Comparative Analysis of Alpha-1 Orthosteric-Site Binding by a Clade of Central American Pit Vipers (Genera Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium)
Abstract
1. Introduction
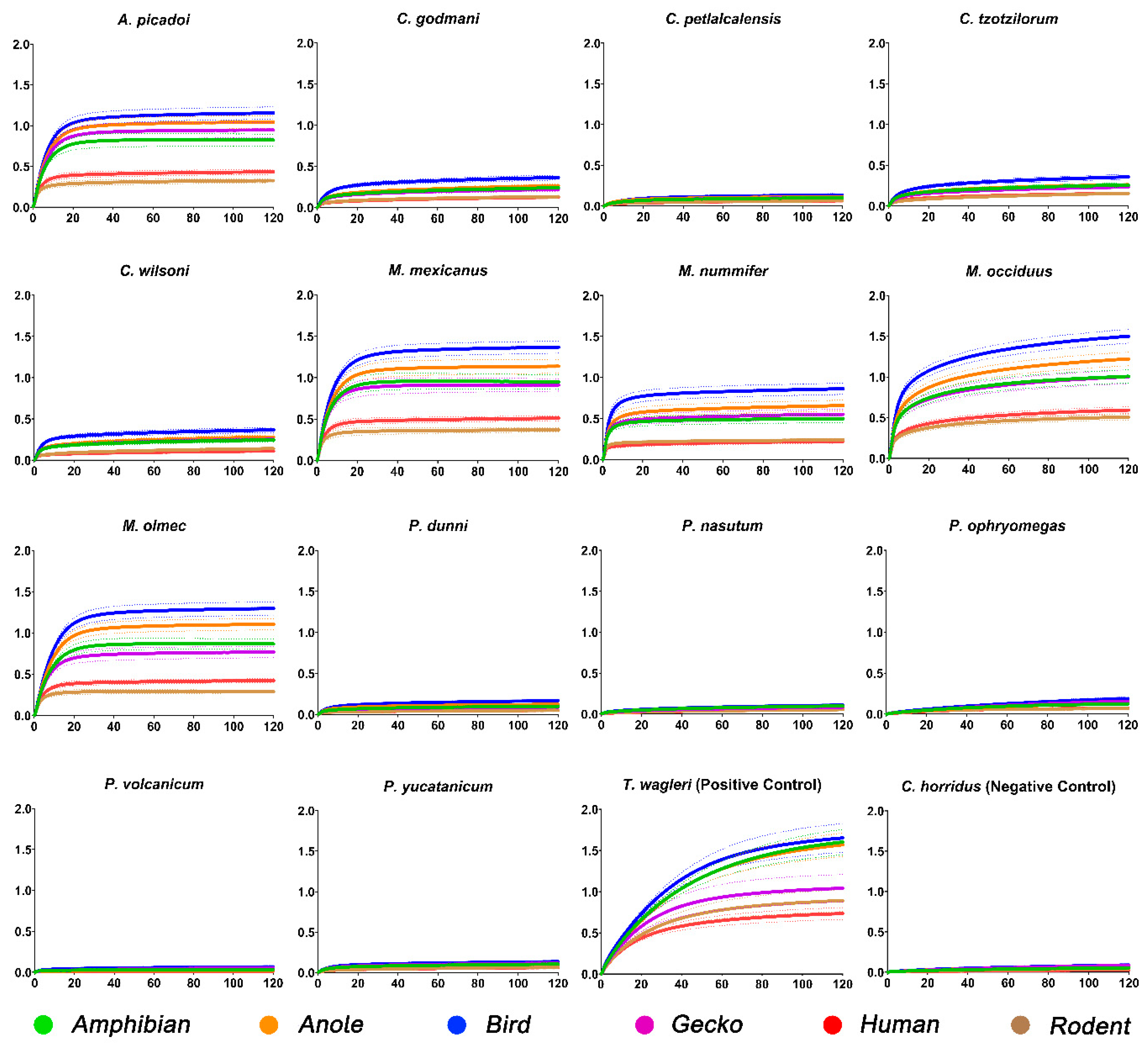
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venoms
5.2. Mimotope Preparation
5.3. Biolayer Interferometry
5.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Casewell, N.R.; Wüster, W.; Vidal, N.; Young, B.; Jackson, T.N. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.J.; Harris, R.J.; Huynh, T.M.; Coster, K.; Sundman, E.; Braun, R.; Naude, A.; Hodgson, W.C.; Fry, B.G. Widespread and differential neurotoxicity in venoms from the Bitis genus of viperid snakes. Neurotox. Res. 2021, 39, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.J.; Peng, Y.-H.; Harris, R.J.; Jones, L.; Llinas, J.; Haworth, M.; Gillett, A.; Fry, B.G. Differential coagulotoxic and neurotoxic venom activity from species of the arboreal viperid snake genus Bothriechis (palm-pitvipers). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109326. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Zdenek, C.N.; Fry, B.G. Diverse and Dynamic Alpha-Neurotoxicity Within Venoms from the Palearctic Viperid Snake Clade of Daboia, Macrovipera, Montivipera, and Vipera. Neurotox. Res. 2022, 40, 1793–1801. [Google Scholar] [CrossRef]
- Harris, R.J.; Zdenek, C.N.; Debono, J.; Harrich, D.; Fry, B.G. Evolutionary interpretations of nicotinic acetylcholine receptor targeting venom effects by a clade of Asian Viperidae snakes. Neurotox. Res. 2020, 38, 312–318. [Google Scholar] [CrossRef]
- Fry, B.G.; Lumsden, N.G.; Wüster, W.; Wickramaratna, J.C.; Hodgson, W.C.; Manjunatha Kini, R. Isolation of a neurotoxin (α-colubritoxin) from a nonvenomous colubrid: Evidence for early origin of venom in snakes. J. Mol. Evol. 2003, 57, 446–452. [Google Scholar] [CrossRef]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; Le Du, M.H.; Ménez, R.; Foo, C.S.; Ménez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef]
- Heyborne, W.H.; Mackessy, S.P. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae). Biochimie 2013, 95, 1923–1932. [Google Scholar] [CrossRef]
- Fry, B.G.; Wüster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Weise, C.; Kasheverov, I.E.; Andreeva, T.V.; Kryukova, E.V.; Zhmak, M.N.; Starkov, V.G.; Hoang, N.A.; Bertrand, D.; Ramerstorfer, J. Azemiopsin from Azemiops feae viper venom, a novel polypeptide ligand of nicotinic acetylcholine receptor. J. Biol. Chem. 2012, 287, 27079–27086. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Spirova, E.N.; Serebryakova, M.V.; Shelukhina, I.V.; Kudryavtsev, D.S.; Kryukova, E.V.; Starkov, V.G.; Kopylova, N.V.; Zhmak, M.N.; Ivanov, I.A. Peptides from puff adder Bitis arietans venom, novel inhibitors of nicotinic acetylcholine receptors. Toxicon 2016, 121, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Xie, B.; Violette, A.; Fourmy, R.; Jaeger, M.; Fry, B.G. Viper venom botox: The molecular origin and evolution of the waglerin peptides used in anti-wrinkle skin cream. J. Mol. Evol. 2017, 84, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.A.; Schmidt, J.J.; Bernheimer, A.W.; Smith, L.A. Characterization and amino acid sequences of two lethal peptides isolated from venom of Wagler’s pit viper, Trimeresurus wagleri. Toxicon 1991, 29, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J.; Zdenek, C.N.; Harrich, D.; Frank, N.; Fry, B.G. An appetite for destruction: Detecting prey-selective binding of α-neurotoxins in the venom of Afro-Asian elapids. Toxins 2020, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; Harris, R.J.; Kuruppu, S.; Youngman, N.J.; Dobson, J.S.; Debono, J.; Khan, M.; Smith, I.; Yarski, M.; Harrich, D. A taxon-specific and high-throughput method for measuring ligand binding to nicotinic acetylcholine receptors. Toxins 2019, 11, 600. [Google Scholar] [CrossRef]
- Serrano, S.M.; Shannon, J.D.; Wang, D.; Camargo, A.C.; Fox, J.W. A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: An approach to understanding venom proteomics. Proteomics 2005, 5, 501–510. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lewin, M.R.; Carter, R.; Soria, R.; Aldridge, M.; Fry, B.G. Extreme Procoagulant Potency in Human Plasma of Venoms from the African Viperid Genera Atheris, Cerastes, and Proatheris and the Relative Efficacy of Antivenoms and Synthetic Enzyme-Inhibitors. Toxins 2022, 14, 836. [Google Scholar] [CrossRef]
- Chowdhury, A.; Zdenek, C.N.; Dobson, J.S.; Bourke, L.A.; Soria, R.; Fry, B.G. Clinical implications of differential procoagulant toxicity of the Palearctic viperid genus Macrovipera, and the relative neutralization efficacy of antivenoms and enzyme inhibitors. Toxicol. Lett. 2021, 340, 77–88. [Google Scholar] [CrossRef]
- Gené, J.; Roy, A.; Rojas, G.; Gutiérrez, J.; Cerdas, L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon 1989, 27, 841–848. [Google Scholar] [CrossRef]
- Isbister, G.K. Procoagulant snake toxins: Laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin. Thromb. Hemost. 2009, 35, 93–103. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Pathophysiol. Haemost. Thromb. 2001, 31, 218–224. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Frank, N.; Afshar, S. De novo assessment and review of pan-american pit viper anticoagulant and procoagulant venom activities via kinetomic analyses. Toxins 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.J.; Debono, J.; Dobson, J.S.; Zdenek, C.N.; Harris, R.J.; Coimbra, F.C.; Naude, A.; Coster, K.; Sundman, E.; Braun, R. Venomous landmines: Clinical implications of extreme coagulotoxic diversification and differential neutralization by antivenom of venoms within the viperid snake genus Bitis. Toxins 2019, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.A.; Zdenek, C.N.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Gutiérrez, J.M.; Sanchez, E.F.; Aldridge, M.; Fry, B.G. Pan-American lancehead pit-vipers: Coagulotoxic venom effects and antivenom neutralisation of Bothrops asper and B. atrox geographical variants. Toxins 2021, 13, 78. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.; Do, M.S.; Fry, B.G. Clinical implications of coagulotoxic variations in Mamushi (Viperidae: Gloydius) snake venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 225, 108567. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.; Nouwens, A.; Ge, L.; Frank, N.; Kwok, H.F.; Fry, B.G. Habu coagulotoxicity: Clinical implications of the functional diversification of Protobothrops snake venoms upon blood clotting factors. Toxicol. Vitr. 2019, 55, 62–74. [Google Scholar] [CrossRef]
- Jones, L.; Youngman, N.J.; Neri-Castro, E.; Guadarrama-Martínez, A.; Lewin, M.R.; Carter, R.; Frank, N.; Fry, B.G. Differential Antivenom and Small-Molecule Inhibition of Novel Coagulotoxic Variations in Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium American Viperid Snake Venoms. Toxins 2022, 14, 511. [Google Scholar] [CrossRef]
- Seneci, L.; Zdenek, C.N.; Rodrigues, C.F.; Chowdhury, A.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Fry, B. A Clot Twist: Potent Coagulotoxicity Found in Mexican Neotropical Rattlesnakes. Front. Immunol. 2021, 12, 552. [Google Scholar] [CrossRef]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.C.; Gillett, A.; Del-Rei, T.H.; Chalkidis, H.d.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M. Coagulotoxicity of Bothrops (lancehead pit-vipers) venoms from Brazil: Differential biochemistry and antivenom efficacy resulting from prey-driven venom variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef]
- Bush, S.P.; Siedenburg, E. Neurotoxicity associated with suspected southern Pacific rattlesnake (Crotalus viridis helleri) envenomation. Wilderness Environ. Med. 1999, 10, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Diaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Neri-Castro, E.; Hernández-Dávila, A.; Olvera-Rodríguez, A.; Cardoso-Torres, H.; Bénard-Valle, M.; Bastiaans, E.; López-Gutierrez, O.; Alagón, A. Detection and quantification of a β-neurotoxin (crotoxin homologs) in the venom of the rattlesnakes Crotalus simus, C. culminatus and C. tzabcan from Mexico. Toxicon X 2019, 2, 100007. [Google Scholar] [CrossRef]
- Segura, Á.; Herrera, M.; Mares, F.R.; Jaime, C.; Sánchez, A.; Vargas, M.; Villalta, M.; Gómez, A.; Gutiérrez, J.M.; León, G. Proteomic, toxicological and immunogenic characterization of Mexican west-coast rattlesnake (Crotalus basiliscus) venom and its immunological relatedness with the venom of Central American rattlesnake (Crotalus simus). J. Proteom. 2017, 158, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wang, Y.-M.; Hseu, M.-J.; Tsai, I.-H. Molecular evolution and structure–function relationships of crotoxin-like and asparagine-6-containing phospholipases A2 in pit viper venoms. Biochem. J. 2004, 381, 25–34. [Google Scholar] [CrossRef]
- Lomonte, B.; Mora-Obando, D.; Fernández, J.; Sanz, L.; Pla, D.; Gutiérrez, J.M.; Calvete, J.J. First crotoxin-like phospholipase A2 complex from a New World non-rattlesnake species: Nigroviriditoxin, from the arboreal Neotropical snake Bothriechis nigroviridis. Toxicon 2015, 93, 144–154. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Lomonte, B.; Valdés, M.; Ponce-López, R.; Bénard-Valle, M.; Borja, M.; Strickland, J.L.; Jones, J.M.; Grünwald, C.; Zamudio, F. Venom characterization of the three species of Ophryacus and proteomic profiling of O. sphenophrys unveils Sphenotoxin, a novel Crotoxin-like heterodimeric β-neurotoxin. J. Proteom. 2019, 192, 196–207. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Guo, Q.; Ma, Z.-R.; Chen, Y.; Wang, Z.-Z.; Wang, X.-M.; Wang, Y.-M.; Tsai, I.-H. Structures and functions of crotoxin-like heterodimers and acidic phospholipases A2 from Gloydius intermedius venom: Insights into the origin of neurotoxic-type rattlesnakes. J. Proteom. 2015, 112, 210–223. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Yang, Y.-E.; Chen, Y.; Cao, J.; Zhang, C.; Liu, L.-L.; Wang, Z.-Z.; Wang, X.-M.; Wang, Y.-M.; Tsai, I.-H. Transcriptome and proteome of the highly neurotoxic venom of Gloydius intermedius. Toxicon 2015, 107, 175–186. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, J.D. Crotoxin, the neurotoxin of South American rattlesnake venom, is a presynaptic toxin acting like β-bungarotoxin. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1977, 296, 159–168. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Sanz, L.; Olvera-Rodríguez, A.; Bénard-Valle, M.; Alagón, A.; Calvete, J.J. Venomics and biochemical analysis of the black-tailed horned pitviper, Mixcoatlus melanurus, and characterization of Melanurutoxin, a novel crotoxin homolog. J. Proteom. 2020, 225, 103865. [Google Scholar]
- Jiménez-Charris, E.; Montealegre-Sanchez, L.; Solano-Redondo, L.; Mora-Obando, D.; Camacho, E.; Castro-Herrera, F.; Fierro-Pérez, L.; Lomonte, B. Proteomic and functional analyses of the venom of Porthidium lansbergii lansbergii (Lansberg’s hognose viper) from the Atlantic Department of Colombia. J. Proteom. 2015, 114, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: Biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteom. 2014, 105, 323–339. [Google Scholar]
- Lomonte, B.; Rey-Suárez, P.; Tsai, W.-C.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics of the pit vipers Porthidium nasutum, Porthidium ophryomegas, and Cerrophidion godmani from Costa Rica: Toxicological and taxonomical insights. J. Proteom. 2012, 75, 1675–1689. [Google Scholar] [CrossRef]
- Méndez, R.; Bonilla, F.; Sasa, M.; Dwyer, Q.; Fernández, J.; Lomonte, B. Proteomic profiling, functional characterization, and immunoneutralization of the venom of Porthidium porrasi, a pitviper endemic to Costa Rica. Acta Trop. 2019, 193, 113–123. [Google Scholar] [CrossRef]
- Ruiz-Campos, M.; Sanz, L.; Bonilla, F.; Sasa, M.; Lomonte, B.; Zaruma-Torres, F.; Terán, M.; Fernández, J.; Calvete, J.J.; Caldeira, C.A. Venomics of the poorly studied hognosed pitvipers Porthidium arcosae and Porthidium volcanicum. J. Proteom. 2021, 249, 104379. [Google Scholar] [CrossRef]
- Angulo, Y.; Escolano, J.; Lomonte, B.; Gutiérrez, J.M.; Sanz, L.; Calvete, J.J. Snake venomics of Central American pitvipers: Clues for rationalizing the distinct envenomation profiles of Atropoides nummifer and Atropoides picadoi. J. Proteome Res. 2008, 7, 708–719. [Google Scholar] [PubMed]
- García-Osorio, B.; Lomonte, B.; Bénard-Valle, M.; de León, J.L.; Román-Domínguez, L.; Mejía-Domínguez, N.R.; Lara-Hernández, F.; Alagón, A.; Neri-Castro, E. Ontogenetic changes in the venom of Metlapilcoatlus nummifer, the mexican jumping viper. Toxicon 2020, 184, 204–214. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Alencar, L.R.; Quental, T.B.; Grazziotin, F.G.; Alfaro, M.L.; Martins, M.; Venzon, M.; Zaher, H. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenetics Evol. 2016, 105, 50–62. [Google Scholar]
- Jin, A.-H.; Israel, M.R.; Inserra, M.C.; Smith, J.J.; Lewis, R.J.; Alewood, P.F.; Vetter, I.; Dutertre, S. δ-Conotoxin SuVIA suggests an evolutionary link between ancestral predator defence and the origin of fish-hunting behaviour in carnivorous cone snails. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150817. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.W.; Imperial, J.S.; Ueberheide, B.; Zhang, M.-M.; Aguilar, M.; Taylor, D.; Watkins, M.; Yoshikami, D.; Showers-Corneli, P.; Safavi-Hemami, H. Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus. Proc. Natl. Acad. Sci. USA 2015, 112, 5087–5092. [Google Scholar] [CrossRef]
- Yang, D.C.; Deuis, J.R.; Dashevsky, D.; Dobson, J.; Jackson, T.N.; Brust, A.; Xie, B.; Koludarov, I.; Debono, J.; Hendrikx, I. The snake with the scorpion’s sting: Novel three-finger toxin sodium channel activators from the venom of the long-glanded blue coral snake (Calliophis bivirgatus). Toxins 2016, 8, 303. [Google Scholar] [CrossRef]
- Campbell, J.A.; Brodie, E.D. Biology of the Pitvipers; Selva: Tyler, TX, USA, 1992. [Google Scholar]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Intraspecific variation in the feeding ecology of the crotaline snake Calloselasma rhodostoma in Southeast Asia. J. Herpetol. 1998, 32, 198–205. [Google Scholar] [CrossRef]
- Otero, R.; Gutiérrez, J.; Mesa, M.a.B.; Duque, E.; Rodŕguez, O.; Arango, J.L.; Gómez, F.; Toro, A.; Cano, F.; Rodŕguez, L.M.a. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef]
- Warrell, D. Snakebites in Central and South America: Epidemiology, clinical features, and clinical management. In the Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Cornell University Press: New York, NY, USA, 2004. [Google Scholar]
- Vulfius, C.A.; Gorbacheva, E.V.; Starkov, V.G.; Osipov, A.V.; Kasheverov, I.E.; Andreeva, T.V.; Astashev, M.E.; Tsetlin, V.I.; Utkin, Y.N. An unusual phospholipase A2 from puff adder Bitis arietans venom–a novel blocker of nicotinic acetylcholine receptors. Toxicon 2011, 57, 787–793. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.; Waite, C.; Neri-Castro, E.; Fry, B.G. Comparative Analysis of Alpha-1 Orthosteric-Site Binding by a Clade of Central American Pit Vipers (Genera Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium). Toxins 2023, 15, 487. https://doi.org/10.3390/toxins15080487
Jones L, Waite C, Neri-Castro E, Fry BG. Comparative Analysis of Alpha-1 Orthosteric-Site Binding by a Clade of Central American Pit Vipers (Genera Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium). Toxins. 2023; 15(8):487. https://doi.org/10.3390/toxins15080487
Chicago/Turabian StyleJones, Lee, Callum Waite, Edgar Neri-Castro, and Bryan G. Fry. 2023. "Comparative Analysis of Alpha-1 Orthosteric-Site Binding by a Clade of Central American Pit Vipers (Genera Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium)" Toxins 15, no. 8: 487. https://doi.org/10.3390/toxins15080487
APA StyleJones, L., Waite, C., Neri-Castro, E., & Fry, B. G. (2023). Comparative Analysis of Alpha-1 Orthosteric-Site Binding by a Clade of Central American Pit Vipers (Genera Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium). Toxins, 15(8), 487. https://doi.org/10.3390/toxins15080487






