Risk Assessment Considering the Bioavailability of 3-β-d-Glucosides of Deoxynivalenol and Nivalenol through Food Intake in Korea
Abstract
1. Introduction
2. Results and Discussion
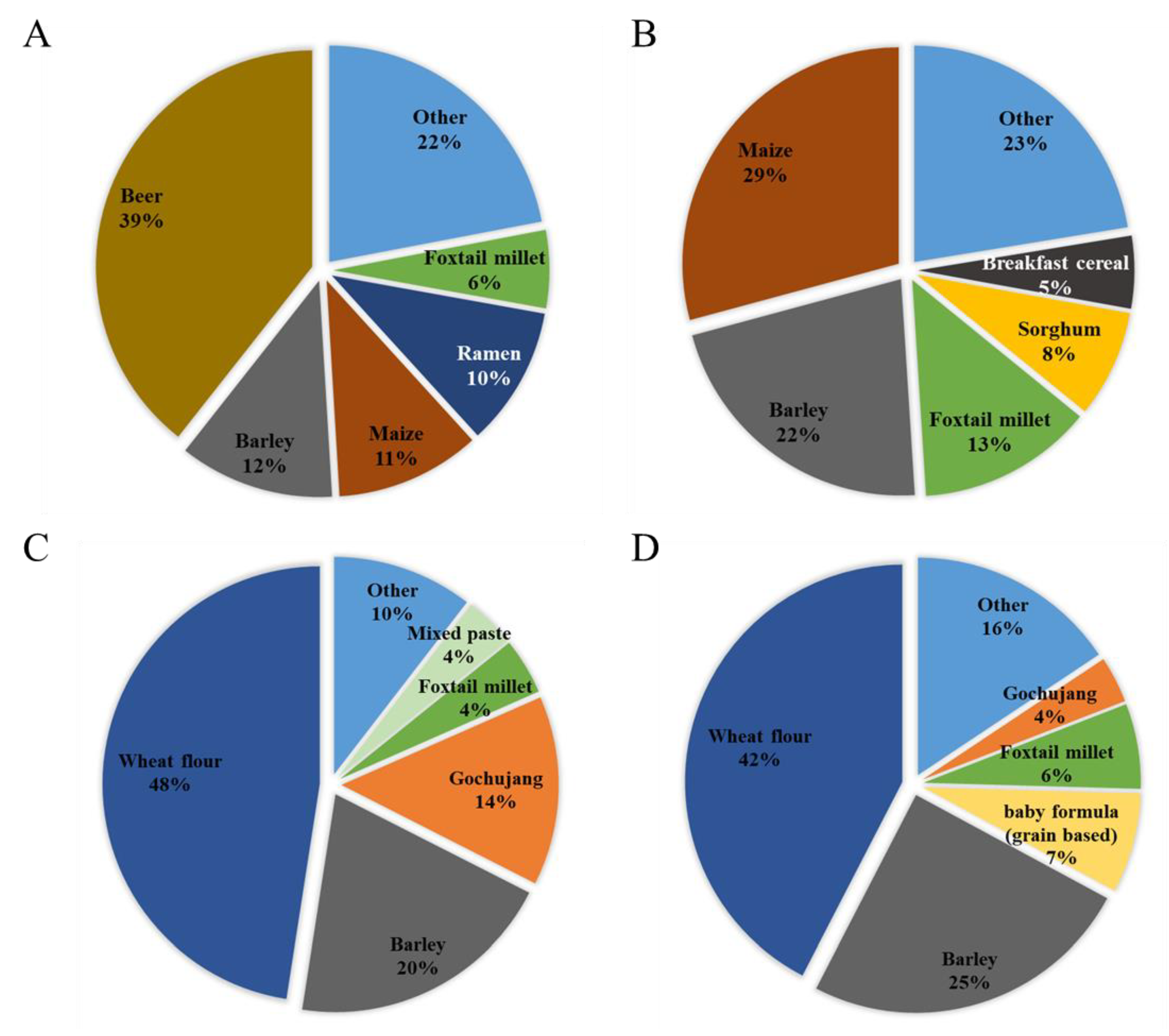
2.1. Estimated Exposure to DON, NIV, and Their Glucosides from Each Food
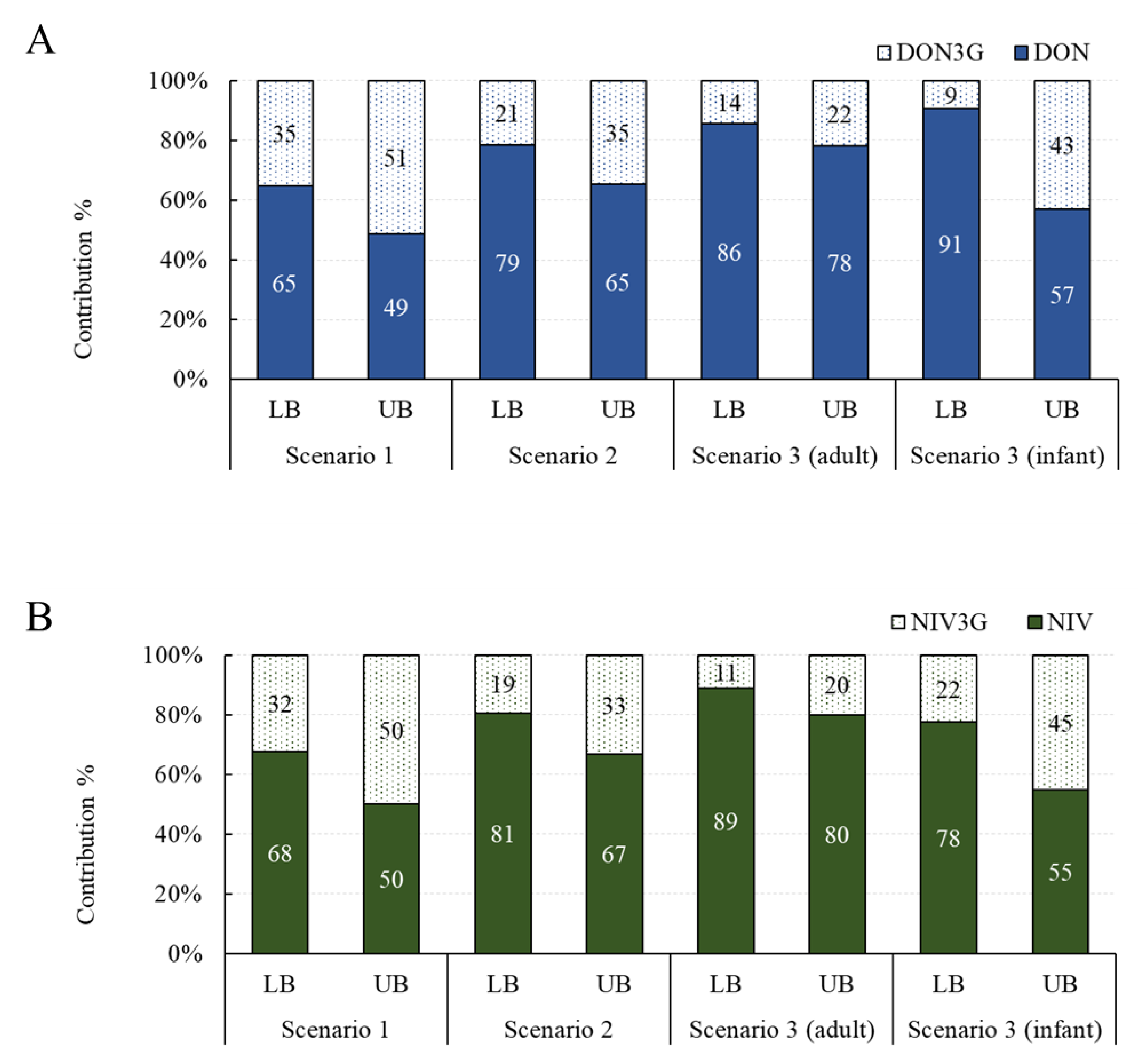
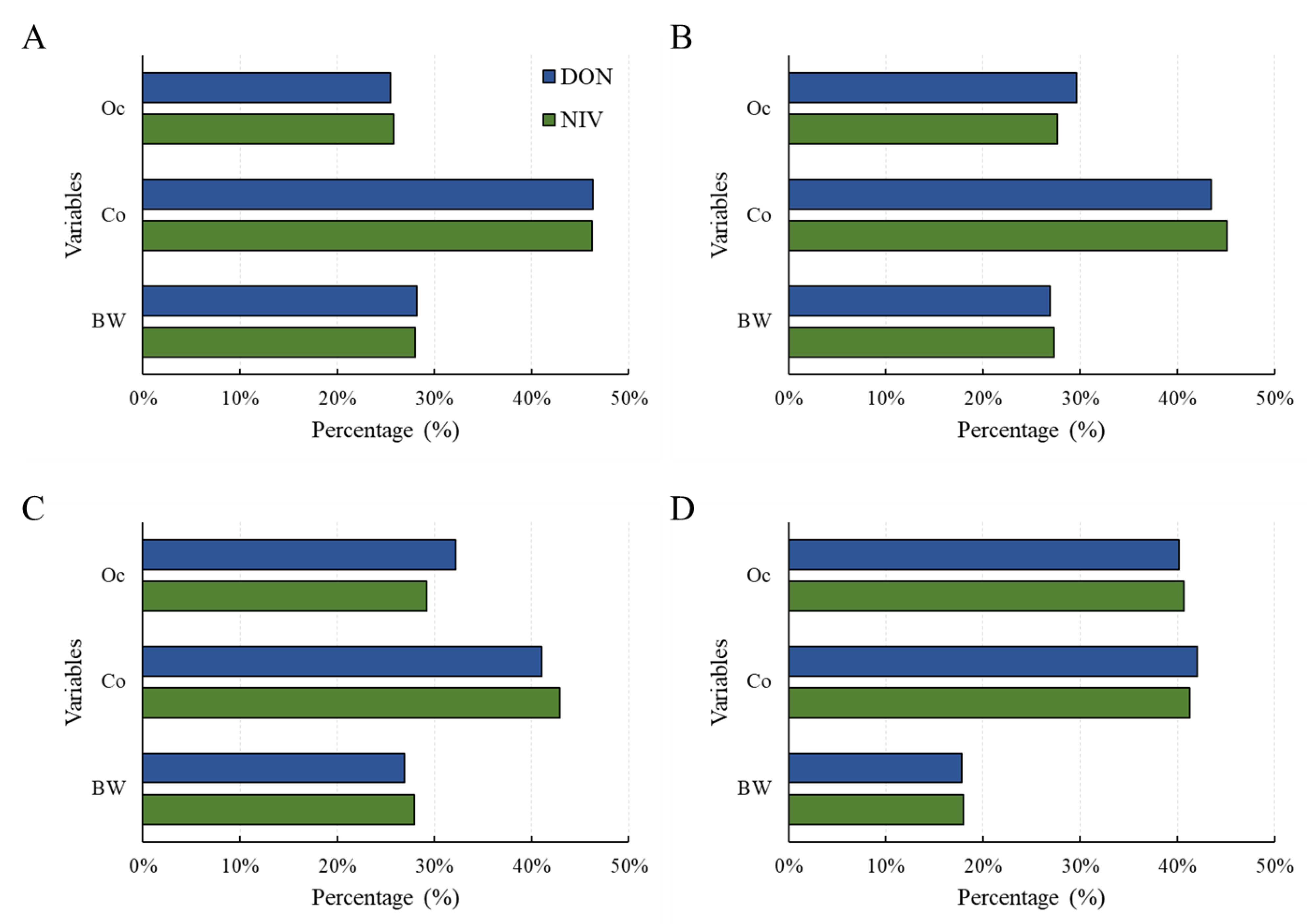
Contribution of Glucoside Conjugates to Exposure
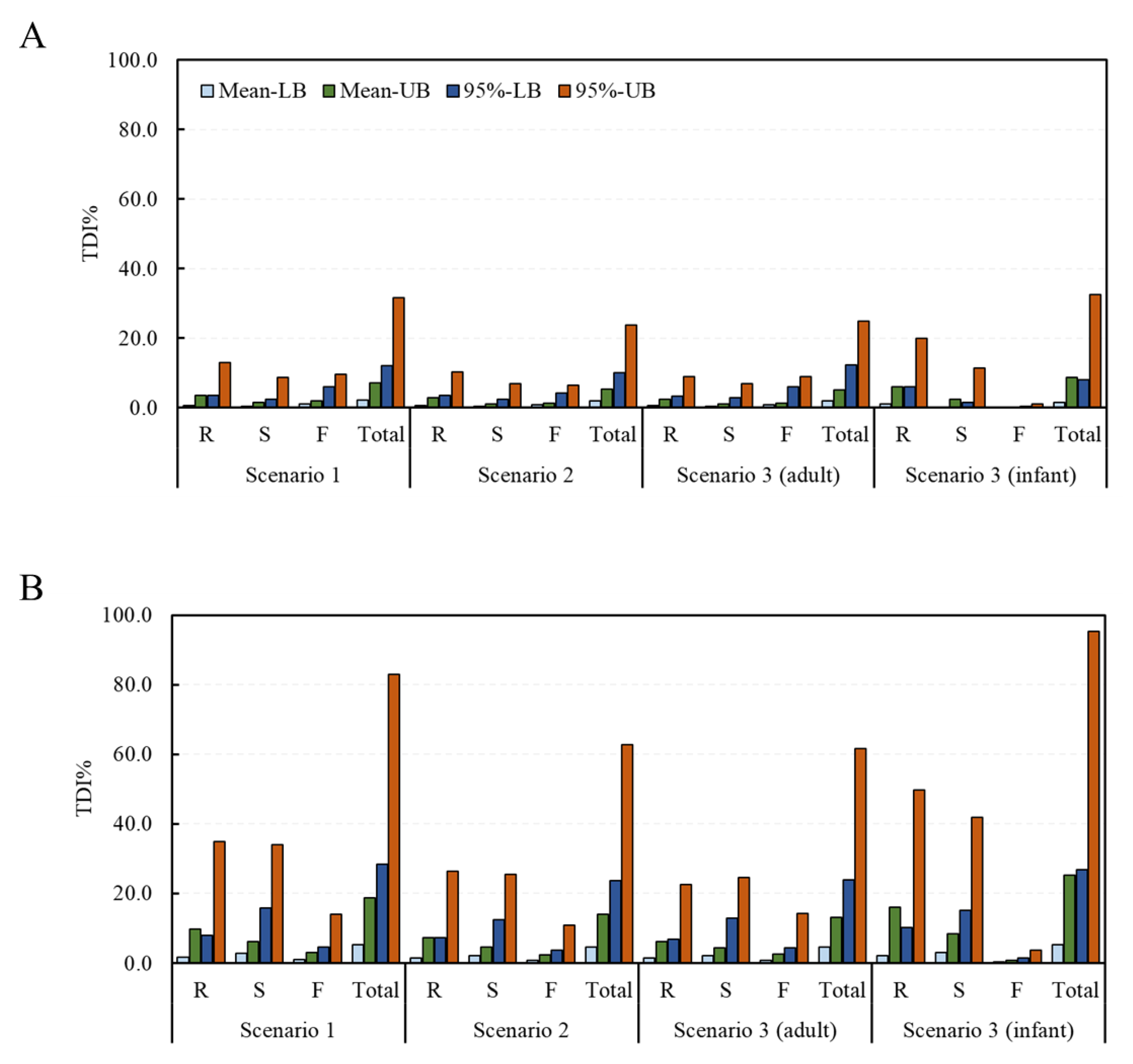
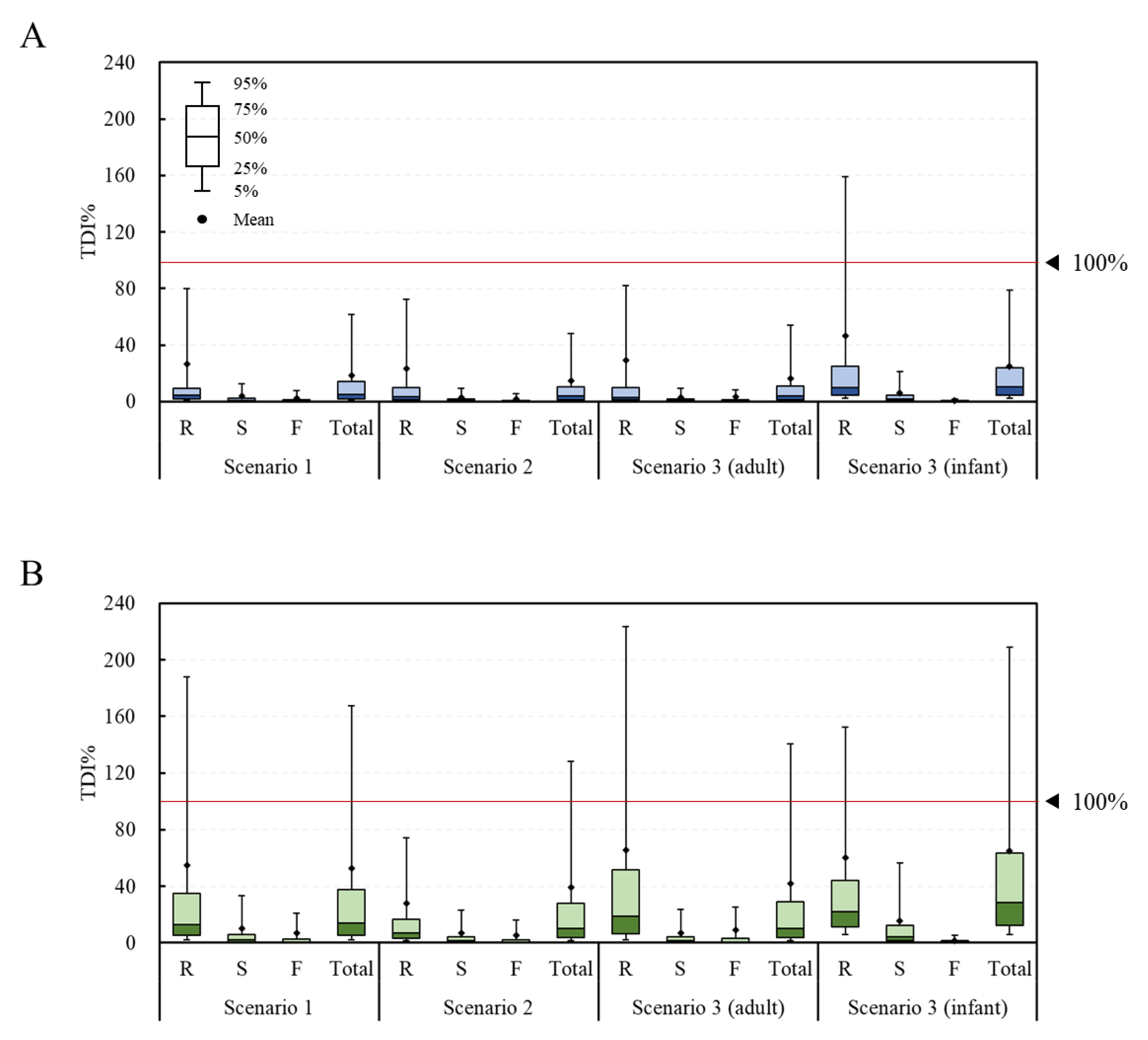
2.2. Estimated Health Risk (TDI%) of DON, NIV, and Their Glucosides for Each Food
2.3. Estimated Health Risk (TDI%) of DON, NIV, and Their Glucosides by Food Processing Type
3. Conclusions
4. Materials and Methods
4.1. Hazard Identification
4.2. Exposure Analysis
4.2.1. Occurrence Data
4.2.2. Determination of Scenarios Considering Absorption Rate of Glucoside Conjugate
4.3. Risk Characterization
4.3.1. Deterministic Approach
4.3.2. Probabilistic Approach
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheat, S.; Gerez, J.R.; Cognié, J.; Alassane-Kpembi, I.; Bracarense, A.P.F.L.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. Nivalenol has a greater impact than deoxynivalenol on pig jejunum mucosa in vitro on explants and in vivo on intestinal loops. Toxins 2015, 7, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Appropriateness to set a group health-based guidance value for nivalenol and its modified forms. EFSA J. 2017, 15, e04751. [Google Scholar] [CrossRef]
- Shin, S.; Son, J.H.; Park, J.C.; Kim, K.H.; Yoon, Y.M.; Cheong, Y.K.; Kim, K.H.; Hyun, J.N.; Park, C.S.; Dill-Macky, R.; et al. Comparative pathogenicity of Fusarium graminearum isolates from wheat kernels in Korea. Plant Pathol. J. 2018, 34, 347–355. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ok, H.E.; Lee, S.Y.; Chun, H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control 2018, 87, 53–59. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Mekete, K.G.; Choi, K.; Kim, B. Occurrence of major type-B trichothecenes and deoxynivalenol-3-glucoside in cereal-based products from Korea. J. Food Compost Anal. 2021, 99, 103851. [Google Scholar] [CrossRef]
- Lee, S.Y.; Woo, S.Y.; Tian, F.; Song, J.; Michlmayr, H.; Kim, J.B.; Chun, H.S. Occurrence of deoxynivalenol, nivalenol, and their glucosides in Korean market foods and estimation of their population exposure through food consumption. Toxins 2020, 12, 89. [Google Scholar] [CrossRef]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of Certain Contaminants in Food: Seventy-Second [72nd] Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011; p. 959. [Google Scholar]
- FSCJ (Food Safety Commission of Japan). Risk Assessment Report. Deoxynivalenol and Nivalenol (Mycotoxin), FS/872/2010. Available online: https://www.fsc.go.jp/english/evaluationreports/nm_toxins/rar_donniv_fs872_2010_nm.pdf (accessed on 28 May 2023).
- EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2017, 61, 1600680. [Google Scholar] [CrossRef]
- Catteuw, A.; Devreese, M.; De Baere, S.; Antonissen, G.; Ivanova, L.; Uhlig, S.; Martens, A.; De Saeger, S.; De Boevre, M.; Croubels, S. Investigation of age-related differences in toxicokinetic processes of deoxynivalenol and deoxynivalenol-3-glucoside in weaned piglets. Arch. Toxicol. 2020, 94, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; van Bergen, T.; Schauvliege, S.; De Boevre, M.; De Saeger, S.; Vanhaecke, L.; Berthiller, F.; Michlmayr, H.; Malachová, A.; et al. In vivo contribution of deoxynivalenol-3-β-D-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017, 91, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, N.; Dänicke, S.; Edler, L.; Gottschalk, C.; Lassek, E.; Marko, D.; Rychlik, M.; Mally, A. A critical evaluation of health risk assessment of modified mycotoxins with a special focus on zearalenone. Mycotoxin Res. 2019, 35, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Rebellato, A.P.; dos Santos Caramês, E.T.; Pallone, J.A.L.; de Oliveira Rocha, L. Mycotoxin bioaccessibility in baby food through in vitro digestion: An overview focusing on risk assessment. Curr. Opin. Food Sci. 2021, 41, 107–115. [Google Scholar] [CrossRef]
- Richardson, G.M. Deterministic versus probabilistic risk assessment: Strengths and weaknesses in a regulatory context. Hum. Ecol. Risk Assess. 1996, 2, 44–54. [Google Scholar] [CrossRef]
- Taghizadeh, S.F.; Rezaee, R.; Badiebostan, H.; Giesy, J.P.; Karimi, G. Occurrence of mycotoxins in rice consumed by Iranians: A probabilistic assessment of risk to health. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 342–354. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Farooq, S. Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits. J. Integr. Agric. 2018, 17, 1676–1690. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, X.; Fu, R.; Yan, H.; Han, S.; Gerelt, K.; Cui, P.; Chen, J.; Qi, K.; Zhou, Y. Determination of six groups of mycotoxins in Chinese dark tea and the associated risk assessment. Environ. Pollut. 2020, 261, 114180. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Tahoun, I.F.; Yamani, R.N.; Rend, E.A.; Shehata, A.B. Natural occurrence of deoxynivalenol, nivalenol and deoxynivalenol-3-glucoside in cereal-derived products from Egypt. Food Control 2022, 137, 108974. [Google Scholar] [CrossRef]
- Martins, C.; Torres, D.; Lopes, C.; Correia, D.; Goios, A.; Assunção, R.; Alvito, P.; Vidal, A.; Boevre, M.D.; Saeger, S.D.; et al. Deoxynivalenol exposure assessment through a modelling approach of food intake and biomonitoring data—A contribution to the risk assessment of an enteropathogenic mycotoxin. Food Res. Int. 2021, 140, 109863. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Dofkova, M.; Blahova, J.; Malir, F.; Kavrik, R.; Rehurkova, I.; Ruprich, J. Dietary exposure assessment of sum deoxynivalenol forms, sum T-2/HT-2 toxins and zearalenone from cereal-based foods and beer. Food Chem. Toxicol. 2020, 139, 111280. [Google Scholar] [CrossRef] [PubMed]
- Nathanail, A.V.; Gibson, B.; Han, L.; Peltonen, K.; Ollilainen, V.; Jestoi, M.; Laitila, A. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016, 203, 448–455. [Google Scholar] [CrossRef]
- Ouwehand, A.; Isolauri, E.; Salminen, S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 2002, 41, i32–i37. [Google Scholar] [CrossRef] [PubMed]
- Sundheim, L.; Lillegaard, I.T.; Fæste, C.K.; Brantsæter, A.L.; Brodal, G.; Eriksen, G.S. Deoxynivalenol exposure in Norway, risk assessments for different human age groups. Toxins 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Ok, H.E.; Kim, D.M.; Kim, D.; Chung, S.H.; Chung, M.S.; Park, K.H.; Chun, H.S. Mycobiota and natural occurrence of aflatoxin, deoxynivalenol, nivalenol and zearalenone in rice freshly harvested in South Korea. Food Control 2014, 37, 284–291. [Google Scholar] [CrossRef]
- Jang, J.Y.; Baek, S.G.; Choi, J.H.; Kim, S.; Kim, J.; Kim, D.W.; Yun, S.H.; Lee, T. Characterization of nivalenol-producing Fusarium asiaticum that causes cereal head blight in Korea. Plant Pathol. J. 2019, 35, 543–552. [Google Scholar] [CrossRef]
- Kostelanska, M.; Dzuman, Z.; Malachova, A.; Capouchova, I.; Prokinova, E.; Skerikova, A.; Hajslova, J. Effects of milling and baking technologies on levels of deoxynivalenol and its masked form deoxynivalenol-3-glucoside. J. Agric. Food Chem. 2011, 59, 9303–9312. [Google Scholar] [CrossRef]
- Wu, Q.; Kuča, K.; Humpf, H.U.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2017, 33, 79–91. [Google Scholar] [CrossRef]
- Taheri, E.; Amin, M.M.; Daniali, S.S.; Abdollahpour, I.; Fatehizadeh, A.; Kelishadi, R. Health risk assessment of exposure to chlorpyrifos in pregnant women using deterministic and probabilistic approaches. PLoS ONE 2022, 17, e0262127. [Google Scholar] [CrossRef]
- Iverson, F.; Armstrong, C.; Nera, E.; Truelove, J.; Fernie, S.; Scott, P.; Stapley, R.; Hayward, S.; Gunner, S. Chronic feeding study of deoxynivalenol in B6C3F1 male and female mice. Teratog. Carcinog. Mutagen. 1995, 15, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.C.; Ohtsubo, K.; Izumiyama, N.; Nakamura, K.; Tanaka, T.; Yamamura, H.; Ueno, Y. The acute and chronic toxicities of nivalenol in mice. Fundam. Appl. Toxicol. 1988, 11, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Ryu, J.C.; Nakamura, K.; Izumiyama, N.; Tanaka, T.; Yamamura, H.; Kobayashi, T.; Ueno, Y. Chronic toxicity of nivalenol in female mice: A 2-year feeding study with Fusarium nivale Fn 2B-moulded rice. Food Chem. Toxicol. 1989, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Gouze, M.E.; Laffitte, J.; Pinton, P.; Dedieux, G.; Galinier, A.; Thouvenot, J.P.; Loiseau, N.; Oswald, I.P.; Galtier, P. Effect of subacute oral doses of nivalenol on immune and metabolic defense systems in mice. Vet. Res. 2007, 38, 635–646. [Google Scholar] [CrossRef]
- Takahashi, M.; Shibutani, M.; Sugita-Konishi, Y.; Aihara, M.; Inoue, K.; Woo, G.-H.; Fujimoto, H.; Hirose, M. A 90-day subchronic toxicity study of nivalenol, a trichothecene mycotoxin, in F344 rats. Food Chem. Toxicol. 2008, 46, 125–135. [Google Scholar] [CrossRef]
- Kubosaki, A.; Aihara, M.; Park, B.J.; Sugiura, Y.; Shibutani, M.; Hirose, M.; Suzuki, Y.; Takatori, K.; Sugita-Konishi, Y. Immunotoxicity of nivalenol after subchronic dietary exposure to rats. Food Chem. Toxicol. 2008, 46, 253–258. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Kubosaki, A.; Takahashi, M.; Park, B.J.; Tanaka, T.; Takatori, K.; Hirose, M.; Shibutani, M. Nivalenol and the targeting of the female reproductive system as well as haematopoietic and immune systems in rats after 90-day exposure through the diet. Food Addit. Contam. Part A. Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1118–1127. [Google Scholar] [CrossRef]
- Dewa, Y.; Kemmochi, S.; Kawai, M.; Saegusa, Y.; Harada, T.; Shimamoto, K.; Mitsumori, K.; Kumagai, S.; Sugita-Konishi, Y.; Shibutani, M. Rapid deposition of glomerular IgA in BALB/c mice by nivalenol and its modifying effect on high IgA strain (HIGA) mice. Exp. Toxicol. Pathol. 2011, 63, 17–24. [Google Scholar] [CrossRef]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef]
- Schwartz-Zimmermann, H.E.; Binder, S.B.; Hametner, C.; Miró-Abella, E.; Schwarz, C.; Michlmayr, H.; Reiterer, N.; Labudova, S.; Adam, G.; Berthiller, F. Metabolism of nivalenol and nivalenol-3-glucoside in rats. Toxicol. Lett. 2019, 306, 43–52. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557. [Google Scholar] [CrossRef]
| Food | Scenario 1 | Scenario 2 | Scenario 3 (Adult) | Scenario 3 (Infant) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean 1 | 95% 2 | Mean | 95% | Mean | 95% | Mean | 95% | |||||||||
| LB 3 | UB 4 | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |
| Baby formula 5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.2 | 2.3 |
| Baby formula 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Barley | 0.3 | 0.3 | 1.4 | 1.8 | 0.3 | 0.3 | 1.3 | 1.6 | 0.2 | 0.3 | 1.3 | 1.5 | 0.3 | 0.4 | 1.6 | 2.0 |
| Barley tea | 0.0 | 0.1 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 |
| Beer | 1.1 | 1.4 | 5.7 | 7.6 | 0.7 | 1.0 | 3.8 | 5.1 | 0.8 | 1.1 | 5.6 | 7.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| Breakfast cereal | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.2 | 0.2 | 0.4 |
| Brown rice | 0.0 | 0.1 | 0.0 | 0.7 | 0.0 | 0.1 | 0.0 | 0.5 | 0.0 | 0.1 | 0.0 | 0.4 | 0.0 | 0.1 | 0.0 | 0.8 |
| Buckwheat | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cheonggukjang | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Chunjang | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.2 | 0.3 |
| Foxtail millet | 0.1 | 0.1 | 0.7 | 0.7 | 0.1 | 0.1 | 0.7 | 0.7 | 0.1 | 0.1 | 0.6 | 0.6 | 0.2 | 0.2 | 1.1 | 1.2 |
| Glutinous rice | 0.0 | 0.1 | 0.0 | 0.4 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.2 | 0.0 | 1.0 |
| Gochujang | 0.0 | 0.1 | 0.2 | 0.7 | 0.0 | 0.1 | 0.2 | 0.5 | 0.1 | 0.1 | 0.3 | 0.5 | 0.0 | 0.0 | 0.1 | 0.2 |
| Job’s tears | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Maize | 0.2 | 0.3 | 1.0 | 1.2 | 0.2 | 0.2 | 0.9 | 1.1 | 0.2 | 0.3 | 1.1 | 1.2 | 0.5 | 0.5 | 2.5 | 3.0 |
| Mixed paste | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mungbean | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Noodle | 0.1 | 0.4 | 0.6 | 2.4 | 0.1 | 0.3 | 0.6 | 1.8 | 0.1 | 0.3 | 0.7 | 2.0 | 0.1 | 0.2 | 0.5 | 1.6 |
| Oat | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pea | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Proso millet | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ramen | 0.2 | 0.4 | 1.3 | 2.8 | 0.2 | 0.3 | 1.3 | 2.4 | 0.2 | 0.4 | 1.5 | 2.5 | 0.0 | 0.1 | 0.1 | 0.2 |
| Red bean | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Rice wine | 0.0 | 0.1 | 0.0 | 0.4 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Snack | 0.0 | 0.1 | 0.1 | 0.8 | 0.0 | 0.1 | 0.1 | 0.6 | 0.0 | 0.1 | 0.1 | 0.5 | 0.1 | 0.5 | 0.3 | 2.4 |
| Sorghum | 0.1 | 0.1 | 0.4 | 0.4 | 0.1 | 0.1 | 0.4 | 0.4 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 | 0.1 | 0.7 | 0.7 |
| Soy sauce | 0.0 | 0.1 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.2 |
| Soybean | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 |
| Soybean paste | 0.0 | 0.1 | 0.1 | 0.4 | 0.0 | 0.1 | 0.1 | 0.3 | 0.0 | 0.1 | 0.1 | 0.3 | 0.0 | 0.1 | 0.1 | 0.4 |
| Soymilk | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.8 |
| Tofu | 0.0 | 0.3 | 0.0 | 1.6 | 0.0 | 0.2 | 0.0 | 1.2 | 0.0 | 0.2 | 0.0 | 1.1 | 0.0 | 0.6 | 0.0 | 3.0 |
| Wheat | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Wheat flour | 0.1 | 0.2 | 0.4 | 0.9 | 0.1 | 0.1 | 0.4 | 0.8 | 0.1 | 0.1 | 0.5 | 0.8 | 0.1 | 0.1 | 0.3 | 0.7 |
| White rice | 0.0 | 2.4 | 0.0 | 7.3 | 0.0 | 1.7 | 0.0 | 5.3 | 0.0 | 1.5 | 0.0 | 4.4 | 0.0 | 4.3 | 0.0 | 11.0 |
| Total | 2.3 | 7.2 | 12.2 | 31.6 | 1.9 | 5.3 | 10.2 | 23.8 | 2.1 | 5.1 | 12.4 | 25.0 | 1.5 | 8.7 | 8.0 | 32.5 |
| Food | Scenario 1 | Scenario 2 | Scenario 3 (Adult) | Scenario 3 (Infant) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean 1 | 95% 2 | Mean | 95% | Mean | 95% | Mean | 95% | |||||||||
| LB 3 | UB 4 | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |
| Baby formula 5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 1.3 | 2.3 | 7.8 |
| Baby formula 6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Barley | 1.1 | 1.3 | 5.5 | 6.6 | 1.0 | 1.1 | 5.0 | 5.7 | 0.9 | 1.0 | 4.7 | 5.2 | 1.3 | 1.5 | 6.3 | 7.4 |
| Barley tea | 0.0 | 0.1 | 0.0 | 0.4 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.2 |
| Beer | 0.0 | 1.1 | 0.0 | 5.7 | 0.0 | 0.9 | 0.0 | 4.5 | 0.0 | 1.1 | 0.0 | 7.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Breakfast cereal | 0.0 | 0.1 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.2 | 0.0 | 0.6 |
| Brown rice | 0.1 | 0.5 | 0.4 | 2.3 | 0.1 | 0.4 | 0.4 | 1.8 | 0.1 | 0.3 | 0.4 | 1.6 | 0.1 | 0.5 | 0.5 | 2.6 |
| Buckwheat | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cheonggukjang | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Chunjang | 0.0 | 0.1 | 0.1 | 0.3 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.7 |
| Foxtail millet | 0.2 | 0.3 | 1.2 | 1.3 | 0.2 | 0.2 | 1.1 | 1.1 | 0.2 | 0.2 | 0.9 | 1.0 | 0.3 | 0.4 | 1.9 | 2.0 |
| Glutinous rice | 0.0 | 0.3 | 0.0 | 1.3 | 0.0 | 0.2 | 0.0 | 0.9 | 0.0 | 0.1 | 0.0 | 0.7 | 0.0 | 0.7 | 0.0 | 2.8 |
| Gochujang | 0.7 | 0.9 | 3.5 | 4.3 | 0.6 | 0.7 | 2.8 | 3.3 | 0.6 | 0.7 | 3.0 | 3.5 | 0.2 | 0.2 | 1.0 | 1.3 |
| Job’s tears | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Maize | 0.1 | 0.2 | 0.3 | 0.9 | 0.1 | 0.2 | 0.3 | 0.7 | 0.1 | 0.2 | 0.3 | 0.7 | 0.1 | 0.4 | 0.7 | 2.1 |
| Mixed paste | 0.1 | 0.2 | 0.8 | 1.1 | 0.1 | 0.2 | 0.7 | 1.0 | 0.2 | 0.2 | 1.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mungbean | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Noodle | 0.0 | 0.8 | 0.0 | 5.1 | 0.0 | 0.6 | 0.0 | 3.6 | 0.0 | 0.5 | 0.0 | 3.5 | 0.0 | 0.4 | 0.0 | 3.3 |
| Oat | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 |
| Pea | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Proso millet | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Ramen | 0.0 | 0.7 | 0.0 | 4.5 | 0.0 | 0.5 | 0.0 | 3.2 | 0.0 | 0.4 | 0.0 | 3.0 | 0.0 | 0.1 | 0.0 | 0.3 |
| Red bean | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| Rice wine | 0.0 | 0.2 | 0.0 | 0.8 | 0.0 | 0.2 | 0.0 | 0.6 | 0.0 | 0.2 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Snack | 0.0 | 0.4 | 0.2 | 2.3 | 0.0 | 0.3 | 0.2 | 1.7 | 0.0 | 0.2 | 0.2 | 1.4 | 0.2 | 1.4 | 0.8 | 6.8 |
| Sorghum | 0.1 | 0.1 | 0.4 | 0.5 | 0.1 | 0.1 | 0.4 | 0.4 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 | 0.1 | 0.7 | 0.8 |
| Soy sauce | 0.0 | 0.1 | 0.2 | 0.6 | 0.0 | 0.1 | 0.2 | 0.5 | 0.0 | 0.1 | 0.2 | 0.5 | 0.0 | 0.2 | 0.2 | 0.6 |
| Soybean | 0.0 | 0.1 | 0.1 | 0.7 | 0.0 | 0.1 | 0.1 | 0.5 | 0.0 | 0.1 | 0.1 | 0.5 | 0.0 | 0.1 | 0.1 | 0.5 |
| Soybean paste | 0.0 | 0.2 | 0.0 | 1.1 | 0.0 | 0.1 | 0.0 | 0.8 | 0.0 | 0.1 | 0.0 | 0.7 | 0.0 | 0.2 | 0.0 | 1.0 |
| Soymilk | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.1 | 0.7 | 0.4 | 2.1 |
| Tofu | 0.0 | 0.9 | 0.0 | 4.7 | 0.0 | 0.6 | 0.0 | 3.3 | 0.0 | 0.6 | 0.0 | 3.0 | 0.0 | 1.7 | 0.0 | 8.5 |
| Wheat | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Wheat flour | 2.7 | 2.9 | 15.5 | 16.7 | 2.1 | 2.2 | 12.3 | 13.0 | 2.1 | 2.3 | 12.5 | 13.3 | 2.2 | 2.4 | 11.5 | 12.3 |
| White rice | 0.0 | 6.9 | 0.0 | 20.8 | 0.0 | 4.9 | 0.0 | 14.8 | 0.0 | 4.1 | 0.0 | 12.4 | 0.0 | 12.3 | 0.0 | 31.1 |
| Total | 5.4 | 18.8 | 28.4 | 82.9 | 4.5 | 14.1 | 23.7 | 62.6 | 4.5 | 13.2 | 24.0 | 61.6 | 5.3 | 25.2 | 26.9 | 95.2 |
| No. | Species | Samples (n) | Route | Toxin | Bioavailability (%) | Relative Bioavailability (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Human (18–62 years) | 20 | Oral | DON, | 64.0 ± 22.8 | ~100 | [14] |
| DON3G | 58.2 ± 16.0 | ||||||
| 2 | Pig (11 weeks) | 6 | Oral | DON, | 81.3 ± 17.4 | ~20 | [15] |
| DON3G | 16.1 ± 5.4 | ||||||
| 3 | Pig (4 weeks) | 8 | Oral | DON, | 77.9 ± 47.7 | ~100 | [13] |
| DON3G | 83.0 ± 69.1 | ||||||
| 4 | Pig (4 weeks) | 4 | Oral | DON, | 84.8 ± 9.7 | ~50 | [41] |
| DON3G | 40.3 ± 10.0 | ||||||
| 5 | Rat (5 months) | 6 | Oral | DON, | 14.9 ± 5.0 | ~25 | [42] |
| DON3G | 3.7 ± 0.7 | ||||||
| 6 | Chicken (3 weeks) | 6 | Oral | DON, | 5.6 ± 2.1 | ~70 | [15] |
| DON3G | 3.8 ± 2.7 | ||||||
| 7 | Rat (6 weeks) | 6 | Oral | NIV, | 3.7 ± 0.7 | ~35 | [43] |
| NIV3G | 1.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Cho, S.; Woo, S.Y.; Hwang, M.; Chun, H.S. Risk Assessment Considering the Bioavailability of 3-β-d-Glucosides of Deoxynivalenol and Nivalenol through Food Intake in Korea. Toxins 2023, 15, 460. https://doi.org/10.3390/toxins15070460
Lee SY, Cho S, Woo SY, Hwang M, Chun HS. Risk Assessment Considering the Bioavailability of 3-β-d-Glucosides of Deoxynivalenol and Nivalenol through Food Intake in Korea. Toxins. 2023; 15(7):460. https://doi.org/10.3390/toxins15070460
Chicago/Turabian StyleLee, Sang Yoo, Solyi Cho, So Young Woo, Myungsil Hwang, and Hyang Sook Chun. 2023. "Risk Assessment Considering the Bioavailability of 3-β-d-Glucosides of Deoxynivalenol and Nivalenol through Food Intake in Korea" Toxins 15, no. 7: 460. https://doi.org/10.3390/toxins15070460
APA StyleLee, S. Y., Cho, S., Woo, S. Y., Hwang, M., & Chun, H. S. (2023). Risk Assessment Considering the Bioavailability of 3-β-d-Glucosides of Deoxynivalenol and Nivalenol through Food Intake in Korea. Toxins, 15(7), 460. https://doi.org/10.3390/toxins15070460






