialysis Patients: Current Challenges and Future Prospects. Int. J. Nephrol. Renov. Dis. 2023, 16, 173–181. [Google Scholar] [CrossRef]
- Wang, A.Y.; Ninomiya, T.; Al-Kahwa, A.; Perkovic, V.; Gallagher, M.P.; Hawley, C.; Jardine, M.J. Effect of Hemodiafiltration or Hemofiltration Compared with Hemodialysis on Mortality and Cardiovascular Disease in Chronic Kidney Failure: A Systematic Review and Meta-Analysis of Randomized Trials. Am. J. Kidney Dis. 2014, 63, 968–978. [Google Scholar] [CrossRef]
- Kawanishi, H. Is There Enough Evidence to Prove That Hemodiafiltration Is Superior? Blood Purif. 2018, 46, 3–6. [Google Scholar] [CrossRef]
- Sars, B.; van der Sande, F.M.; Kooman, J.P. Intradialytic Hypotension: Mechanisms and Outcome. Blood Purif. 2020, 49, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Carfagna, F.; Vecchio, L.D.; Milia, V.L. Haemodialysis or Haemodiafiltration: That Is the Question. Nephrol. Dial. Transplant. 2018, 33, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Waniewski, J.; Pietribiasi, M.; Pstras, L. Calculation of the Gibbs–Donnan Factors for Multi-Ion Solutions with Non-Permeating Charge on Both Sides of a Permselective Membrane. Sci. Rep. 2021, 11, 22150. [Google Scholar] [CrossRef] [PubMed]
- Filippo, S.D.; Manzoni, C.; Andrulli, S.; Tentori, F.; Locatelli, F. Sodium Removal during Pre-Dilution Haemofiltration. Nephrol. Dial. Transplant. 2003, 18, vii31–vii36. [Google Scholar] [CrossRef][Green Version]
- Redaelli, B.; Limido, D.; Sforzini, S.; Beretta, P.; Bonoldi, G.; Dadone, C.; Filippo, G.D.; Mariani, P.; Mascia, F.; Pincella, G.; et al. Forecasting Correct Sodium Balance in Hemodiafiltration Procedures Involving Infusions. Blood Purif. 1991, 9, 123–128. [Google Scholar] [CrossRef]
- Milia, V.L.; Ravasi, C.; Carfagna, F.; Alberghini, E.; Baragetti, I.; Buzzi, L.; Ferrario, F.; Furiani, S.; Barbone, G.S.; Pontoriero, G. Sodium Removal and Plasma Tonicity Balance Are Not Different in Hemodialysis and Hemodiafiltration Using High-Flux Membranes. J. Nephrol. 2019, 32, 461–469. [Google Scholar] [CrossRef]
- Rodriguez, A.; Morena, M.; Bargnoux, A.; Chenine, L.; Leray-Moragues, H.; Cristol, J.P.; Canaud, B. Quantitative Assessment of Sodium Mass Removal Using Ionic Dialysance and Sodium Gradient as a Proxy Tool: Comparison of High-Flux Hemodialysis versus Online Hemodiafiltration. Artif. Organs 2021, 45, E280–E292. [Google Scholar] [CrossRef]
- Maggiore, Q.; Pizzarelli, F.; Sisca, S.; Zoccali, C.; Parlongo, S.; Nicolò, F.; Creazzo, G. Blood Temperature and Vascular Stability during Hemodialysis and Hemofiltration. Trans.—Am. Soc. Artif. Intern. Organs 1982, 28, 523–527. [Google Scholar]
- Donauer, J.; Schweiger, C.; Rumberger, B.; Krumme, B.; Böhler, J. Reduction of Hypotensive Side Effects during Online-Haemodiafiltration and Low Temperature Haemodialysis. Nephrol. Dial. Transplant. 2003, 18, 1616–1622. [Google Scholar] [CrossRef]
- Kumar, S.; Khosravi, M.; Massart, A.; Potluri, M.; Davenport, A. Haemodiafiltration Results in Similar Changes in Intracellular Water and Extracellular Water Compared to Cooled Haemodialysis. Am. J. Nephrol. 2013, 37, 320–324. [Google Scholar] [CrossRef]
- Sande, F.M.V.D.; Kooman, J.P.; Konings, C.J.; Leunissen, K.M.L. Thermal Effects and Blood Pressure Response during Postdilution Hemodiafiltration and Hemodialysis: The Effect of Amount of Replacement Fluid and Dialysate Temperature. J. Am. Soc. Nephrol. 2001, 12, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T. Lower Cardiovascular Mortality with High-Volume Hemodiafiltration: A Cool Effect? Nephrol. Dial. Transplant. 2016, 31, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Rosales, L.M.; Schneditz, D.; Morris, A.T.; Rahmati, S.; Levin, N.W. Isothermic Hemodialysis and Ultrafiltration. Am. J. Kidney Dis. 2000, 36, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gotch, F.A.; Keen, M.L.; Yarian, S.R. An Analysis of Thermal Regulation in Hemodialysis with One and Three Compartment Models. ASAIO J. 1989, 35, 622–624. [Google Scholar] [CrossRef]
- Reeves, P.B.; Causland, F.R.M. Mechanisms, Clinical Implications, and Treatment of Intradialytic Hypotension. Clin. J. Am. Soc. Nephrol. 2018, 13, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A. Why Is Intradialytic Hypotension the Commonest Complication of Outpatient Dialysis Treatments? Kidney Int. Rep. 2023, 8, 405–418. [Google Scholar] [CrossRef]
- Pizzarelli, F.; Cerrai, T.; Dattolo, P.; Tetta, C.; Maggiore, Q. Convective Treatments with On-Line Production of Replacement Fluid: A Clinical Experience Lasting 6 Years. Nephrol. Dial. Transplant. 1998, 13, 363–369. [Google Scholar] [CrossRef][Green Version]
- Canaud, B.; Blankestijn, P.J.; Grooteman, M.P.C.; Davenport, A. Why and How High Volume Hemodiafiltration May Reduce Cardiovascular Mortality in Stage 5 Chronic Kidney Disease Dialysis Patients? A Comprehensive Literature Review on Mechanisms Involved. Semin. Dial. 2022, 35, 117–128. [Google Scholar] [CrossRef]
- Locatelli, F.; Mastrangelo, F.; Redaelli, B.; Ronco, C.; Marcelli, D.; Greca, G.L.; Orlandini, G.; Group, T.I.C.D.S. Effects of Different Membranes and Dialysis Technologies on Patient Treatment Tolerance and Nutritional Parameters. Kidney Int. 1996, 50, 1293–1302. [Google Scholar] [CrossRef]
- Portales-Castillo, I.; Yee, J.; Tanaka, H.; Fenves, A.Z. Beta-2 Microglobulin Amyloidosis: Past, Present, and Future. Kidney360 2020, 1, 1447–1455. [Google Scholar] [CrossRef]
- Campistol, J.M. Dialysis-Related Amyloidosis After Renal Transplantation. Semin. Dial. 2001, 14, 99–102. [Google Scholar] [CrossRef]
- Venkataraman, S.; Kendrick, J. Barriers to Kidney Transplantation in ESKD. Semin. Dial. 2020, 33, 523–532. [Google Scholar] [CrossRef]
- Cheung, A.K.; Rocco, M.V.; Yan, G.; Leypoldt, J.K.; Levin, N.W.; Greene, T.; Agodoa, L.; Bailey, J.; Beck, G.J.; Clark, W.; et al. Serum β-2 Microglobulin Levels Predict Mortality in Dialysis Patients: Results of the HEMO Study. J. Am. Soc. Nephrol. 2006, 17, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Assounga, A.G. Beta 2 Microglobulin in Kidney Failure: A Review and an Algorithm for Renal Replacement Therapy. Saudi J. Kidney Dis. Transplant. 2021, 32, 1214–1220. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, X.; Sawashita, J.; Yao, J.; Zhang, B.; Qian, J.; Tomozawa, H.; Mori, M.; Ando, Y.; Naiki, H.; et al. Mouse Model to Study Human A Beta2M Amyloidosis: Generation of a Transgenic Mouse with Excessive Expression of Human Beta2-Microglobulin. Amyloid Int. J. Exp. Clin. Investig. 2010, 17, 50–62. [Google Scholar] [CrossRef]
- Yamamoto, S. Molecular Mechanisms Underlying Uremic Toxin-Related Systemic Disorders in Chronic Kidney Disease: Focused on Β2-Microglobulin-Related Amyloidosis and Indoxyl Sulfate-Induced Atherosclerosis—Oshima Award Address 2016. Clin. Exp. Nephrol. 2019, 23, 151–157. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayakawa, H.; Fujita, Y.; Takai, I.; Morita, H.; Emoto, Y.; Miyazaki, T.; Shinzato, T. Effectiveness of Push/Pull Hemodiafiltration Using Large-Pore Membrane for Shoulder Joint Pain in Long-Term Dialysis Patients. Artif. Organs 1990, 14, 321–327. [Google Scholar] [CrossRef]
- Kim, S.; Yamamoto, C.; Asabe, H.; Sato, T.; Takamiya, T. On-line Haemodiafiltration: Effective Removal of High Molecular Weight Toxins and Improvement in Clinical Manifestations of Chronic Haemodialysis Patients. Nephrology 1996, 2, S183–S186. [Google Scholar] [CrossRef]
- Sato, T.; Koga, N. Centralized On-Line Hemodiafiltration System Utilizing Purified Dialysate as Substitution Fluid. Artif. Organs 1998, 22, 285–290. [Google Scholar] [CrossRef]
- Kayalar, A.O.; Basturk, T.; Koc, Y.; Yilmaz, F.; Caglayan, F.B.; Sakaci, T.; Ahbap, E.; Ünsal, A. Comparison of Long-Term Complications in Patients on Haemodialysis and Peritoneal Dialysis Longer than 10 Years. J. Clin. Diagnostic. Res. 2016, 10, OC05-8. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Kiernan, M.C. Neurological Complications of Chronic Kidney Disease. Nat. Rev. Neurol. 2009, 5, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Malberti, F.; Surian, M.; Farina, M.; Vitelli, E.; Mandolfo, S.; Guri, L.; Petri, G.C.D.; Castellani, A. Effect of Hemodialysis and Hemodiafiltration on Uremic Neuropathy. Blood Purif. 1991, 9, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Pussell, B.A.; Pianta, T.J.; Grinius, V.; Lin, C.S.-Y.; Kiernan, M.C.; Howells, J.; Jardine, M.J.; Krishnan, A.V. Effects of Hemodiafiltration and High Flux Hemodialysis on Nerve Excitability in End-Stage Kidney Disease. PLoS ONE 2013, 8, e59055. [Google Scholar] [CrossRef] [PubMed]
- Karkar, A.; Abdelrahman, M.; Locatelli, F. A Randomized Trial on Health-Related Patient Satisfaction Level with High-Efficiency Online Hemodiafiltration versus High-Flux Dialysis. Blood Purif. 2015, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ji, F.; Chen, Z.-W.; Huang, Q.-L. Comparison of High-Flux Hemodialysis with Hemodialysis Filtration in Treatment of Uraemic Pruritus: A Randomized Controlled Trial. Int. Urol. Nephrol. 2016, 48, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Arzhan, S.; Roumelioti, M.-E.; Unruh, M.L. Itch and Ache on Dialysis: New Approaches to Manage Uremic Pruritus and Restless Legs. Blood Purif. 2020, 49, 222–227. [Google Scholar] [CrossRef]
- Sakurai, K.; Saito, T.; Hosoya, H.; Kurihara, Y.; Yamauchi, F. Therapeutic Effect of High-Efficiency Online Hemodiafiltration for Recurrent Restless Legs Syndrome in Dialysis Patients. J. Artif. Organs 2020, 23, 296–301. [Google Scholar] [CrossRef]
- Kang, A.; Arnold, R.; Gallagher, M.; Snelling, P.; Green, J.; Fernando, M.; Kiernan, M.C.; Hand, S.; Grimley, K.; Burman, J.; et al. Effect of Hemodiafiltration on the Progression of Neuropathy with Kidney Failure: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2020, 16, 1365–1375. [Google Scholar] [CrossRef]
- Nistor, I.; Palmer, S.C.; Craig, J.C.; Saglimbene, V.; Vecchio, M.; Covic, A.; Strippoli, G.F. Haemodiafiltration, Haemofiltration and Haemodialysis for End-stage Kidney Disease. Cochrane Database Syst. Rev. 2015, CD006258. [Google Scholar] [CrossRef]
- Moura, A.; Madureira, J.; Alija, P.; Fernandes, J.C.; Oliveira, J.G.; Lopez, M.; Filgueiras, M.; Amado, L.; Sameiro-Faria, M.; Miranda, V.; et al. Predictors of Health-Related Quality of Life Perceived by End-Stage Renal Disease Patients under Online Hemodiafiltration. Qual. Life Res. 2015, 24, 1327–1335. [Google Scholar] [CrossRef]
- Suwabe, T.; Barrera-Flores, F.J.; Rodriguez-Gutierrez, R.; Ubara, Y.; Takaichi, K. Effect of Online Hemodiafiltration Compared with Hemodialysis on Quality of Life in Patients with ESRD: A Systematic Review and Meta-Analysis of Randomized Trials. PLoS ONE 2018, 13, e0205037. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Larkin, J.; Poli-de-Figueiredo, C.E.; Cuvello-Neto, A.L.; Barra, A.B.L.; Gonçalves, P.B.; Sheth, S.; Guedes, M.; Han, M.; Calice-Silva, V.; et al. Effect of Hemodiafiltration on Measured Physical Activity: Primary Results of the HDFIT Randomized Controlled Trial. Nephrol. Dial. Transplant. 2020, 36, gfaa173. [Google Scholar] [CrossRef] [PubMed]
- Mazairac, A.H.A.; de Wit, G.A.; Grooteman, M.P.C.; Penne, E.L.; van der Weerd, N.C.; den Hoedt, C.H.; Lévesque, R.; van den Dorpel, M.A.; Nubé, M.J.; ter Wee, P.M.; et al. Effect of Hemodiafiltration on Quality of Life over Time. Clin. J. Am. Soc. Nephrol. 2013, 8, 82–89. [Google Scholar] [CrossRef][Green Version]
- Kantartzi, K.; Panagoutsos, S.; Mourvati, E.; Roumeliotis, A.; Leivaditis, K.; Devetzis, V.; Passadakis, P.; Vargemezis, V. Can Dialysis Modality Influence Quality of Life in Chronic Hemodialysis Patients? Low-Flux Hemodialysis versus High-Flux Hemodiafiltration: A Cross-Over Study. Renal. Fail. 2013, 35, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kashgary, A.; Khojah, A.; Bamalan, B.; Alafari, S.; Sindi, M.; Alahmari, A.; Gasm, I.; Alkhateeb, L.; Khojah, Y.; Abdelsalam, M. Effect of Hemodiafiltration Versus Hemodialysis on Cognitive Function among Patients with End-Stage Renal Disease: A Multicenter Study. Cureus 2021, 13, e19719. [Google Scholar] [CrossRef]
- Han, M.; Guedes, M.; Larkin, J.; Raimann, J.G.; Barra, A.B.L.; Canziani, M.E.F.; Neto, A.L.C.; Poli-de-Figueiredo, C.E.; Kotanko, P.; Pecoits-Filho, R. Effect of Hemodiafiltration on Self-Reported Sleep Duration: Results from a Randomized Controlled Trial. Blood Purif. 2020, 49, 168–177. [Google Scholar] [CrossRef]
- Hays, R.D.; Peipert, J.D.; Kallich, J.D. Problems with Analyses and Interpretation of Data in “Use of the KDQOL-36TM for Assessment of Health-Related Quality of Life among Dialysis Patients in the United States”. BMC Nephrol. 2019, 20, 447. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Fischer, K.I.; Barth, C.; Cromm, K.; Canaud, B.; Davenport, A.; Grobbee, D.E.; Hegbrant, J.; Roes, K.C.; Rose, M.; et al. Benefits and Harms of High-Dose Haemodiafiltration versus High-Flux Haemodialysis: The Comparison of High-Dose Haemodiafiltration with High-Flux Haemodialysis (CONVINCE) Trial Protocol. BMJ Open 2020, 10, e033228. [Google Scholar] [CrossRef]
- Caskey, F.J.; Procter, S.; MacNeill, S.J.; Wade, J.; Taylor, J.; Rooshenas, L.; Liu, Y.; Annaw, A.; Alloway, K.; Davenport, A.; et al. The High-Volume Haemodiafiltration vs High-Flux Haemodialysis Registry Trial (H4RT): A Multi-Centre, Unblinded, Randomised, Parallel-Group, Superiority Study to Compare the Effectiveness and Cost-Effectiveness of High-Volume Haemodiafiltration and High-Flux Haemodialysis in People with Kidney Failure on Maintenance Dialysis Using Linkage to Routine Healthcare Databases for Outcomes. Trials 2022, 23, 532. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) Developed and Tested Its First Wave of Adult Self-Reported Health Outcome Item Banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Peipert, J.D.; Hays, R.D. Methodological Considerations in Using Patient Reported Measures in Dialysis Clinics. J. Patient-Rep. Outcomes 2017, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Moreso, F.; Mora-Macià, J.; Pons, M.; Ramos, R.; Carreras, J.; Soler, J.; Torres, F.; En Nombre del Grupo del Estudio ESHOL. Reanálisis Del Estudio ESHOL: Mortalidad Por Todas Las Causas Considerando Riesgos de Competición y Tiempo-Dependientes Para Trasplante Renal. Nefrología 2016, 36, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Barbieri, C.; Marcelli, D.; Bellocchio, F.; Bowry, S.; Mari, F.; Amato, C.; Gatti, E. Optimal Convection Volume for Improving Patient Outcomes in an International Incident Dialysis Cohort Treated with Online Hemodiafiltration. Kidney Int. 2015, 88, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Peters, S.A.E.; Bots, M.L.; Canaud, B.; Grooteman, M.P.C.; Asci, G.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Higher Convection Volume Exchange with Online Hemodiafiltration Is Associated with Survival Advantage for Dialysis Patients: The Effect of Adjustment for Body Size. Kidney Int. 2016, 89, 193–199. [Google Scholar] [CrossRef]
- Mercadal, L.; Franck, J.-E.; Metzger, M.; Torres, P.U.; de Cornelissen, F.; Edet, S.; Béchade, C.; Vigneau, C.; Drüeke, T.; Jacquelinet, C.; et al. Hemodiafiltration Versus Hemodialysis and Survival in Patients With ESRD: The French Renal Epidemiology and Information Network (REIN) Registry. Am. J. Kidney Dis. 2016, 68, 247–255. [Google Scholar] [CrossRef]
- See, E.J.; Hedley, J.; Agar, J.W.M.; Hawley, C.M.; Johnson, D.W.; Kelly, P.J.; Lee, V.W.; Mac, K.; Polkinghorne, K.R.; Rabindranath, K.S.; et al. Patient Survival on Haemodiafiltration and Haemodialysis: A Cohort Study Using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol. Dial. Transplant. 2018, 34, 326–338. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Predilution Online Hemodiafiltration Is Associated with Improved Survival Compared with Hemodialysis. Kidney Int. 2019, 95, 929–938. [Google Scholar] [CrossRef]
- Valderrama, L.A.; Barrera, L.; Cantor, E.J.; Muñoz, J.; Arango, J.; Tobon, C.; Canaud, B. Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study. Kidney Dial. 2022, 2, 209–220. [Google Scholar] [CrossRef]
- Guedes, M.; Dambiski, A.C.; Canhada, S.; Barra, A.B.L.; Poli-de-Figueiredo, C.E.; Neto, A.L.C.; Canziani, M.E.F.; Strogoff-de-Matos, J.P.; Raimann, J.G.; Larkin, J.; et al. Achieving High Convective Volume in Hemodiafiltration: Lessons Learned after Successful Implementation in the HDFit Trial. Hemodial. Int. 2021, 25, 50–59. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.M.; Vernooij, R.W.M.; Bots, M.L.; Peters, S.A.E.; Dorresteijn, J.A.N.; Visseren, F.L.J.; Blankestijn, P.J.; Debray, T.P.A.; Bots, M.L.; Blankestijn, P.J.; et al. Personalizing Treatment in End-Stage Kidney Disease: Deciding between Haemodiafiltration and Haemodialysis Based on Individualized Treatment Effect Prediction. Clin. Kidney J. 2022, 15, 1924–1931. [Google Scholar] [CrossRef]
- Golper, T.A. Hemodiafiltration Outcomes in Special Situations. Semin. Dial. 2022, 35, 431–435. [Google Scholar] [CrossRef] [PubMed]
| Study Parameter | CONTRAST Study [14] | Turkish Study [15] | ESHOL Study [16] | FRENCHIE Study [17] | CONVINCE Study [21] |
|---|---|---|---|---|---|
| Comparison | LF-HD (n = 358) versus HDF (n = 356 | HF-HD (n = 391) versus HDF (n = 391) | HF-HD (n = 450) versus HDF (n = 456) | HF-HD (n = 191) versus HDF (n = 190) | HF-HD (n = 677) versus HDF (n = 683) |
| Mean follow-up (years) | 3.04 | 1.89 | 1.91 | 1.64 | 2.5 |
| Delivered convection volume | 20.7 L per session | >17.2 L per session + net UF | 22.9–23.9 L per session | 21 L per session | 24.8–25.7 L per session |
| Primary outcome | All-cause mortality | All-cause mortality + nonfatal CV events | All-cause mortality | Intradialytic tolerance | All-cause mortality |
| HR for primary outcome (95% CI) | 0.95 [0.75–1.20] | 0.82 [0.59–1.16] | 0.70 [0.53–0.92] | 0.94 [0.51–1.76] | 0.77 [0.65–0.93] |
| Secondary outcomes (HR, 95% CI) | Fatal + nonfatal CV events (1.07, 0.83–1.39) | CV and overall mortality (0.72, 0.45–1.13) | CV mortality (0.67, 0.44–1.02); infection-related mortality (0.45, 0.21–0.96) | All-cause mortality (0.83 0.52–1.33) | CV mortality (0.81, 0.49–1.33); infection-related mortality (0.69, 0.49–0.96) |
| Potential limitations | Target volume was not achieved in 50–66% patients. The control group used low-flux HD. Mortality significance was observed only with post hoc analysis. | Significance in mortality reduction only observed with post hoc analysis. Underpowered (lower-than-anticipated event rate). | HDF group was younger, less diabetic, and had a lower Charlson Comorbidity Index (CCI). RRF not monitored. | This study was underpowered (recruitment target not met). Mortality for entire kidney failure population was low. | The sample size was smaller than calculated, and it is difficult to interpret data related to cardiovascular mortality and hospitalizations due to the COVID-19 pandemic. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedreros-Rosales, C.; Jara, A.; Lorca, E.; Mezzano, S.; Pecoits-Filho, R.; Herrera, P. Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond. Toxins 2023, 15, 531. https://doi.org/10.3390/toxins15090531
Pedreros-Rosales C, Jara A, Lorca E, Mezzano S, Pecoits-Filho R, Herrera P. Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond. Toxins. 2023; 15(9):531. https://doi.org/10.3390/toxins15090531
Chicago/Turabian StylePedreros-Rosales, Cristian, Aquiles Jara, Eduardo Lorca, Sergio Mezzano, Roberto Pecoits-Filho, and Patricia Herrera. 2023. "Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond" Toxins 15, no. 9: 531. https://doi.org/10.3390/toxins15090531
APA StylePedreros-Rosales, C., Jara, A., Lorca, E., Mezzano, S., Pecoits-Filho, R., & Herrera, P. (2023). Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond. Toxins, 15(9), 531. https://doi.org/10.3390/toxins15090531





