Insights into the Ecotoxicology of Radicinin and (10S,11S)-(—)-epi-Pyriculol, Fungal Metabolites with Potential Application for Buffelgrass (Cenchrus ciliaris) Biocontrol
Abstract
1. Introduction
2. Results and Discussion
2.1. Toxicity of Radicinin and EPYR against Representative Organisms
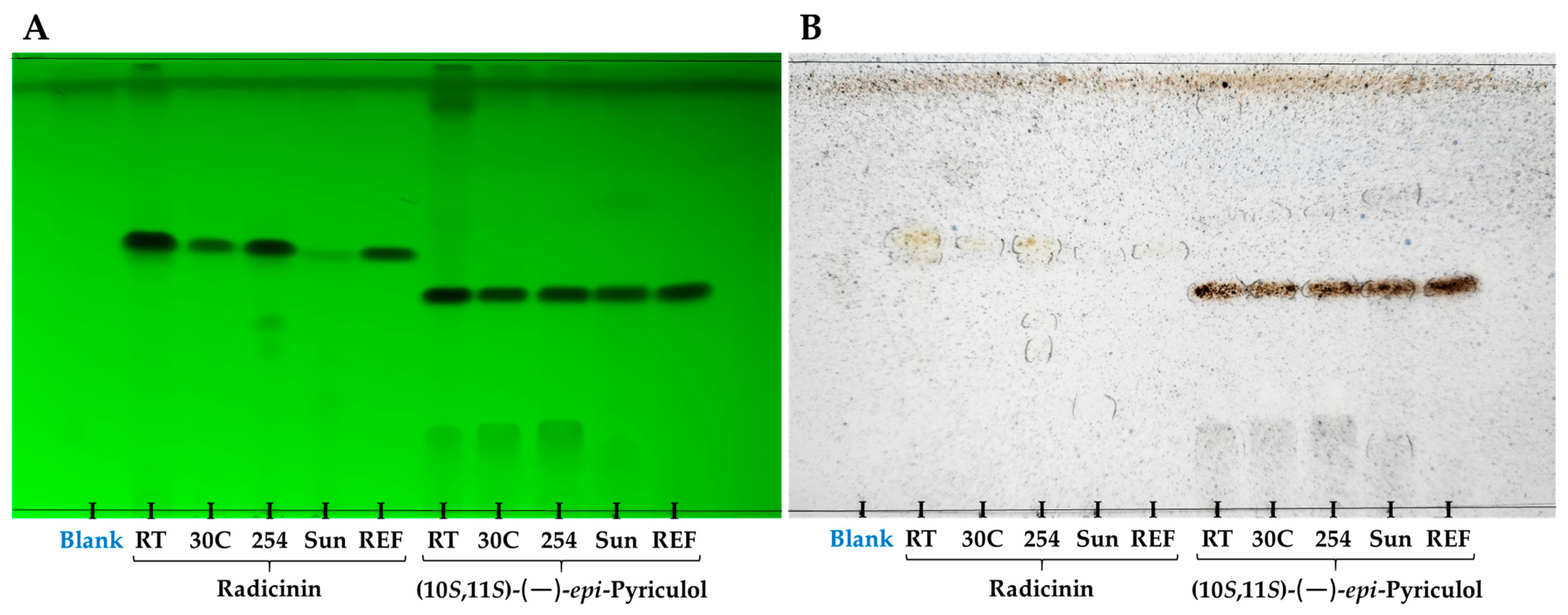
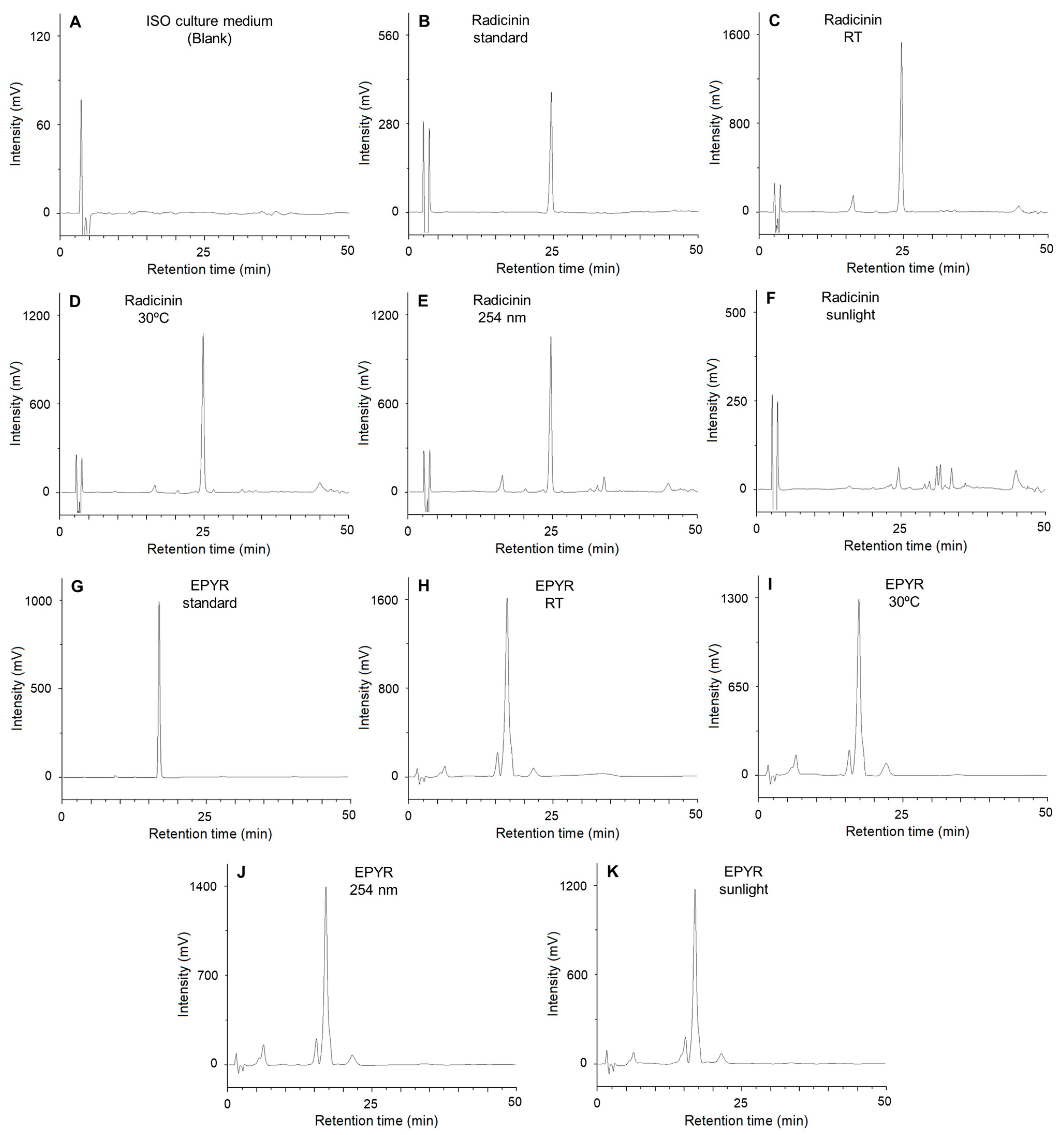
2.2. Stability Assays of EPYR and Radicinin
3. Conclusions
4. Materials and Methods
4.1. Obtaining and Identification of Radicinin and EPYR
4.2. Toxicity Assays
4.3. Degradation Assays
4.4. Thin-Layer Chromatography and HPLC Analyses
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, V.M.; Lewis, M.M.; Ostendorf, B. Buffel grass (Cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: A review. J. Arid Environ. 2012, 78, 1–12. [Google Scholar] [CrossRef]
- Farrell, H.L.; Funk, J.; Law, D.; Gornish, E.S. Impacts of drought and native grass competition on buffelgrass (Pennisetum ciliare). Biol. Invasions 2022, 24, 697–708. [Google Scholar] [CrossRef]
- Gerst, K.L.; Crimmins, T.M.; Posthumus, E.; Marsh, R.L.; Switzer, J.; Wallace, C. The USA National Phenology Network’s Buffelgrass Green-up Forecast map products. Ecol. Solut. Evid. 2021, 2, e12109. [Google Scholar] [CrossRef]
- Edwards, K.M.; Schlesinger, C.; Ooi, M.K.; French, K.; Gooden, B. Invasive grass affects seed viability of native perennial shrubs in arid woodlands. Biol. Invasions 2019, 21, 1763–1774. [Google Scholar] [CrossRef]
- de Albuquerque, F.S.; Macías-Rodríguez, M.Á.; Búrquez, A.; Astudillo-Scalia, Y. Climate change and the potential expansion of buffelgrass (Cenchrus ciliaris L., Poaceae) in biotic communities of Southwest United States and northern Mexico. Biol. Invasions 2019, 21, 3335–3347. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Handbook of Sustainable Weed Management; Singh, H.P., Batish, D.R., Kohli, R.K., Eds.; The Haworth Press Inc.: New York, NY, USA, 2006. [Google Scholar]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.L.; Gornish, E.S. Pennisetum ciliare: A review of treatment efficacy, competitive traits, and restoration opportunities. Invasive Plant Sci. Manag. 2019, 12, 203–213. [Google Scholar] [CrossRef]
- Berestetskiy, A. Modern approaches for the development of new herbicides based on natural compounds. Plants 2023, 12, 234. [Google Scholar] [CrossRef]
- Castaldi, S.; Zorrilla, J.G.; Petrillo, C.; Russo, M.T.; Ambrosino, P.; Masi, M.; Cimmino, A.; Isticato, R. Alternaria alternata isolated from infected pears (Pyrus communis) in Italy produces non-host toxins and hydrolytic enzymes as infection mechanisms and exhibits competitive exclusion against Botrytis cinerea in co-infected host fruits. J. Fungi 2023, 9, 326. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Kong, L.C.; Jiang, D.H.; Yin, C.P.; Cai, Q.M.; Chen, Q.; Zheng, J.Y. Phytotoxic and antifungal metabolites from Curvularia sp. FH01 isolated from the gut of Atractomorpha sinensis. Bioresour. Technol. 2011, 102, 3575–3577. [Google Scholar] [CrossRef]
- Varejão, E.V.; Demuner, A.J.; Barbosa, L.C.; Barreto, R.W. The search for new natural herbicides–Strategic approaches for discovering fungal phytotoxins. Crop Prot. 2013, 48, 41–50. [Google Scholar] [CrossRef]
- Masi, M.; Zorrilla, J.G.; Meyer, S. Bioactive metabolite production in the genus Pyrenophora (Pleosporaceae, Pleosporales). Toxins 2022, 14, 588. [Google Scholar] [CrossRef]
- Macías, F.A.; Velasco, R.F.; Castellano, D.; Galindo, J.C. Application of Hansch’s model to guaianolide ester derivatives: A quantitative structure−activity relationship study. J. Agric. Food Chem. 2005, 53, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Roireau, J.H.; Rosano, R.J.; Lazzara, N.C.; Chen, T.; Bajsa-Hirschel, J.; Schrader, K.K.; Duke, S.O.; Wykoff, D.; Giuliano, R.M. Synthesis of pyranopyrans related to diplopyrone and evaluation as antibacterials and herbicides. J. Agric. Food Chem. 2020, 68, 9906–9916. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Nord, F.F. Radicinin: A metabolite from Stemphylium radicinum. I. Chemistry and action. Arch. Biochem. Biophys. 1955, 59, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Santoro, E.; Mazzeo, G.; Marsico, G.; Masi, M.; Longhi, G.; Superchi, S.; Evidente, A.; Abbate, S. Assignment through chiroptical methods of the absolute configuration of fungal dihydropyranpyran-4-5-diones phytotoxins, potential herbicides for buffelgrass (Cenchrus ciliaris) biocontrol. Molecules 2019, 24, 3022. [Google Scholar] [CrossRef] [PubMed]
- Rolshausen, P.; Roper, C.; Maloney, K. Greenhouse Evaluation of Grape-Vine Microbial Endophytes and Fungal Natural Products for Control of Pierce’s Disease. Final Report of CDFA Agreement. 16-0512; 2018. Available online: https://static.cdfa.ca.gov/PiercesDisease/reports/2018/Rolshausen%2016-0512-SA_Final%20Report.pdf (accessed on 9 June 2023).
- Suzuki, M.; Sugiyama, T.; Watanabe, M.; Murayama, T.; Yamashita, K. Syntheses of all four stereoisomers of pyriculol. Agric. Biol. Chem. 1987, 51, 2161–2166. [Google Scholar]
- Leyva, A.; Blum, F.E.; Ley, S.V. A new synthesis of (−)-epipyriculol: A phytotoxic metabolite. Tetrahedron 2008, 64, 4711–4717. [Google Scholar] [CrossRef]
- Kono, Y.; Sekido, S.; Yamaguchi, I.; Kondo, H.; Suzuki, Y.; Neto, G.C.; Sakurai, A.; Yaegashi, H. Structures of two novel pyriculol-related compounds and identification of naturally produced epipyriculol from Pyricularia oryzae. Agric. Biol. Chem. 1991, 55, 2785–2791. [Google Scholar] [CrossRef]
- Yang, Y.H.; Yang, D.S.; Lei, H.M.; Li, C.Y.; Li, G.H.; Zhao, P.J. Griseaketides A–D, new aromatic polyketides from the pathogenic fungus Magnaporthe grisea. Molecules 2019, 25, 72. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Evidente, A. Effect of cultural conditions on the production of radicinin, a specific fungal phytotoxin for buffelgrass (Cenchrus ciliaris) biocontrol, by different Cochlioboulus australiensis strains. Nat. Prod. Res. 2021, 35, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, J.G.; Masi, M.; Clement, S.; Cimmino, A.; Meyer, S. Production of (10S,11S)-(—)-epi-pyriculol and its HPLC quantification in liquid cultures of Pyricularia grisea, a potential mycoherbicide for the control of buffelgrass (Cenchrus ciliaris). J. Fungi 2023, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Marsico, G.; Ciccone, M.S.; Masi, M.; Freda, F.; Cristofaro, M.; Evidente, A.; Superchi, S.; Scafato, P. Synthesis and herbicidal activity against buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules 2019, 24, 3193. [Google Scholar] [CrossRef]
- ISO 8692:2012; Water Quality–Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. International Organisation for Standardisation: Geneva, Switzerland, 2012.
- ISO 11348-3:2007; Water Quality–Determination of the Inhibitory Effect of Water Samples on the Light Emission of Aliivibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria. International Organisation for Standardisation: Geneva, Switzerland, 2007.
- ISO 6341:2012; Water Quality-Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organisation for Standardisation: Geneva, Switzerland, 2012.
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 5 Rev. Ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2013. [Google Scholar]
- Thompson, D.G.; Kreutzweiser, D.P. A review of the environmental fate and effects of natural “reduced-risk” pesticides in Canada. In Crop Protection Products for Organic Agriculture; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Handayani, D.; Putri, R.A.; Ismed, F.; Hertiani, T.; Ariantari, N.P.; Proksch, P. Bioactive metabolite from marine sponge-derived fungus Cochliobolus geniculatus WR12. Rasayan J. Chem. 2020, 13, 417–422. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Vitti, C.; De Girolamo, A.; Visconti, A.; Logrieco, A.; Fanizzi, F.P. Radicinols and radicinin phytotoxins produced by Alternaria radicina on carrots. J. Agric. Food Chem. 2004, 52, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Tylkowska, K.; Grabarkiewicz-Szczesna, J.; Szopinska, D.; Dorna, H.; Solfrizzo, M.; De Girolamo, A. Effects of temperature and incubation period on production of toxic metabolites by Alternaria radicina and A. alternata. Pol. Bot. J. 2005, 57, 7–17. [Google Scholar] [CrossRef]
- Tylkowska, K.; Bagniewska-Zadworna, A.; Grabarkiewicz-Szczesna, J.; Szopinska, D.; Dorna, H.; Zenkteler, E. Histopathology of Daucus carota L. root cells treated with toxic metabolites produced by Alternaria radicina and A. alternata. Acta Biol. Crac. Ser. Bot. 2008, 50, 27–34. [Google Scholar]
- Grove, J.F. Metabolic products of Stemphylium radicinum. Part III. Biosynthesis of radicinin and pyrenophorin. J. Chem. Soc. C 1970, 13, 1860–1865. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakuno, E.; Ishihara, A.; Tamura, J.I.; Nakajima, H. Conversions of deoxyradicinin to radicinin and of radicinin to 3-epi-radicinin in the phytopathogenic fungus Bipolaris coicis. Phytochemistry 2012, 75, 14–20. [Google Scholar] [CrossRef]
- Jacob, S.; Grötsch, T.; Foster, A.J.; Schüffler, A.; Rieger, P.H.; Sandjo, L.P.; Liermann, J.G.; Opatz, T.; Thines, E. Unravelling the biosynthesis of pyriculol in the rice blast fungus Magnaporthe oryzae. Microbiology 2017, 163, 541. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, Y.; Motoyama, T.; Nogawa, T.; Hayashi, T.; Hirota, H.; Kiyota, H.; Kamakura, T.; Osada, H. Controlling the production of phytotoxin pyriculol in Pyricularia oryzae by aldehyde reductase. Biosci. Biotechnol. Biochem. 2021, 85, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, Y.; Jiao, B.; Zuo, H.; Dong, F.; Wu, X.; Pan, X.; Xu, J. Photodegradation of enestroburin in water by simulated sunlight irradiation: Kinetics, isomerization, transformation products identification and toxicity assessment. Sci. Total Environ. 2022, 849, 157725. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce’s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Temussi, F.; Cermola, F.; DellaGreca, M.; Iesce, M.R.; Passananti, M.; Previtera, L.; Zarrelli, A. Determination of photostability and photodegradation products of indomethacin in aqueous media. J. Pharm. Biomed. Anal. 2011, 56, 678–683. [Google Scholar] [CrossRef]
- Nakajima, H.; Ishida, T.; Otsuka, Y.; Hamasaki, T.; Ichinoe, M. Phytotoxins and related metabolites produced by Bipolaris coicis, the pathogen of Job’s tears. Phytochemistry 1997, 45, 41–45. [Google Scholar] [CrossRef]

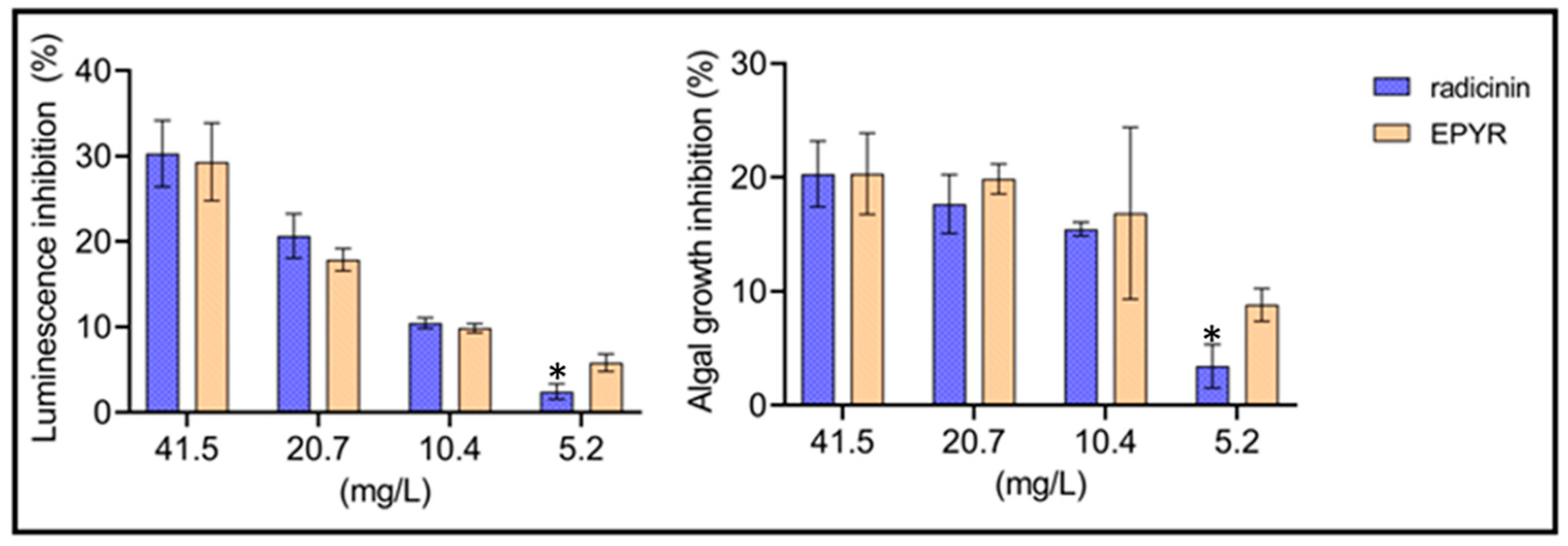
| Compounds | EC5 | EC10 | EC20 | EC50 |
|---|---|---|---|---|
| Radicinin | 0.62 (0.03–4.45) | 0.86 (0.04–5.52) | 1.68 (0.08–8.52) | 19.14 (12.49–27.71) |
| (10S,11S)-(—)-epi-Pyriculol (EPYR) | 0.49 (0.04–2.96) | 0.72 (0.06–3.88) | 1.57 (0.13–3.88) | 24.82 (19.87–31.64) |
| Compound and Degradation Method | Concentration for Analysis (mg/mL) | Retention Time (min) | Amount of Compound Detected (µg) in 20 µL | % of Compound Degraded after 72 h |
|---|---|---|---|---|
| Radicinin (standard) | 0.5 | 24.583 | - | - |
| Radicinin (room temperature) | 0.5 | 24.560 | 4.049 ± 0.011 | 59.51 |
| Radicinin (30 °C) | 0.5 | 24.603 | 2.629 ± 0.004 | 73.71 |
| Radicinin (254 nm) Radicinin (sunlight) | 0.5 | 24.510 | 2.618 ± 0.005 | 73.82 |
| 0.5 | 24.513 | 0.110 ± 0.001 | 98.90 | |
| EPYR (standard) | 1.0 | 17.381 | - | - |
| EPYR (room temperature) | 1.0 | 17.347 | 10.148 ± 0.029 | 49.26 |
| EPYR (30 °C) | 1.0 | 17.433 | 7.983 ± 0.014 | 60.09 |
| EPYR (254 nm) | 1.0 | 17.380 | 8.178 ± 0.014 | 59.11 |
| EPYR (sunlight) | 1.0 | 17.310 | 6.936 ± 0.012 | 65.32 |
| Blank | 1.0 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A.; Zorrilla, J.G.; Saviano, L.; Cimmino, A.; Guida, M.; Masi, M.; Meyer, S. Insights into the Ecotoxicology of Radicinin and (10S,11S)-(—)-epi-Pyriculol, Fungal Metabolites with Potential Application for Buffelgrass (Cenchrus ciliaris) Biocontrol. Toxins 2023, 15, 405. https://doi.org/10.3390/toxins15060405
Siciliano A, Zorrilla JG, Saviano L, Cimmino A, Guida M, Masi M, Meyer S. Insights into the Ecotoxicology of Radicinin and (10S,11S)-(—)-epi-Pyriculol, Fungal Metabolites with Potential Application for Buffelgrass (Cenchrus ciliaris) Biocontrol. Toxins. 2023; 15(6):405. https://doi.org/10.3390/toxins15060405
Chicago/Turabian StyleSiciliano, Antonietta, Jesús G. Zorrilla, Lorenzo Saviano, Alessio Cimmino, Marco Guida, Marco Masi, and Susan Meyer. 2023. "Insights into the Ecotoxicology of Radicinin and (10S,11S)-(—)-epi-Pyriculol, Fungal Metabolites with Potential Application for Buffelgrass (Cenchrus ciliaris) Biocontrol" Toxins 15, no. 6: 405. https://doi.org/10.3390/toxins15060405
APA StyleSiciliano, A., Zorrilla, J. G., Saviano, L., Cimmino, A., Guida, M., Masi, M., & Meyer, S. (2023). Insights into the Ecotoxicology of Radicinin and (10S,11S)-(—)-epi-Pyriculol, Fungal Metabolites with Potential Application for Buffelgrass (Cenchrus ciliaris) Biocontrol. Toxins, 15(6), 405. https://doi.org/10.3390/toxins15060405











