Simultaneous Determination of Pyrethrins, Pyrethroids, and Piperonyl Butoxide in Animal Feeds by Liquid Chromatography-Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. LC-MS/MS Conditions
2.2. Method Validation Results
2.3. Method Application in Real Feed Samples
2.4. Method Application to a Toxicological Case
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. LC-MS/MS Instrument Analysis
4.4. Method Validation
4.5. Real Feed Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anadon, A.; Martinez-Larranaga, M.R.; Martinez, M.A. Use and Abuse of Pyrethrins and Synthetic Pyrethroids in Veterinary Medicine. Vet. J. 2009, 182, 7–20. [Google Scholar] [CrossRef]
- Ravula, A.R.; Yenugu, S. Pyrethroid Based Pesticides–Chemical and Biological Aspects. Crit. Rev. Toxicol. 2021, 51, 117–140. [Google Scholar] [CrossRef]
- Anadón, A.; Arés, I.; Martínez, M.A.; Martínez-Larrañaga, M.R. Pyrethrins and Synthetic Pyrethroids: Use in Veterinary Medicine. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 4061–4086. [Google Scholar]
- Ensley, S.M. Chapter 39–Pyrethrins and Pyrethroids. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 515–520. [Google Scholar]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, Pyrethrins, Pyrethroids and Insect Sodium Channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 Inhibitors and Insecticides: Challenges and Opportunities. Pest Manag. Sci. 2015, 71, 793–800. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Ahamad, A.; Kumar, J. Pyrethroid Pesticides: An Overview on Classification, Toxicological Assessment and Monitoring. J. Hazard. Mater. Adv. 2023, 10, 100284. [Google Scholar] [CrossRef]
- Chrustek, A.; Holynska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wroblewski, M.; Cwynar, A.; Olszewska-Slonina, D. Current Research on the Safety of Pyrethroids used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef]
- Holynska-Iwan, I.; Szewczyk-Golec, K. Pyrethroids: How they Affect Human and Animal Health? Medicina 2020, 56, 582. [Google Scholar] [CrossRef] [PubMed]
- Tuck, S.; Furey, A.; Crooks, S.; Danaher, M. A Review of Methodology for the Analysis of Pyrethrin and Pyrethroid Residues in Food of Animal Origin. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 911–940. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.G.; Kurz, M.; Guimaraes, M.; Martins, M.L.; Prestes, O.D.; Zanella, R.; Ribeiro, J.; Goncalves, F.F. Development and Validation of a Method for the Analysis of Pyrethroid Residues in Fish using GC-MS. Food Chem. 2019, 297, 124944. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, W.; Wang, J.; Yin, J.; Liu, Y.; Liu, Z. New Strategy to Enhance the Extraction Efficiency of Pyrethroid Pesticides in Fish Samples using a Modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) Method. Anal. Methods 2012, 4, 449–453. [Google Scholar] [CrossRef]
- Kim, B.J.; Yang, S.; Choi, H. Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry. Foods 2022, 11, 3634. [Google Scholar] [CrossRef] [PubMed]
- Khay, S.; Abd El-Aty, A.M.; Choi, J.; Shin, E.; Shin, H.; Kim, J.; Chang, B.; Lee, C.; Shin, S.; Jeong, J.Y.; et al. Simultaneous Determination of Pyrethroids from Pesticide Residues in Porcine Muscle and Pasteurized Milk using GC. J. Sep. Sci. 2009, 32, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Santilio, A.; Cataldi, L.; Dommarco, R. Multiresidue Analysis of Organochlorine and Pyrethroid Pesticides in Ground Beef Meat by Gas Chromatography-Mass Spectrometry. J. Environ. Sci. Health B 2009, 44, 350–356. [Google Scholar] [CrossRef]
- Barbini, D.A.; Vanni, F.; Girolimetti, S.; Dommarco, R. Development of an Analytical Method for the Determination of the Residues of Four Pyrethroids in Meat by GC-ECD and Confirmation by GC-MS. Anal. Bioanal. Chem. 2007, 389, 1791–1798. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, M.; Yu, Y.; Wang, X.; Zhang, H.; Ding, L.; Jin, H. Determination of Pyrethroids in Porcine Tissues by Matrix Solid-Phase Dispersion Extraction and High-Performance Liquid Chromatography. Meat Sci. 2009, 82, 407–412. [Google Scholar] [CrossRef]
- Moloney, M.; Tuck, S.; Ramkumar, A.; Furey, A.; Danaher, M. Determination of Pyrethrin and Pyrethroid Residues in Animal Fat using Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1077–1078, 60–70. [Google Scholar] [CrossRef]
- Zheng, W.; Choi, J.; Abd El-Aty, A.M.; Yoo, K.; Park, D.; Kim, S.; Kang, Y.; Hacımüftüoğlu, A.; Wang, J.; Shim, J.; et al. Simultaneous Determination of Spinosad, Temephos, and Piperonyl Butoxide in Animal-Derived Foods using LC-MS/MS. Biomed. Chromatogr. 2019, 33, e4493. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Faas, J.; Krska, R.; Khiaosa-Ard, R.; Zebeli, Q. Residues of Pesticides and Veterinary Drugs in Diets of Dairy Cattle from Conventional and Organic Farms in Austria. Environ. Pollut. 2023, 316, 120626. [Google Scholar] [CrossRef]
- US EPA. Tolerances and Exemptions for Pesticide Chemical Residues in Food. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-180/subpart-C (accessed on 20 March 2023).
- Caloni, F.; Cortinovis, C.; Rivolta, M.; Davanzo, F. Animal Poisoning in Italy: 10 Years of Epidemiological Data from the Poison Control Centre of Milan. Vet. Rec. 2012, 170, 415. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.L.; Cortinovis, C.; Spicer, L.J.; Perego, M.C.; Caloni, F. Long-Established and Emerging Pesticide Poisoning in Horses. Equine Vet. Educ. 2019, 31, 496–500. [Google Scholar] [CrossRef]
- He, F.S.; Deng, H.; Ji, X.; Zhang, Z.W.; Sun, J.X.; Yao, P.P. Changes of Nerve Excitability and Urinary Deltamethrin in Sprayers. Int. Arch. Occup. Environ. Health 1991, 62, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Tavengwa, N.T. Recent Developments and Applications of QuEChERS Based Techniques on Food Samples during Pesticide Analysis. J. Food Compost. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]
- US FDA Foods Program. Guidelines for the Validation of Chemical Methods for the Foods Program. Available online: https://www.fda.gov/food/laboratory-methods-food/foods-program-methods-validation-processes-and-guidelines (accessed on 20 March 2023).
- Kappenberg, A.; Juraschek, L.M. Development of a LC-MS/MS Method for the Simultaneous Determination of the Mycotoxins Deoxynivalenol (DON) and Zearalenone (ZEA) in Soil Matrix. Toxins 2021, 13, 470. [Google Scholar] [CrossRef]
- Carballo, D.; Font, G.; Ferrer, E.; Berrada, H. Evaluation of Mycotoxin Residues on Ready-to-Eat Food by Chromatographic Methods Coupled to Mass Spectrometry in Tandem. Toxins 2018, 10, 243. [Google Scholar] [CrossRef]
- Maneeboon, T.; Chuaysrinule, C.; Mahakarnchanakul, W. Optimization and Validation of Dispersive Liquid-Liquid Microextraction for Simultaneous Determination of Aflatoxins B1, B2, G1, and G2 in Senna Leaves and Pods using HPLC-FLD with Pre-Column Derivatization. Toxins 2023, 15, 277. [Google Scholar] [CrossRef]
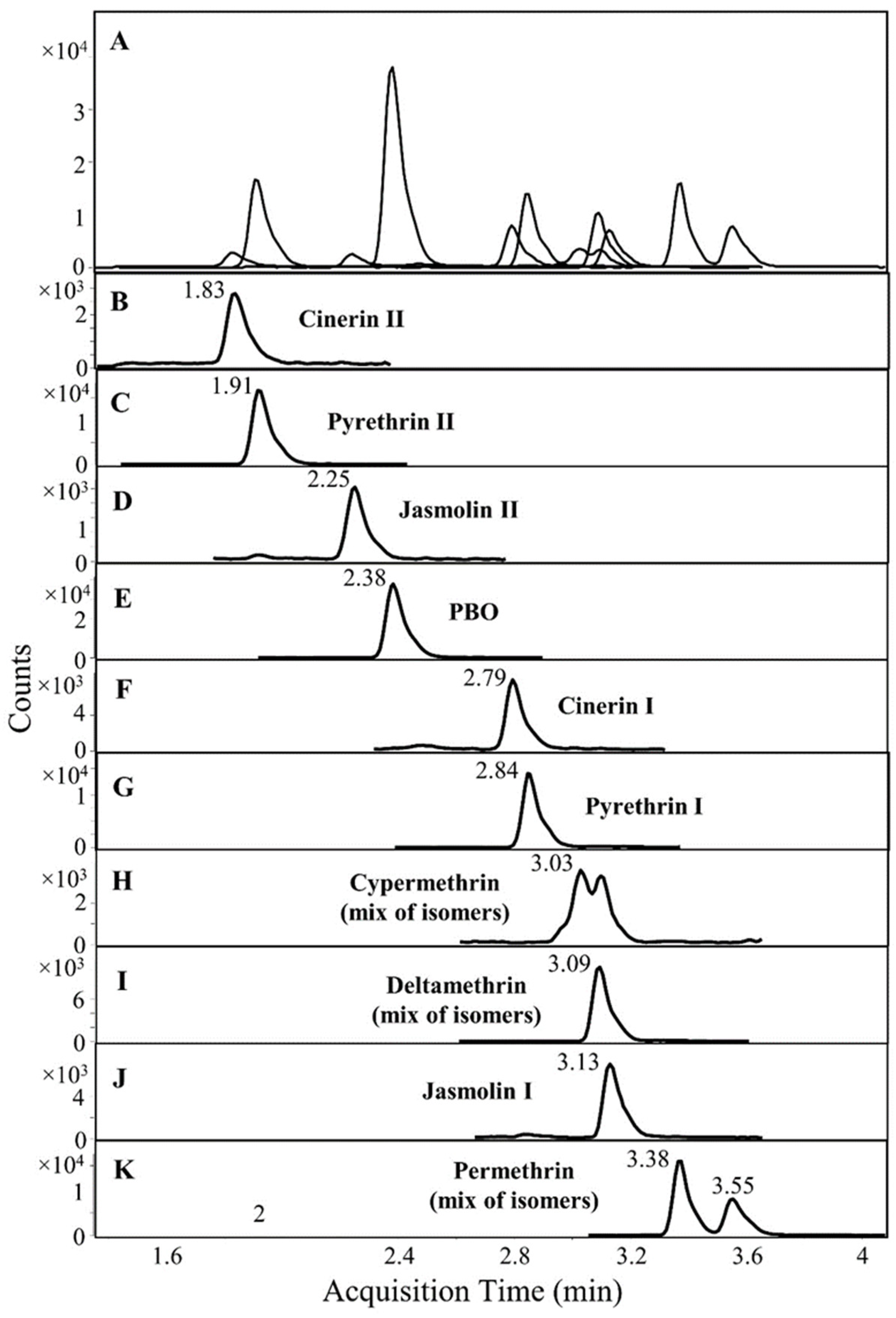
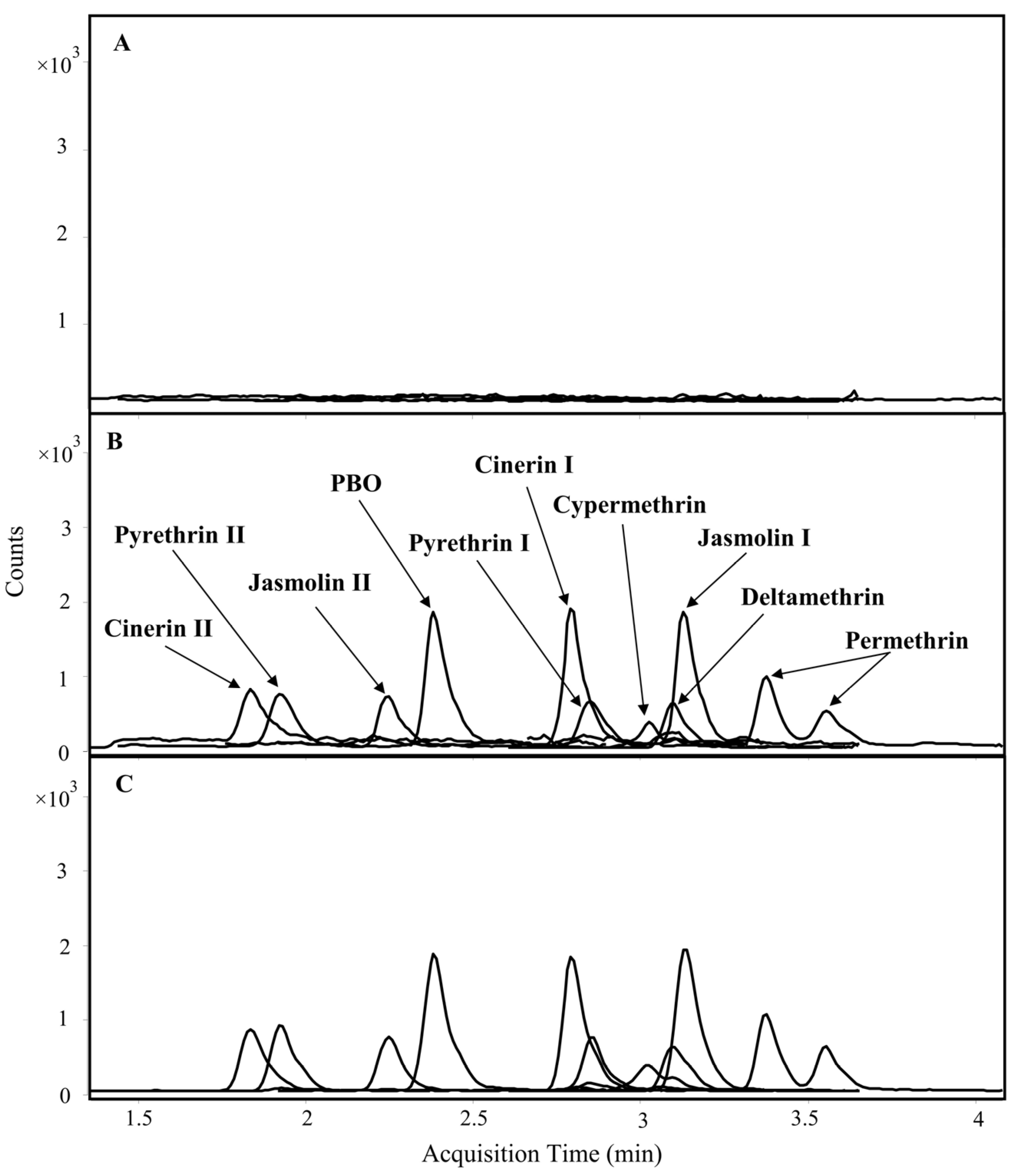
| Compound | Quantitation Range (µg/kg) | LOD (µg/kg) | LOQ (µg/kg) | Matrix Effects (%) |
|---|---|---|---|---|
| PBO | 1–50 | 0.15 | 1 | 108 |
| Cypermethrin | 10–500 | 3 | 10 | 82 |
| Deltamethrin | 10–500 | 3 | 10 | 77 |
| Permethrin | 10–500 | 3 | 10 | 76 |
| Pyrethrin I | 5.61–280.5 | 1.68 | 5.61 | 84 |
| Pyrethrin II | 2.78–139 | 0.83 | 2.78 | 82 |
| Cinerin I | 2.85–28.5 | 0.86 | 2.85 | 88 |
| Cinerin II | 1.9–19 | 0.57 | 1.9 | 86 |
| Jasmolin I | 2–20 | 0.60 | 2 | 87 |
| Jasmolin II | 1.35–13.5 | 0.41 | 1.35 | 84 |
| Compound | Analytical Technique | Sample Type | Sample Size (g or mL) | Run Time (min) | LOD (µg/kg) | LOQ (µg/kg) | Reference |
|---|---|---|---|---|---|---|---|
| Pyrethroids | GC/MS | Fish tissue | 10 | 25 | N.A. | 5–10 | [12] |
| Pyrethroids | GC-MS/MS | Beef, pork, chicken, milk, and eggs | 5 | 26.83 | N.A. | 10 | [14] |
| Pyrethroids, Organochlorine | GC/MS | Beef meat | 10 | ~60 | N.A. | 13–125 | [16] |
| Pyrethrins, Pyrethroids | LC-MS/MS | Animal fat | 1 | 21 | N.A. | 50 | [19] |
| N.A. | 10–500 | ||||||
| PBO, Other insecticides | LC-MS/MS | Porcine muscle, eel, flatfish, shrimp, milk, andwhole egg | 2 | 8 | 0.3–0.7 | 0.6–1.5 | [20] |
| PBO, Other pesticides Veterinary drugs | LC-MS/MS | Dairy cattle feed | 5 | 21 | 5 | 15 | [21] |
| Pyrethrins | LC-MS/MS | Various animal feed for cattle, horses, goats, sheep, pigs, and poultry | 0.1 | <7 | 0.41–1.68 | 1.35–5.61 | This study |
| Pyrethroids | 3 | 10 | |||||
| PBO | 0.15 | 1 |
| Compound | Spiked Level (µg/kg) | Intra-Day (%, n = 5) | Inter-Day (%, n = 15) | ||
|---|---|---|---|---|---|
| Accuracy | Precision | Accuracy | Precision | ||
| PBO | 1 | 112 | 4.2 | 109 | 3.9 |
| 20 | 97 | 1.4 | 97 | 1.2 | |
| 50 | 104 | 1.7 | 103 | 1.8 | |
| Cypermethrin | 10 | 92 | 7.2 | 94 | 9.1 |
| 200 | 86 | 4.6 | 87 | 3.9 | |
| 500 | 84 | 2.9 | 86 | 2.8 | |
| Deltamethrin | 10 | 91 | 7.6 | 99 | 8.0 |
| 200 | 103 | 3.8 | 102 | 2.8 | |
| 500 | 110 | 2.2 | 108 | 2.2 | |
| Permethrin | 10 | 115 | 4.3 | 109 | 8.2 |
| 200 | 106 | 7.6 | 103 | 6.3 | |
| 500 | 114 | 7.4 | 109 | 6.8 | |
| Pyrethrin I | 5.61 | 111 | 7.5 | 107 | 6.3 |
| 112.2 | 111 | 4.0 | 110 | 3.3 | |
| 280.5 | 114 | 4.4 | 112 | 3.5 | |
| Pyrethrin II | 2.78 | 114 | 5.3 | 111 | 4.6 |
| 55.6 | 112 | 4.1 | 111 | 3.5 | |
| 139 | 114 | 3.5 | 113 | 3.2 | |
| Cinerin I | 2.85 | 111 | 6.2 | 110 | 4.7 |
| 11.4 | 112 | 5.1 | 109 | 4.2 | |
| 28.5 | 113 | 4.1 | 111 | 3.0 | |
| Cinerin II | 1.9 | 107 | 9.0 | 106 | 7.0 |
| 7.6 | 109 | 5.1 | 108 | 4.6 | |
| 19 | 113 | 4.2 | 111 | 3.6 | |
| Jasmolin I | 2 | 112 | 4.6 | 111 | 4.2 |
| 8 | 110 | 3.8 | 109 | 3.2 | |
| 20 | 112 | 4.7 | 111 | 3.8 | |
| Jasmolin II | 1.35 | 113 | 4.5 | 110 | 4.3 |
| 5.4 | 111 | 4.9 | 110 | 4.4 | |
| 13.5 | 113 | 4.0 | 111 | 3.6 | |
| Sample | PBO | Cypermethrin | Deltamethrin | Permethrin | Pyrethrins |
|---|---|---|---|---|---|
| Cattle feed (µg/kg) | |||||
| Sample 1 | 3.59 | ND | Traces | ND | ND |
| Sample 2 | 20.1 | ND | Traces | ND | ND |
| Sample 3 | 186.5 | ND | 10.3 | ND | ND |
| Sample 4 | 18.3 | ND | Traces | ND | ND |
| Sample 5 | 6.2 | ND | Traces | ND | ND |
| Sample 6 | 1.17 | ND | ND | ND | ND |
| Horse feed (µg/kg) | |||||
| Sample 1 | 4.02 | ND | ND | ND | ND |
| Sample 2 | 13 | ND | Traces | ND | ND |
| Sample 3 | 90.1 | ND | Traces | ND | ND |
| Goat feed (µg/kg) | |||||
| Sample 1 | 895 | ND | 89.6 | ND | ND |
| Sample 2 | 7.87 | ND | ND | ND | ND |
| Sheep feed (µg/kg) | |||||
| Sample 1 | 83.4 | ND | 55 | ND | ND |
| Sample 2 | 14.7 | ND | 10.4 | ND | ND |
| Sample 3 | 7.6 | ND | ND | ND | ND |
| Sample 4 | 4.95 | ND | ND | ND | ND |
| Pig feed (µg/kg) | |||||
| Sample 1 | 6.04 | 77.2 | ND | ND | ND |
| Sample 2 | 1.18 | ND | ND | ND | ND |
| Sample 3 | 32.2 | Traces | ND | ND | ND |
| Sample 4 | 11.6 | Traces | ND | ND | ND |
| Poultry feed (µg/kg) | |||||
| Sample 1 | 4.75 | ND | ND | ND | ND |
| Sample 2 | 6.28 | 93.5 | Traces | ND | ND |
| Sample 3 | 2.46 | ND | ND | ND | ND |
| Toxicology case (µg/kg) | |||||
| Lot #1 Sample | 1040 | ND | 38.4 | ND | ND |
| Lot #2 Sample | 2480 | ND | 90.6 | ND | ND |
| Compound | Precursor Ion (m/z) | Product Ion for Quantification (m/z) | Product Ion for Qualification (m/z) | Collision Energy (eV) * | Internal Standard |
|---|---|---|---|---|---|
| PBO | 356.2 | 177.0 | 119.0 | 9, 41 | PBO-d9 |
| Cypermethrin | 433.1 | 190.9 | 416.1 | 13, 5 | Cypermethrin-d5 |
| Deltamethrin | 523.0 | 280.9 | 506.0 | 13, 5 | Deltamethrin-d5 |
| Permethrin | 408.1 | 183.0 | 355.1 | 21, 5 | Permethrin-d5 |
| Pyrethrin I | 329.2 | 160.9 | 143.0 | 5, 17 | Cypermethrin-d5 |
| Pyrethrin II | 373.2 | 161.1 | 133.1 | 5, 25 | Cypermethrin-d5 |
| Cinerin I | 317.2 | 149.1 | 107.0 | 5, 21 | Cypermethrin-d5 |
| Cinerin II | 361.2 | 149.0 | 107.0 | 5, 25 | Cypermethrin-d5 |
| Jasmolin I | 331.2 | 163.1 | 107.0 | 5, 21 | Cypermethrin-d5 |
| Jasmolin II | 375.2 | 163.1 | 107.0 | 5, 33 | Cypermethrin-d5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Murphy, L.A. Simultaneous Determination of Pyrethrins, Pyrethroids, and Piperonyl Butoxide in Animal Feeds by Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2023, 15, 401. https://doi.org/10.3390/toxins15060401
Xu X, Murphy LA. Simultaneous Determination of Pyrethrins, Pyrethroids, and Piperonyl Butoxide in Animal Feeds by Liquid Chromatography-Tandem Mass Spectrometry. Toxins. 2023; 15(6):401. https://doi.org/10.3390/toxins15060401
Chicago/Turabian StyleXu, Xin, and Lisa A. Murphy. 2023. "Simultaneous Determination of Pyrethrins, Pyrethroids, and Piperonyl Butoxide in Animal Feeds by Liquid Chromatography-Tandem Mass Spectrometry" Toxins 15, no. 6: 401. https://doi.org/10.3390/toxins15060401
APA StyleXu, X., & Murphy, L. A. (2023). Simultaneous Determination of Pyrethrins, Pyrethroids, and Piperonyl Butoxide in Animal Feeds by Liquid Chromatography-Tandem Mass Spectrometry. Toxins, 15(6), 401. https://doi.org/10.3390/toxins15060401





