Effect of Fungicide Treatment on Multi-Mycotoxin Occurrence in French Wheat during a 4-Year Period
Abstract
1. Introduction
2. Results
2.1. Fungicide Treatments
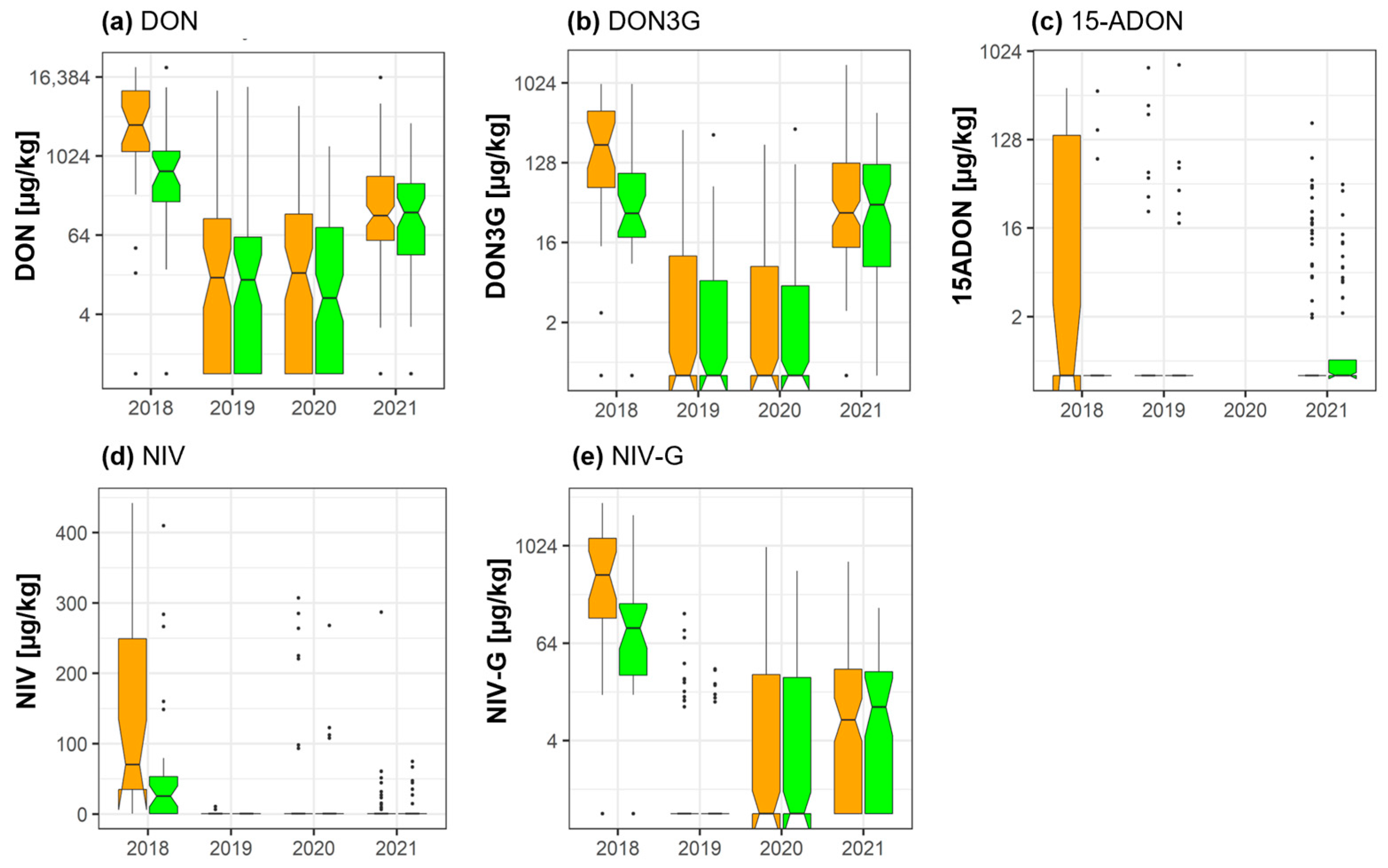
2.2. DON, NIV, and Their Masked Forms
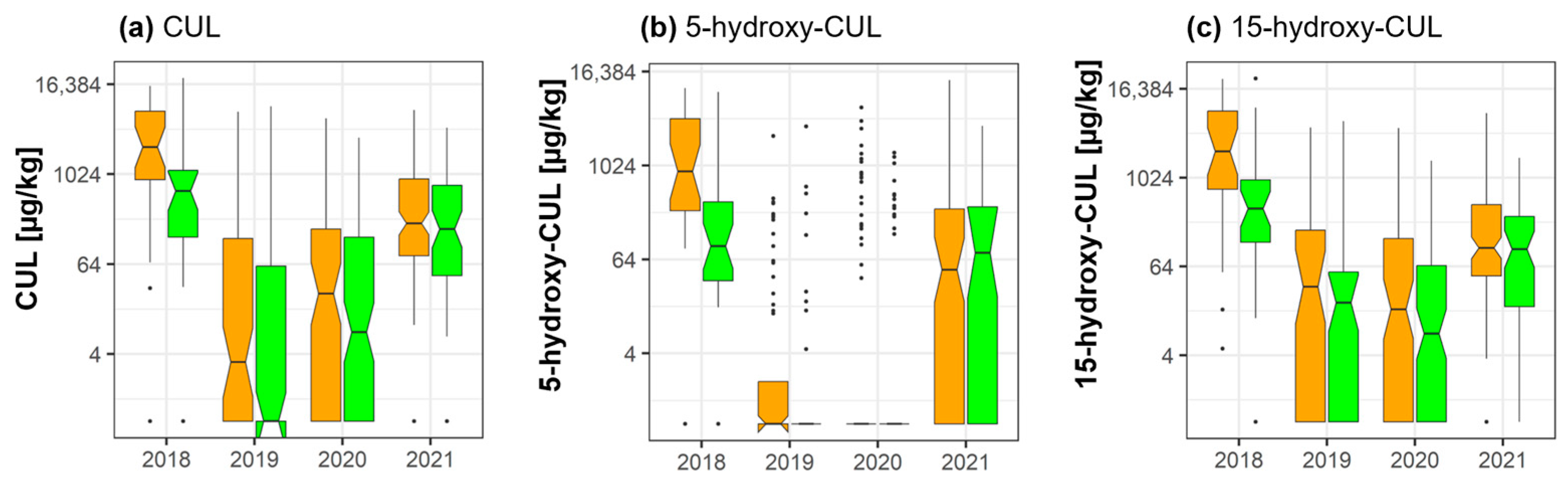
2.3. Culmorin and Its Derivatives
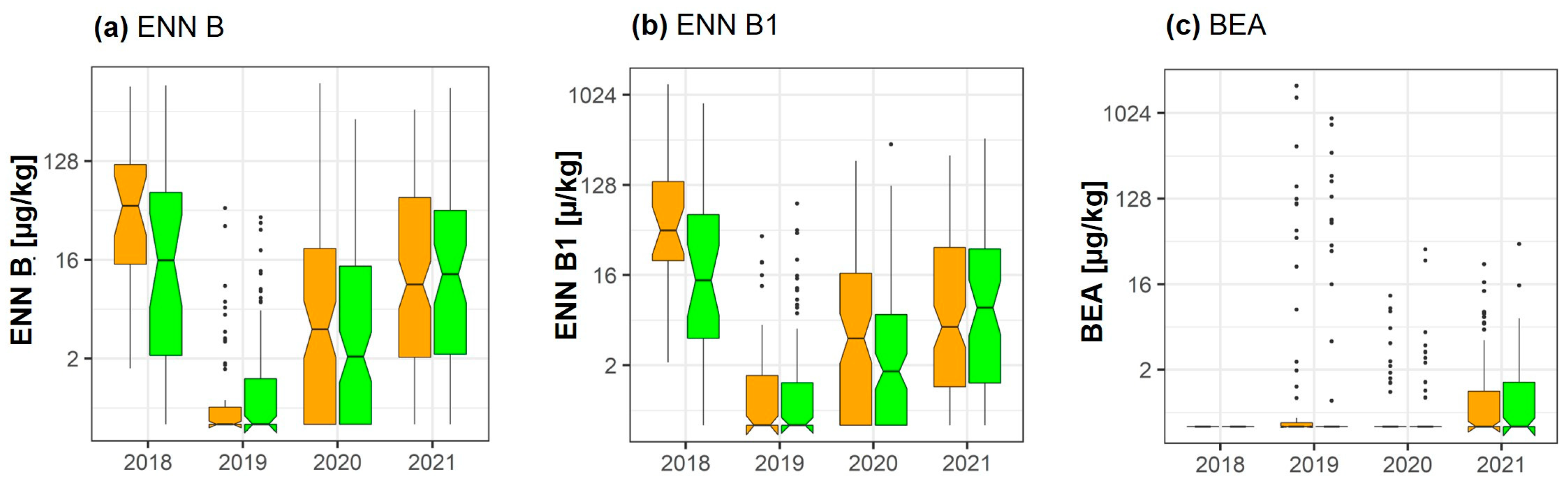
2.4. Enniatins and Beauvericin
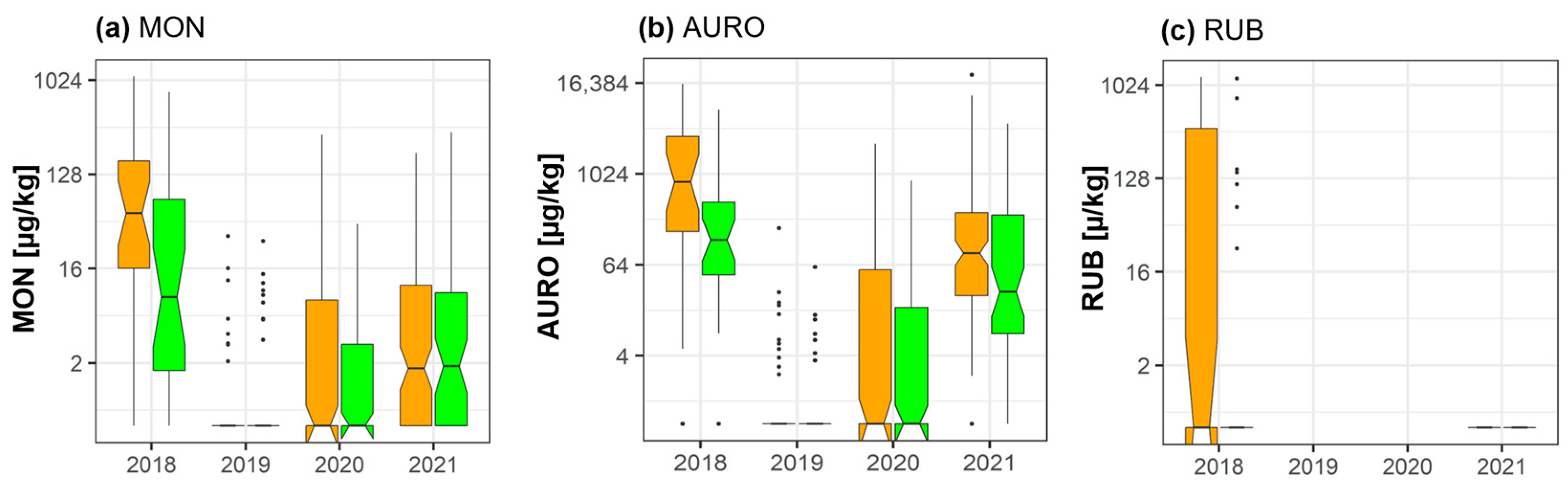
2.5. Moniliformin, Aurofusarin, and Rubrofusarin
2.6. Alternaria Mycotoxins
2.7. Butenolide, Tryptophol, and Chrysogin
2.8. Overview of Fungicide Effects
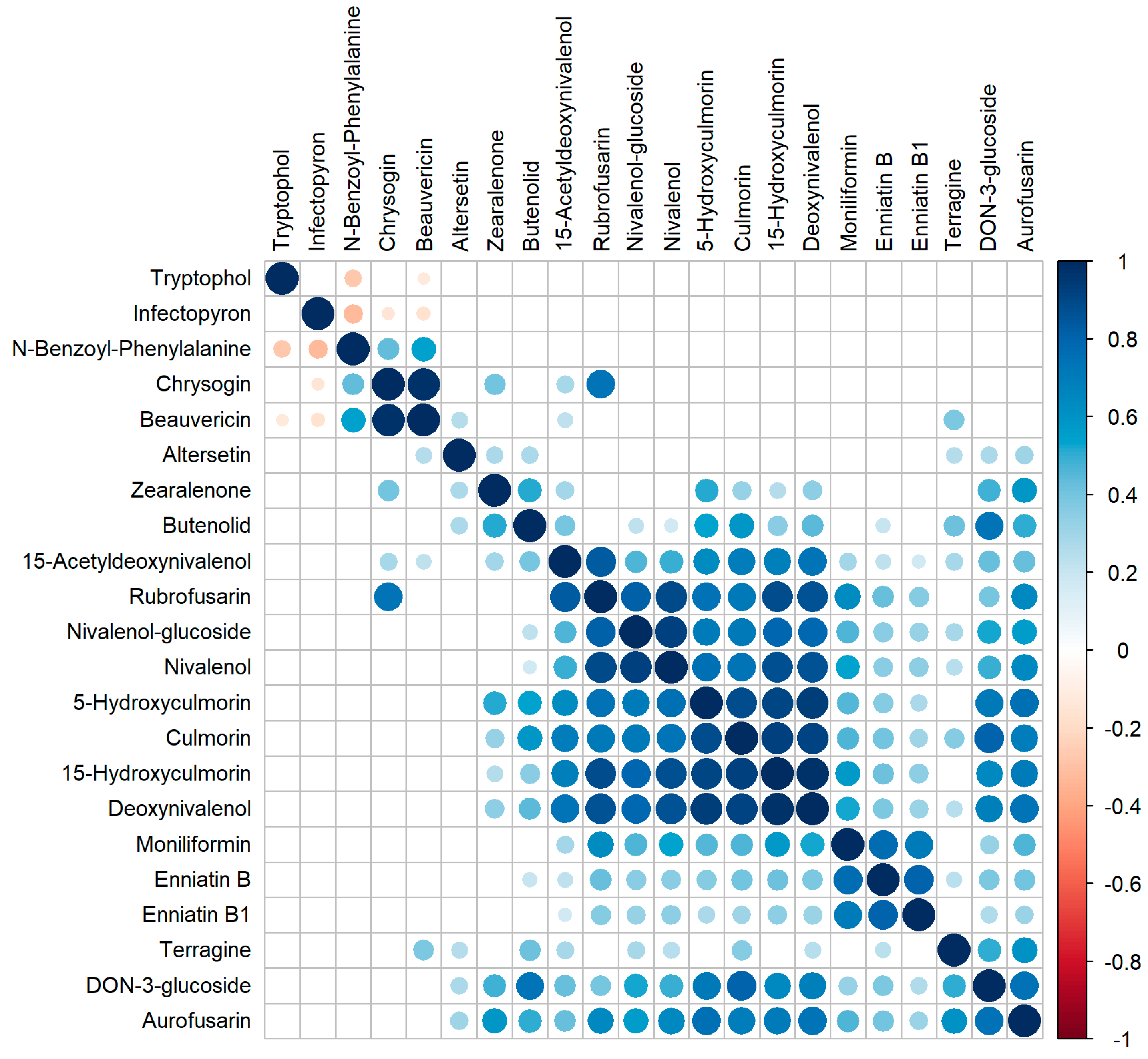
2.9. Correlation of Metabolite Occurrence
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Fungicide Application
4.3. Quantification of Mycotoxins via LC-MS/MS Multi-Analyte Method
4.4. Statistical Analysis
4.4.1. Filtering and Processing
4.4.2. Clustering
4.4.3. Differences between Control and Treatment
4.4.4. Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations (FAO). Cereal Supply and Demand Brief. In FAO Cereal Supply and Demand Situation; Food and Agricultural Organization: Rome, Italy, 2016. [Google Scholar]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited “FAO Estimate” of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Pinotti, L.; Novacco, M.; Ottoboni, M.; Tretola, M.; Orto, V.D. Mycotoxins in Wheat and Mitigation Measures. In Wheat Improvement, Management and Utilization; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar]
- Smith, J.E.; Solomons, G.; Lewis, C.; Anderson, J.G. Role of Mycotoxins in Human and Animal Nutrition and Health. Nat. Toxins 1995, 3, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin Production by Aspergillus, Fusarium and Penicillium Species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of Milling Procedures on Mycotoxin Distribution in Wheat Fractions: A Review. LWT—Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A Review of Worldwide Contamination of Cereal Grains and Animal Feed with Fusarium Mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide Occurrence of Mycotoxins in Commodities, Feeds and Feed Ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the Toxicity, Occurrence, Metabolism, Detoxification, Regulations and Intake of Zearalenone: An Oestrogenic Mycotoxin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Neuhof, T.; Koch, M.; Rasenko, T.; Nehls, I. Occurrence of Zearalenone in Wheat Kernels Infected with Fusarium culmorum. World Mycotoxin J. 2008, 1, 429–435. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A Three-Year Survey on the Worldwide Occurrence of Mycotoxins in Feedstuffs and Feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin Occurrence in Commodities, Feeds and Feed Ingredients Sourced in the Middle East and Africa. Food Addit. Contam. Part B Surveill. 2011, 4, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-Occurrence of Regulated, Masked and Emerging Mycotoxins and Secondary Metabolites in Finished Feed and Maize—An Extensive Survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria Mycotoxins: Occurrence, Toxicity and Toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and Validation of a Liquid Chromatography/Tandem Mass Spectrometric Method for the Determination of 39 Mycotoxins in Wheat and Maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- Sulyok, M.; Krska, R.; Schuhmacher, R. Application of an LC–MS/MS Based Multi-Mycotoxin Method for the Semi-Quantitative Determination of Mycotoxins Occurring in Different Types of Food Infected by Moulds. Food Chem. 2010, 119, 408–416. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked Mycotoxins: A Review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for Preventing, Decontaminating and Minimizing the Toxicity of Mycotoxins in Feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Matumba, L.; Namaumbo, S.; Ngoma, T.; Meleke, N.; De Boevre, M.; Logrieco, A.F.; De Saeger, S. Five Keys to Prevention and Control of Mycotoxins in Grains: A Proposal. Glob. Food Sec. 2021, 30, 100562. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How Will Climate Change Affect Mycotoxins in Food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Bencze, S.; Puskás, K.; Vida, G.; Karsai, I.; Balla, K.; Komáromi, J.; Veisz, O. Rising Atmospheric CO2 Concentration May Imply Higher Risk of Fusarium Mycotoxin Contamination of Wheat Grains. Mycotoxin Res. 2017, 33, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Schatzmayr, G.; Streit, E. Global Occurrence of Mycotoxins in the Food and Feed Chain: Facts and Figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin Occurrence in Feed and Feed Raw Materials Worldwide: Long-Term Analysis with Special Focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Oswald, I.P. Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef]
- Pancosma, S.A. Pancosma & Associates’ 2015 Survey: Threat of Multi-Mycotoxin Contamination. 2015. Available online: http://en.engormix.com/MA-mycotoxins/articles/pancosma-associates-2015-survey-t3648/p0.htm (accessed on 2 March 2023).
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural Co-Occurrence of Mycotoxins in Wheat Grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Mushongi, A.A.; Kangéthe, E.K. Enhancing Food Safety through Adoption of Long-Term Technical Advisory, Financial, and Storage Support Services in Maize Growing Areas of East Africa. Sustainability 2019, 11, 2827. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Extent and Drivers of Mycotoxin Contamination: Inferences from a Survey of Kenyan Maize Mills. Phytopathology 2014, 104, 1221–1231. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Faas, J.; Doupovec, B.; Aleschko, M.; Stoiber, C.; Höbartner-Gußl, A.; Schöndorfer, K.; Killinger, M.; Zebeli, Q.; Schatzmayr, D. Metabolism of Zearalenone in the Rumen of Dairy Cows with and without Application of a Zearalenone-Degrading Enzyme. Toxins 2021, 13, 84. [Google Scholar] [CrossRef]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.-E.; Sărăndan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal Degradation of Fumonisin B1 by Carboxylesterase FumD Prevents Fumonisin Induced Alteration of Sphingolipid Metabolism in Turkey and Swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef]
- Grenier, B.; Bracarense, A.-P.F.L.; Schwartz, H.E.; Lucioli, J.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Biotransformation Approaches To Alleviate the Effects Induced by Fusarium Mycotoxins in Swine. J. Agric. Food Chem. 2013, 61, 6711–6719. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.D.; Wang, M.; Elmore, S.E.; Hearon, S.E.; Wang, J.-S. NovaSil Clay for the Protection of Humans and Animals from Aflatoxins and Other Contaminants. Clays Clay Miner. 2019, 67, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.A.; Do Cam, T.; Tri, N.N.; Santos, G.A.; Encarnação, P.; Hung, L.T. Aflatoxin B1 (AFB1) Reduces Growth Performance, Physiological Response, and Disease Resistance in Tra Catfish (Pangasius hypophthalmus). Aquac. Int. 2018, 26, 921–936. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Kumari, P. Management of Mycotoxin Contamination in Preharvest and Post Harvest Crops: Present Status and Future Prospects. J. Phytol. 2010, 2, 655. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of Agricultural Practices on Fusarium Infection of Cereals and Subsequent Contamination of Grain by Trichothecene Mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Lemmens, M.; Hartl, L.; Doldi, L.; Steiner, B.; Stierschneider, M.; Ruckenbauer, P. Molecular Mapping of QTLs for Fusarium Head Blight Resistance in Spring Wheat. I. Resistance to Fungal Spread (Type II Resistance). Theor. Appl. Genet. 2002, 104, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Steiner, B.; Buerstmayr, M.; Michel, S.; Schweiger, W.; Lemmens, M.; Buerstmayr, H. Breeding Strategies and Advances in Line Selection for Fusarium Head Blight Resistance in Wheat. Trop. Plant Pathol. 2017, 42, 165–174. [Google Scholar] [CrossRef]
- Mesterházy, A. Types and Components of Resistance to Fusarium Head Blight of Wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural Mechanisms for Cereal Resistance to the Accumulation of Fusarium Trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the Mycotoxin Deoxynivalenol in Fusarium Resistant and Susceptible Near Isogenic Wheat Lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Bekalu, Z.E.; Krogh Madsen, C.; Dionisio, G.; Bæksted Holme, I.; Jørgensen, L.N.; Fomsgaard, I.S.; Brinch-Pedersen, H. Overexpression of Nepenthesin HvNEP-1 in Barley Endosperm Reduces Fusarium Head Blight and Mycotoxin Accumulation. Agronomy 2020, 10, 203. [Google Scholar] [CrossRef]
- Machado, A.K.; Brown, N.A.; Urban, M.; Kanyuka, K.; Hammond-Kosack, K.E. RNAi as an Emerging Approach to Control Fusarium Head Blight Disease and Mycotoxin Contamination in Cereals. Pest Manag. Sci. 2018, 74, 790–799. [Google Scholar] [CrossRef]
- Majumdar, R.; Rajasekaran, K.; Cary, J.W. RNA Interference (RNAi) as a Potential Tool for Control of Mycotoxin Contamination in Crop Plants: Concepts and Considerations. Front. Plant Sci. 2017, 8, 200. [Google Scholar] [CrossRef]
- Janssen, E.M.; Mourits, M.C.M.; van der Fels-Klerx, H.J.; Lansink, A.G.J.M.O. Pre-Harvest Measures against Fusarium Spp. Infection and Related Mycotoxins Implemented by Dutch Wheat Farmers. Crop Prot. 2019, 122, 9–18. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van Der Lee, T.; Brodal, G. Associations between Fusarium Species and Mycotoxins in Oats and Spring Wheat from Farmers Fields in Norway over a Six-Year Period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Qiu, J.; Dong, F.; Yu, M.; Xu, J.; Shi, J. Effect of Preceding Crop on Fusarium Species and Mycotoxin Contamination of Wheat Grains. J. Sci. Food Agric. 2016, 96, 4536–4541. [Google Scholar] [CrossRef]
- Steinkellner, S.; Langer, I. Impact of Tillage on the Incidence OfFusarium spp. in Soil. Plant Soil 2004, 267, 13–22. [Google Scholar] [CrossRef]
- Podolska, G.; Bryła, M.; Sulek, A.; Waskiewicz, A.; Szymczyk, K.; Jedrzejczak, R. Influence of the Cultivar and Nitrogen Fertilisation Level on the Mycotoxin Contamination in Winter Wheat. Qual. Assur. Saf. Crops Foods 2017, 9, 451–461. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.F.; Doré, T.; Rossignol, L. Influence of Cropping System on Fusarium Head Blight and Mycotoxin Levels in Winter Wheat. Crop Prot. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-Harvest Control Strategies: Minimizing Mycotoxins in the Food Chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Torelli, E.; Firrao, G.; Bianchi, G.; Saccardo, F.; Locci, R. The Influence of Local Factors on the Prediction of Fumonisin Contamination in Maize. J. Sci. Food Agric. 2012, 92, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Dill-Macky, R. Impact of Moisture, Host Genetics and Fusarium Graminearum Isolates on Fusarium Head Blight Development and Trichothecene Accumulation in Spring Wheat. Mycotoxin Res. 2012, 28, 45–58. [Google Scholar] [CrossRef]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of Fungicides on the Development of Fusarium Head Blight, Yield and Deoxynivalenol Accumulation in Wheat Inoculated under Field Conditions with Fusarium Graminearum and Fusarium culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A Unified Effort to Fight an Enemy of Wheat and Barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakajima, T.; Tomimura, K.; Suzuki, F.; Arai, M.; Miyasaka, A. Effect of the Timing of Fungicide Application on Fusarium Head Blight and Mycotoxin Contamination in Wheat. Plant Dis. 2012, 96, 845–851. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of Fungicide Application to Control Fusarium Head Blight and 20 Fusarium and Alternaria Mycotoxins in Winter Wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Ibanez-Vea, M.; Lizarraga, E.; Gonzalez-Penas, E.; Lopez de Cerain, A. Co-occurrence of type-A and type-B thrichothecenes in barley from a northern region of Spain. Food Control 2012, 25, 81–88. [Google Scholar] [CrossRef]
- Zhang, H.; van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population Analysis of the Fusarium Graminearum Species Complex from Wheat in China Show a Shift to More Aggressive Isolates. PLoS ONE 2012, 7, e0031722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Van Der Lee, T.; Chen, W.Q.; Xu, J.; Xu, J.S.; Yang, L.; Yu, D.; Waalwijk, C.; Feng, J. Population Genetic Analyses of Fusarium Asiaticum Populations from Barley Suggest a Recent Shift Favoring 3ADON Producers in Southern China. Phytopathology 2010, 100, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Del Ponte, E.M.; Fernandes, J.M.C.; Pavan, W.; Baethgen, W.E. A Model-Based Assessment of the Impacts of Climate Variability on Fusarium Head Blight Seasonal Risk in Southern Brazil. J. Phytopathol. 2009, 157, 675–681. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Spolti, P.; Ward, T.J.; Gomes, L.B.; Nicolli, C.P.; Kuhnem, P.R.; Silva, C.N.; Tessmann, D.J. Regional and Field-Specific Factors Affect the Composition of Fusarium Head Blight Pathogens in Subtropical No-Till Wheat Agroecosystem of Brazil. Phytopathology® 2014, 105, 246–254. [Google Scholar] [CrossRef]
- Palazzini, J.; Fumero, V.; Yerkovich, N.; Barros, G.; Cuniberti, M.; Chulze, S. Correlation between Fusarium Graminearum and Deoxynivalenol during the 2012/13 Wheat Fusarium Head Blight Outbreak in Argentina. Cereal Res. Commun. 2015, 43, 627–637. [Google Scholar] [CrossRef]
- Spolti, P.; Shah, D.A.; Fernandes, J.M.C.; Bergstrom, G.C.; Del Ponte, E.M. Disease Risk, Spatial Patterns, and Incidence-Severity Relationships of Fusarium Head Blight in No-till Spring Wheat Following Maize or Soybean. Plant Dis. 2015, 99, 1360–1366. [Google Scholar] [CrossRef]
- Panwar, V.; Aggarwal, A.; Paul, S.; Kumar, J.; Saharan, M.S. Distribution Dynamics of Fusarium Spp. Causing Fusarium Head Blight (FHB) in Wheat at Different Geographical Locations in India. South Asian J. Exp. Biol. 2017, 6, 167–177. [Google Scholar] [CrossRef]
- Windels, C.E. Economic and Social Impacts of Fusarium Head Blight: Changing Farms and Rural Communities in the Northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar] [CrossRef]
- Minnaar-Ontong, A.; Herselman, L.; Kriel, W.-M.; Leslie, J.F. Morphological Characterization and Trichothecene Genotype Analysis of a Fusarium Head Blight Population in South Africa. Eur. J. Plant Pathol. 2017, 148, 261–269. [Google Scholar] [CrossRef]
- Xue, A.G.; Chen, Y.; Seifert, K.; Guo, W.; Blackwell, B.A.; Harris, L.J.; Overy, D.P. Prevalence of Fusarium Species Causing Head Blight of Spring Wheat, Barley and Oat in Ontario during 2001–2017. Can. J. Plant Pathol. 2019, 41, 392–402. [Google Scholar] [CrossRef]
- Aboukhaddour, R.; Fetch, T.; McCallum, B.D.; Harding, M.W.; Beres, B.L.; Graf, R.J. Wheat Diseases on the Prairies: A Canadian Story. Plant Pathol. 2020, 69, 418–432. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Sulyok, M.; Krska, R.; Logrieco, A.F.; Vogelgsang, S. Raised Concerns about the Safety of Barley Grains and Straw: A Swiss Survey Reveals a High Diversity of Mycotoxins and Other Fungal Metabolites. Food Control 2021, 125, 107919. [Google Scholar] [CrossRef]
- Hao, W.; Guan, S.; Li, A.; Wang, J.; An, G.; Hofstetter, U.; Schatzmayr, G. Mycotoxin Occurrence in Feeds and Raw Materials in China: A Five-Year Investigation. Toxins 2023, 15, 63. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin Contamination of the Feed Supply Chain: Implications for Animal Productivity and Feed Security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their In Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.-C.; Nielsen, E.; et al. Assessment of Information as Regards the Toxicity of Fumonisins for Pigs, Poultry and Horses. EFSA J. 2022, 20, e07534. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; del Mazo, J.K.C.J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.-C.; Nielsen, E.; Ntzani, E.; et al. Assessment of Information as Regards the Toxicity of Deoxynivalenol for Horses and Poultry. EFSA J. 2023, 21, e07806. [Google Scholar] [CrossRef]
- Del Puerto, O.; Gonçalves, N.P.F.; Medana, C.; Prevot, A.B.; Roslev, P. Attenuation of toxicity and occurrence of degradation products of the fungicide tebuconazole after combined vacuum UV and UVC treatment of drinking water. Environ. Sci. Pollut. Res. Int. 2022, 29, 58312–58325. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, M.C.; Neeff, D.V.d.; Jager, A.V.; Corassin, C.H.; Carão, Á.C.d.P.; de Albuquerque, R.; de Azevedo, A.C.; Oliveira, C.A.F. Mineral Adsorbents for Prevention of Mycotoxins in Animal Feeds. Toxin Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-Based Dilute-and-Shoot Approach for the Quantification of >500 Mycotoxins and Other Secondary Metabolites in Food Crops: Challenges and Solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Volume 2, p. 2019. [Google Scholar]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. R Package “Corrplot”: Visualization of a Correlation Matrix. Statistician 2017, 56, 316–324. [Google Scholar]


| 2018 | 2019 | 2020 | 2021 | |||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | GW | PW | GW | PW | GW | PW | GW | PW |
| DON | 🡫 −59.8% (p = 0.044) | 🡫 −59.8% (p = 5.51 × 10−6) | 🡫 −65.4% (p = 4.13 × 10−5) | 🡫 −47.1% (p = 3.7 × 10−7) | ||||
| DON3G | 🡫 −53.9% (p = 0.044) | 🡫 −53.9% (p = 0.00031) | 🡫 −61.7% (p = 0.0002) | 🡫 −18.2% (p = 1.67 × 10−7) | ||||
| 15-ADON | 🡫 −71% (p = 0.048) | 🡫 −49.6% (p = 0.0009) | ||||||
| NIV | 🡫 −59.1% (p = 0.044) | 🡫 −59.1% p = 0.00031 | 🡫 −58.3% (p = 0.006) | 🡫 −15.6% (p = 0.019) | ||||
| NIV-G | 🡫 −57% (p = 0.044) | 🡫 −57% (p = 0.002) | ||||||
| CUL | 🡫 −54.2% (p = 0.046) | 🡫 −54.2% (p = 0.0005) | 🡫 −64.5% (p = 0.0002) | 🡫 −40.3% (p = 3.7 × 10−7) | ||||
| 5-hydroxy-CUL | 🡫 −62% (p = 0.044) | 🡫 −62% (p = 0.0003) | 🡫 −74% (p = 0.0005) | 🡫 −39.8% (p = 2.13 × 10−5) | ||||
| 15-hydroxy-CUL | 🡫 −57.3% (p = 0.044) | 🡫 −57.3% (p = 0.00003) | 🡫 −64.4% (p = 4.13 × 10−5) | 🡫 −45.5% (p = 4.17 × 10−7) | ||||
| ENN B | 🡫 −62.4% (p = 0.022) | |||||||
| ENN B1 | 🡫 −40.5% (p = 0.043) | |||||||
| BEA | ||||||||
| MON | ||||||||
| AURO | 🡫 −69.8% (p = 0.045) | 🡫 −69.8% (p = 0.0003) | 🡫 −64.3% (p = 0.0001) | 🡫 −55.1% (p = 3.7 × 10−7) | ||||
| RUB | 🡫 −59.3% (p = 0.029) | |||||||
| IP | 🡩 +19.8% (p = 0.011) | |||||||
| BUT | 🡫 −47.7% (p = 1.14 × 10−6) | |||||||
| TRYP | 🡩 +72.2% (p = 0.001) | 🡩 +72.2% (p = 0.01) | ||||||
| CHRY | 🡫 −57.5% (p = 0.043) | 🡫 −57.5% (p = 2.21 × 10−6) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleber, A.; Gruber-Dorninger, C.; Platzer, A.; Payet, C.; Novak, B. Effect of Fungicide Treatment on Multi-Mycotoxin Occurrence in French Wheat during a 4-Year Period. Toxins 2023, 15, 443. https://doi.org/10.3390/toxins15070443
Kleber A, Gruber-Dorninger C, Platzer A, Payet C, Novak B. Effect of Fungicide Treatment on Multi-Mycotoxin Occurrence in French Wheat during a 4-Year Period. Toxins. 2023; 15(7):443. https://doi.org/10.3390/toxins15070443
Chicago/Turabian StyleKleber, Alexandra, Christiane Gruber-Dorninger, Alexander Platzer, Clarisse Payet, and Barbara Novak. 2023. "Effect of Fungicide Treatment on Multi-Mycotoxin Occurrence in French Wheat during a 4-Year Period" Toxins 15, no. 7: 443. https://doi.org/10.3390/toxins15070443
APA StyleKleber, A., Gruber-Dorninger, C., Platzer, A., Payet, C., & Novak, B. (2023). Effect of Fungicide Treatment on Multi-Mycotoxin Occurrence in French Wheat during a 4-Year Period. Toxins, 15(7), 443. https://doi.org/10.3390/toxins15070443





