Abstract
We conducted a comparative analysis to unveil the divergence among venoms from a subset of Old World habu snakes (Protobothrops) in terms of venomic profiles and toxicological and enzymatic activities. A total of 14 protein families were identified in the venoms from these habu snakes, and 11 of them were shared among these venoms. The venoms of five adult habu snakes were overwhelmingly dominated by SVMP (32.56 ± 13.94%), PLA2 (22.93 ± 9.26%), and SVSP (16.27 ± 4.79%), with a total abundance of over 65%, while the subadult P. mangshanensis had an extremely low abundance of PLA2 (1.23%) but a high abundance of CTL (51.47%), followed by SVMP (22.06%) and SVSP (10.90%). Apparent interspecific variations in lethality and enzymatic activities were also explored in habu snake venoms, but no variations in myotoxicity were found. Except for SVSP, the resemblance of the relatives within Protobothrops in other venom traits was estimated to deviate from Brownian motion evolution based on phylogenetic signals. A comparative analysis further validated that the degree of covariation between phylogeny and venom variation is evolutionarily labile and varies among clades of closely related snakes. Our findings indicate a high level of interspecific variation in the venom proteomes of habu snakes, both in the presence or absence and the relative abundance of venom protein families, and that these venoms might have evolved under a combination of adaptive and neutral mechanisms.
Keywords:
Protobothrops; venom proteome; biochemical activity; phylogenetic signal; interspecific variation Key Contribution:
Remarkable variations have been explored in proteomic profiles and toxicological and enzymatic activities of venoms from a subset of habu snakes, and these venoms might have evolved under a combination of adaptive and neutral mechanisms.
1. Introduction
Snake venom has been widely validated to be composed of enzymes and proteins without enzymatic activity that belong to a few protein families and are regarded as an innovative biochemical weapon to assist different venomous snakes in capturing and digesting different prey [1,2]. The general concept is that the evolution of snake venom was driven by natural selection to adapt to the diversity of diets during the radiation of snakes [3,4]. It has further been claimed that properties of snake venom are associated with the resource spectrum in the habitat of the snakes, and that a highly productive environment or phylogenetically diverse diets could have promoted the development of venom with more complexity and potency [5,6,7]. However, an alternative “overkill” concept proposes that the evolution of snake venom might be subject to relaxed selection because of its high toxicity and a far higher injected dose than minimally necessary for capturing prey [8,9], by virtue of which the complexity of snake venom may be closely related to the phylogeny of the snakes [10]. This prediction is supported by empirical observations [11]; however, two other studies on phylogeny-related venom variation found that none of the major protein families were associated with phylogeny, and one family (CRISP) was even significantly associated with diet [10,12]. However, it was still declared that venom divergence can track the phylogeny of the genus Agkistrodon to a greater extent than that of Sistrurus, based on a comparison of phylogenetic signal strength [12]. The conclusion from a recent investigation of two habu snake species (Protobothrops jerdonii and P. mucrosquamatus) at a microevolutionary level strikingly integrated these two concepts mentioned above, i.e., that snake venom evolved under a combination of adaptive and neutral mechanisms [13]. Thus, it would be worthwhile examining whether this conclusion is applicable to the variations in venom composition at the macroevolutionary level in habu snakes (genus Protobothrops).
As Old-World pit vipers, habu snakes have been highly concerned in their phylogenetic relationships, biogeological patterns, and evolutionary histories. These species mainly occur in South Asia, Southeast Asia, and East Asia, and have been inferred to originate from ~20.64 mya and diverged within the genus from ~17.20 mya, with experience of 38 dispersal and 11 vicariant events [14]. They were initially considered to comprise eight species, including P. cornutus, P. elegans, P. flavoviridis, P. jerdonii, P. kaulbacki, P. mucrosquamatus, P. tokarensis, and P. xiangchengensis [15]. Due to seven more members (P. dabieshanensis, P. himalayanus, P. kelomohy, P. mangshanensis, P. maolanensis, P. sieversorum, and P. trungkhanensis) having been designated or revised from synonymous species over the last two decades, habu snakes were recently recognized to comprise 15 validated species [16,17,18,19,20]. Phylogenetic analysis based on multilocus gene markers robustly classified 14 habu snakes into four clades, in which the first clade comprises P. himalayanus, P. kaulbacki, and P. sieversorum and the second clade only comprises P. mangshanensis, while the other two clades contain the remaining four and six Protobothrops species, respectively [21]. The first clade should also contain a very recently described species (P. kelomohy) [20]. Of note, the phylogenetic relationships of the habu snakes in some clades are still very complicated and should be further clarified [21].
On the other hand, a complicated natural history and taxonomical relationships might indicate a diversity in venom profiles and snakebite envenomation mechanisms, which have also resulted in habu snakes receiving varying degrees of attention. Among these, P. mucrosquamatus might potentially cause the heaviest snakebite burden, as this species occupies the largest distribution area and threatens the greatest proportion of the human population (8.04% of the world’s population), and thus is well-known as the highest medically important habu snake [22,23]. P. flavoviridis is another highest medically important habu snake, for which epidemiological and clinical data have been exactly tracked, although this species ranges over a relatively small distribution area and threatens a very small proportion of the human population (<0.01% of the world’s population) [23,24]. Victims envenomed by P. mucrosquamatus and P. flavoviridis always suffer similar symptoms, such as local swelling, necrosis, bleeding at the bite site, vomiting, loss of consciousness, hypotension, and even acute renal failure, in addition to other species-specific symptoms (e.g., severe pain and burning sensation, festering, dizziness, and blurred vision from P. mucrosquamatus; cyanosis and compartment syndrome from P. flavoviridis) [25,26]. Epidemiological data indicate that the regional snakebite frequency of P. mucrosquamatus ranges from 1.40% (Nanchang, Jiangxi; 2017–2019) to 95.04% (Chongqin; 2019–2020) in China [27,28], and the snakebite incidence has been estimated to range from 0.16 to 1.16/100,000 population per year, whereas the snakebite incidence caused by P. flavoviridis was estimated to be 35/1,000,000 population per year during 1977–1984 in Japan [29]. To better understand and treat the snakebites from these two species, their venom compositions have been elucidated and are mainly comprised of snake venom metalloproteinase (SVMP), snake venom serine proteinase (SVSP), phospholipase A2 (PLA2), cysteine-rich secretory protein (CRISP), bradykinin-potentiating and C-type natriuretic peptides (BPP/CNP), and C-type lectin (CTL) [30,31], and the relative abundance of the same protein family can also shift due to the different sources of venom and -omics technology [32,33,34,35]. However, most habu snakes are mainly endemic to small areas or lack enough detailed epidemiological and clinical data, and are classified as secondary medically important venomous snakes [23]. Except for P. elegans, P. kelomohy, and P. mangshanensis [33,36,37], the global venom composition of the remaining habu snakes has been less studied. Thus, the mystery of the venom profiles across the genus Protobothrops still needs to be comprehensively solved.
Here, we employed a combined proteomic strategy [38,39] to conduct a relative quantitative investigation of venom profiles from six habu snakes (P. cornutus, P. jerdonii, P. mangshanensis, P. mucrosquamatus, P. sieversorum, and P. xiangchengensis; Figure 1), and used mice and specific substrates to determine the toxicological and enzymatic activities of these venoms. We also integrated the venom profiles from four other habu snakes that were previously characterized to calculate the phylogenetic signal strength of major venom components, and venom complexity and potency across the genus Protobothrops. Our results will enrich the background data for systematically exploring the interspecific variations in venom composition and function of the habu snakes, and preliminarily reveal whether these variations are related to phylogeny.
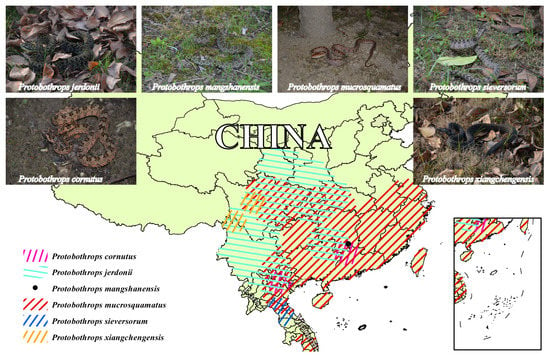
Figure 1.
Major geographical distribution regions of the six habu snakes in the current study. The distribution areas of these snakes were illustrated by ArcGIS based on the data from the GBIF website and complied with the detailed descriptions in “Snakes of China” edited by Ermi Zhao [40]. The animal images were photographed by Jian-Fang Gao; the P. mangshanensis was a subadult specimen and the other habu snakes were adult specimens. A high-resolution image can be found in Figure S1.
2. Results and Discussion
2.1. Chromatographic and Electrophoretic Profiles of Venoms from Six Habu Snakes
Conventionally, the apparent properties of venom proteins can be simply distinguished by chromatography and electrophoresis [41,42,43]. In the current study, interspecific variations could be clearly observed in the chromatographic and the electrophoretic profiles of the venoms from six habu snakes (Figure 2). Specifically, a total amount of 29–38 chromatographic fractions could be resolved from these venoms, among which the P. xiangchengensis and P. jerdonii venoms expressed the least and the most fractions, respectively. The retention time of most fractions ranged from 25 to 125 min using the current separation procedure, and apparently varied among all the venoms. The components of the first 3–5 chromatographic fractions from these venoms could not be visualized in the electrophoretogram, while the remaining fractions could be further separated by electrophoresis and stained with CBB-250. The fractions expressing simple protein bands were mainly located at the retention times before 72 min in P. cornutus, P. jerdonii, P. mangshanensis, and P. murcosquamatus venoms, while those were located at the retention times before 60 and 75 min in P. xiangchengensis and P. sieversorum venoms, respectively. Moreover, the fractions with long retention times expressed more protein bands in the electrophoretogram in all venoms, among which P. jerdonii and P. sieversorum venoms were resolved with relatively more such fractions.
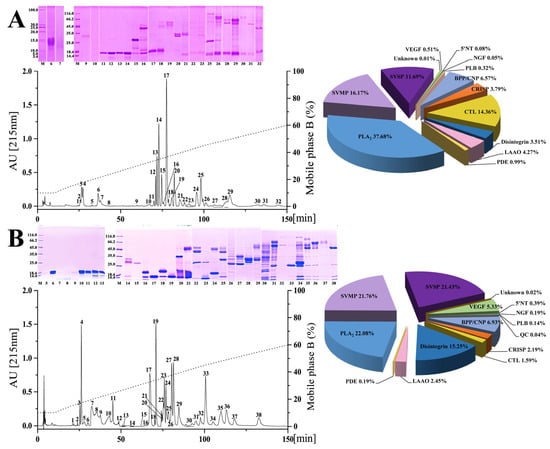
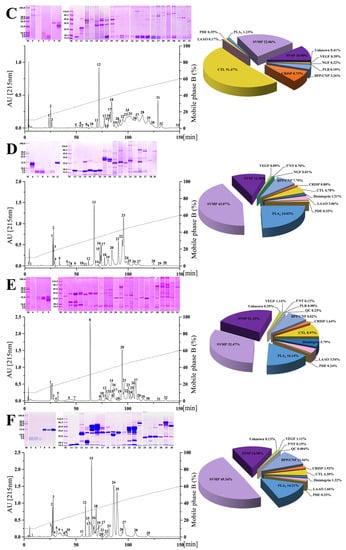
Figure 2.
The venom proteomic profiles of six habu snakes. The venom proteins of P. cornutus (A), P. jerdonii (B), P. mangshanensis (C), P. mucrosquamatus (D), P. sieversorum (E), and P. xiangchengensis (F) were fractionated on a C18 column as described in the Materials and Methods. The RP-HPLC eluted fractions were collected and separated by SDS-PAGE under reducing conditions (on the left of panels). Protein bands from gels were digested with trypsin and identified by MALDI-TOF/TOF-MS and nESI-MS/MS. The details of the sequenced peptides and assigned protein families are listed in Supplementary Tables S1–S6. The pie charts (on the right of panels) display the relative abundance of the toxin families in the relevant venoms of habu snakes. The venom of P. mangshanensis was collected from subadult specimens, and the venom of the other habu snakes were collected from adult specimens. SVMP, snake venom metalloproteinase; SVSP, snake venom serine proteinase; PLA2, phospholipase A2; CRISP, cysteine-rich secretory protein; BPP/CNP, bradykinin-potentiating and C-type natriuretic peptides; CTL, C-type lectin; LAAO, L-amino acid oxidase; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; PDE, phosphodiesterase; 5′NT, 5′-nucleotidase; PLB, phospholipase B; QC, glutaminyl-peptide cyclotransferase. A high-resolution image can be found in Figure S2.
2.2. Proteomic Profiles of Venoms from Six Habu Snakes
Snake venom can potentially serve as a resource library with phylogeny clues, and exploration of its detailed composition by mass spectrometry technology has been highly concerned and advanced over the past ten years [38,44]. Here, based on the chromatographic and electrophoretic profiles, 4–8 chromatographic fractions from each venom were found to express a low abundance of proteins or contain components that cannot be displayed by electrophoresis, and thus were conducted by in-solution tryptic digestion and identified by nESI-MS/MS analysis. The remaining chromatographic fractions with visible protein bands were subjected to in-gel tryptic digestion, and then the peptides were identified by MALDI-TOF-MS/MS or nESI-MS/MS analysis. In general, the lowest and the highest number of protein bands for mass spectrometry analysis were 89 (P. cornutus venom) and 181 (P. jerdonii venom), respectively; more than 150 protein bands were identified in both P. jerdonii and P. sieversorum venoms (Figure 2 and Supplementary Tables S1–S6). Similar to the findings where the venom components could be classified into a few protein families in most venomous snakes [45], the mass spectrometry analysis identified 14 protein families in total in the fractions and protein bands of the venoms from six habu snakes, including SVMP (snake venom metalloproteinase), SVSP (snake venom serine protease), PLA2 (phospholipase A2), CRISP (cysteine-rich secretory protein), disintegrin, BPP/CNP (bradykinin-potentiating peptide/C-type natriuretic peptides), CTL (C-type lectin), LAAO (L-amino acid oxidase), NGF (nerve growth factor), VEGF (vascular endothelial growth factor), PDE (phosphodiesterase), PLB (phospholipase B), 5′NT (5′-nucleotidase), and QC (glutamine peptide cyclotransferase).
Remarkable variations were observed between the venom proteomes of five adult habu snakes in the current study, both in the presence or absence and the relative abundance of venom protein families (pie charts in Figure 2 and Table 1). Specifically, 11 protein families were shared among these habu snake venoms, with SVMP (32.56 ± 13.94% of the total venom proteome), PLA2 (22.93 ± 9.26%), and SVSP (16.27 ± 4.79%) as the three predominate protein families, which were expressed with a total abundance of higher than 65% in all five snake venoms with that in P. mucrosquamatus venom even up to 79.88%. The other common protein families between these habu snake venoms with a relatively low abundance included CRISP which accounted for 0.88–3.79% of the total venom proteome, disintegrin (1.21–15.25%), BPP/CNP (6.57–11.04%), CTL (1.59–14.26%), LAAO (1.66–4.27%), VEGF (0.51–5.33%), PDE (0.19–0.99%), and 5′NT (0.08–0.7%). The remaining three protein families including NGF, PLB, and QC with an average relative abundance of less than 0.15% were inconsistently present in these venoms, and they were only found in no more than three habu snake venoms. Moreover, four of these protein families (5′NT, NGF, PLB, and QC) can be considered trace components due to their very low average relative abundance (<0.3%) in these five snake venoms. These adult habu snakes were estimated to share relatively low similarities between their venom proteomes with an average protein similarity coefficient (PSC) of 17.7% (ranging from 7.6% to 39.2%, Table 2), which was similar to those estimated within the genera Atropoides (14–16%) [43] and Bitis (7–28%) [1], but apparently lower than those within the genus Bothrops (65–70%) [46]. Thus, these results might indicate a high level of interspecific variation in venom proteomes within the genus Protobothrops. Moreover, the criteria applied to determine the same or highly similar toxin proteins in the current study were not as strict as those in previous studies [1,43], and thus the real PSCs of these habu snake venoms may be lower than those in Table 2.
These results are consistent with the previous investigations on the venom proteomes of three other habu snakes, which were also overwhelmingly dominated by SVMP, PLA2, and SVSP (P. elegans 71.4%, P. flavoviridis 87.92%, and P. kelomohy 86.27% of the total abundance; Table 1) [31,33,36]; P. flavoviridis undoubtedly expressed the highest abundance of PLA2 (55.14%) in its venom proteome among all adult habu snakes. However, the relative abundances of these three protein families were highly variable among these venoms, especially the abundance of SVSP in P. kelomohy which was 20.8-fold that of P. flavoviridis. Apparent uniformity in the presence or absence, as well as the relative abundance, of the remaining 11 protein families, could also be observed in these habu snake venoms. As for the venom proteome of P. mucrosquamatus, a moderate level of geographical variation in the relative abundance of each protein family could be found between the samples from mainland China (current study) and China’s Taiwan (venom proteome decomplexed by Villalta and her colleagues, [30]) (Table 1); however, a great divergence in the presence or absence and relative abundance of components could be observed between the venom samples from these two populations, one of which from China’s Taiwan was elucidated by Liu and his colleagues based on genomic data [32]. Such differences could be due to the different sensitivities of the strategies for proteomic identification, as well as the differences in methodologies for relative abundance quantitation and the database for peptide assignment [47].
As an endangered species with an eye-catching appearance, P. mangshanensis was still mysterious due to the rare incidence of snakebites, and once received attention for some key venom components and their functions as well as a two-dimensional electrophoresis profile of its venom proteins, although its global venom proteome has never been decomplexed [48,49,50,51]. PLA2 was one of these studied components and accounted for 58% of the total abundance of adult venom proteins, and is also considered the major component inducing the edema, local inflammation, and muscle necrosis in victims envenomed by P. mangshanensis [50]. Of note, the subadult P. mangshanensis venom in the current study had an extremely low abundance of PLA2 (1.23%) (pie charts in Figure 2 and Table 1); the adult venom contained 47.2-fold more PLA2than the subadult venom of P. mangshanensis, which might be attributed to an ontogenetic shift in snake venom composition. In the global proteome profile of subadult P. mangshanensis venom, CTL was the most abundant component (51.47% of the total venom proteome), followed by SVMP (22.06%) and SVSP (10.90%). Unfortunately, the growth status and whereabouts of these two subadult specimens could not be continuously tracked after contacting the anonymous reptile enthusiast again, and thus we could not conduct global proteome analyses on the adult venom.
Statistical analyses further indicated the degrees of variation in the relative abundance of the 14 protein families in the venoms of eight adult habu snakes (three in previous studies [31,33,36] and five in the current study) ranged from 32.5 to 233.0 (coefficients of variations, CV), and the average CV of three highly abundant protein families (47.8 for SVMP, SVSP, and PLA2) was much lower than that of trace protein families (206.6 for NGF, PLB, 5′NT, and QC). Similar trends were found in Agkistrodon snakes with an average CV of 19.5 estimated for highly abundant protein families (SVMP, SVSP, and PLA2) and 71.3 for low abundance families (CRISP, disintegrin, CTL, LAAO, and NGF) [12], as well as in Sistrurus snakes with CV values of 23.9 and 125.4, respectively [10]. These results could be because the components with a high abundance rather than those with a low (trace) abundance are generally considered the major contributors to the function of the snake venom, and thus it is more important to maintain the stability of their relative abundance than that of the latter [52].

Table 1.
Overview of the relative abundance of protein families in venoms from habu snakes.
Table 1.
Overview of the relative abundance of protein families in venoms from habu snakes.
| Species | % of Total Venom Proteins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P. cornutus | P. jerdonii | P. mangshanensis | P. mucrosquamatus | P. sieversorum | P. xiangchengensis | P. elegans | P. flavoviridis | P. kelomohy | ||
| Location | Mainland China | Mainland China | Mainland China | Mainland China | China’s Taiwan | Vietnam | Mainland China | Japan | Japan | Thailand |
| Reference | Current study | Current study | Current study | Current study | Villalta et al. [30] | Current study | Current study | Aird et al. [33], Yamakawa et al. [53] | Huang et al. [54], Damm et al. [31] | Chanhome et al. [36] |
| LD50 (µg/g) | 0.61 (0.46–0.81) | 1.91 (1.73–2.11) | 1.23 (0.98–1.54) | 3.82 (3.50–4.17) | 2.17 | 1.23 (1.07–1.41) | 1.79 (1.51–2.11) | 4.85 | 2.60 | 0.67 (0.58–0.78) |
| Separation | RP-HPLC SDS-PAGE | RP-HPLC SDS-PAGE | RP-HPLC SDS-PAGE | RP-HPLC SDS-PAGE | RP-HPLC SDS-PAGE | RP-HPLC SDS-PAGE | RP-HPLC SDS-PAGE | - | RP-HPLC SDS-PAGE | SDS-PAGE |
| Digestion | In-gel | In-gel | In-gel | In-gel | In-gel | In-gel | In-gel | In-solution | In-gel | In-gel |
| MS | LC-MS/MS | MALDI-TOF LC-MS/MS | LC-MS/MS | LC-MS/MS | MALDI-TOF LC-MS/MS | LC-MS/MS | MALDI-TOF LC-MS/MS | LC-MS/MS | LC-MS/MS | LC-MS/MS |
| SVMP | 16.17 | 21.76 | 22.06 | 43.07 | 43.40 | 32.47 | 49.34 | 39.40 | 31.34 | 40.85 |
| SVSP | 11.69 | 21.43 | 10.90 | 12.30 | 10.40 | 21.33 | 14.58 | 9.50 | 1.44 | 29.93 |
| PLA2 | 37.68 | 22.10 | 1.23 | 24.51 | 23.50 | 16.14 | 14.21 | 22.50 | 55.14 | 15.49 |
| CRISP | 3.79 | 2.19 | 8.73 | 0.88 | 0.80 | 1.64 | 1.92 | 1.80 | 1.83 | 1.41 |
| Disintegrin | 3.51 | 15.25 | - | 1.21 | 0.80 | 5.79 | 1.32 | - | - | 0.35 |
| BPP/CNP | 6.57 | 6.93 | 3.26 | 7.79 | 3.60 | 8.02 | 11.04 | 0.10 | - | - |
| CTL | 14.36 | 1.59 | 51.47 | 4.78 | 3.90 | 8.97 | 4.20 | 2.90 | 2.78 | - |
| LAAO | 4.27 | 2.45 | 0.17 | 3.06 | 2.00 | 3.54 | 1.66 | 9.80 | 0.71 | 3.87 |
| VEGF | 0.51 | 5.33 | 0.39 | 0.89 | - | 1.14 | 1.11 | 1.80 | - | 0.70 |
| NGF | 0.05 | 0.19 | 0.22 | 0.01 | - | - | - | 0.50 | - | 1.76 |
| PDE | 0.99 | 0.19 | 1.06 | 0.80 | - | 0.24 | 0.33 | 4.10 | 0.07 | - |
| 5′NT | 0.08 | 0.39 | - | 0.70 | - | 0.12 | 0.15 | 2.80 | 0.02 | - |
| PLB | 0.32 | 0.14 | 0.10 | - | - | 0.08 | - | 2.20 | - | - |
| QC | - | 0.04 | - | - | - | 0.23 | 0.004 | 1.40 | - | - |
| Others | - | - | - | - | 3.30 | - | - | 1.00 | 6.39 | 5.63 |
| Unknown | 0.01 | 0.02 | 0.41 | - | - | 0.29 | 0.13 | 0.30 | 0.27 | - |
The venom of P. mangshanensis was collected from subadult specimens. SVMP, snake venom metalloproteinase; SVSP, snake venom serine proteinase; PLA2, phospholipase A2; CRISP, cysteine-rich secretory protein; BPP/CNP, bradykinin-potentiating and C-type natriuretic peptides; CTL, C-type lectin; LAAO, L-amino acid oxidase; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; PDE, phosphodiesterase; 5′NT, 5′-nucleotidase; PLB, phospholipase B; QC, glutaminyl-peptide cyclotransferase. LD50: dose that induces death in 50% of mice, confidence limits (95%) are listed in parentheses.

Table 2.
Protein similarity coefficients (%) of the venoms from five adult habu snakes.
Table 2.
Protein similarity coefficients (%) of the venoms from five adult habu snakes.
| P. cornutus | P. jerdonii | P. mucrosquamatus | P. sieversorum | P. xiangchengensis | |
|---|---|---|---|---|---|
| P. cornutus | - | 14.4 | 15.8 | 18.9 | 9.6 |
| P. jerdonii | - | - | 15.2 | 14.3 | 23.7 |
| P. mucrosquamatus | - | - | - | 39.2 | 18.0 |
| P. sieversorum | - | - | - | - | 7.6 |
| P. xiangchengensis | - | - | - | - | - |
2.3. Toxicological and Enzymatic Activities of Venoms from Six Habu Snakes
As a complex arsenal, snake venom has complicated biochemical functions, which can facilitate the prediction of the clinical manifestations of envenomations, as well as the diagnosis and treatment of snakebites [55]. Given the distinct proteomic profiles of habu snake venoms that were unraveled in the current study, we further determined their toxicological and enzymatic activities to explore the potential variations in biochemical functions among these venoms. The venoms from adult habu snakes in the current study showed relatively high variability in their toxicity to ICR mice, among which the P. cornutus and P. mucrosquamatus venoms, respectively, produced the strongest (LD50 = 0.61 μg/g) and the weakest (LD50 = 3.82 μg/g) toxicities among these venoms, and the former was 6-fold more toxic than the latter (Table 1). These venoms all seemed more toxic than P. elegans venom (LD50 = 4.85 μg/g, [53]) and only the P. cornutus venom showed a strong toxicity similar to that of P. kelomohy venom (LD50 = 0.67 μg/g, [36]). The other habu snake venoms investigated in a previous study [31] and the current study had moderate levels of toxicity. Moreover, ontogenetic shift (LD50 of subadult and adult P. mangshanensis venom was 1.23 and 4.0 μg/g, respectively [48]; the difference in toxicity could be attributed to the difference in CTL relative abundance between these two venoms) and geographical variation (LD50 of mainland China and China’s Taiwan P. mucrosquamatus venom was 3.82 and 2.17 μg/g, respectively [30]) in the toxicity of venoms could also be observed in the habu snakes, which should be considered in the clinical diagnosis and treatment of snakebites caused by them. In regard to the toxicity of venoms from the less clinically important habu snakes, such as P. cornutus, P. kelomohy, P. sieversorum, and P. xiangchengensis, the toxicity was apparently strong among all venoms; therefore, the envenomations caused by them and their clinical treatment should be given more attention.
The myotoxicity caused by viperid venom is mainly attributed to the basic Asp49 and Lys49 PLA2s and their homologues, which can damage the skeletal muscle cell plasma membrane [56,57]. Here, these two PLA2s could be found in both adult and subadult habu snake venoms (Supplementary Tables S1–S6), and thus can apparently cause higher creatine kinase activity (1598.6–2382.4 U/L) in mice compared to the saline-treated mice (612.2 U/L) (all p < 0.05) (Figure 3A). Although the abundance of these two PLA2s varied among adult habu snakes, Student’s t-tests indicated that their myotoxicities were relatively similar to each other (all p ≥ 0.14). Compared to the adult habu snake venoms, the subadult P. mangshanensis venom contained the lowest abundance of PLA2 (1.23%), but the highest apparent value of myotoxicity (2382.4 U/L) (Figure 2 and Figure 3A). Given that the habu snakes are notorious for causing severe muscle necrosis in victims, the gastrocnemius muscle of the ICR mice that were injected with these habu snake venoms was subsequently dissected and observed. Unsurprisingly, these mice suffered from significant swelling and varying degrees of muscle necrosis, which were roughly correlated with the intensity of creatine kinase activity: P. xiangchengensis and P. mangshanensis venoms caused the strongest submuscular symptoms with large reddish brown areas of muscle necrosis in mice, followed by P. cornutus and P. jerdonii venoms, whereas P. mucrosquamatus and P. sieversorum venoms caused relatively mild submuscular symptoms with small reddish brown areas of muscle necrosis in mice (Figure 4). Moreover, the subcutaneous capillaries near the injection site were also observed with varying degrees of bleeding, which could further verify the conclusion that the habu snake venom can induce strong hemorrhagic symptoms in victims [58,59,60]. In total, it could be inferred that the myotoxicity of these venoms is not rigidly correlated with the relative abundance of basic Asp49 and Lys49 PLA2s, but may be correlated with the potential capability of the unique catalytic domains of each PLA2 to damage the skeletal muscle cell plasma membrane. For instance, a basic Asp49 PLA2 (Q90W39) was highly expressed in P. cornutus venom, but the venom caused low myotoxicity according to the description in the UniProt database. In line with a previous study [12], the myotoxicity and enzymatic activity of PLA2s in these venoms were not correlated, as some acidic PLA2s showed a lack of myotoxicity and some myotoxic Lys49 PLA2s may be enzymatically inactive according to the UniProt database (Supplementary Tables S1–S6). Among the adult habu snake venoms, the P. cornutus venom expressed the highest amount of enzymatically active PLA2s (37.68%), followed by P. jerdonii (22.10%), P. xiangchengensis (10.56%), P. mucrosquamatus (8.47%), and P. sieversorum (0.36%) venoms; in addition, these venoms showed a strong dose-dependent relationship between hydrolytic activity towards soybean lecithin and the relative abundance of enzymatically active PLA2s with a strong Spearman’s rank correlation coefficient (ρ = 1.0) as well as a strong Pearson’s correlation coefficient (R = 0.94, F1,3 = 24.39, p = 0.016) (Supplementary Tables S1, S2 and S4–S6, and Figure 3B).
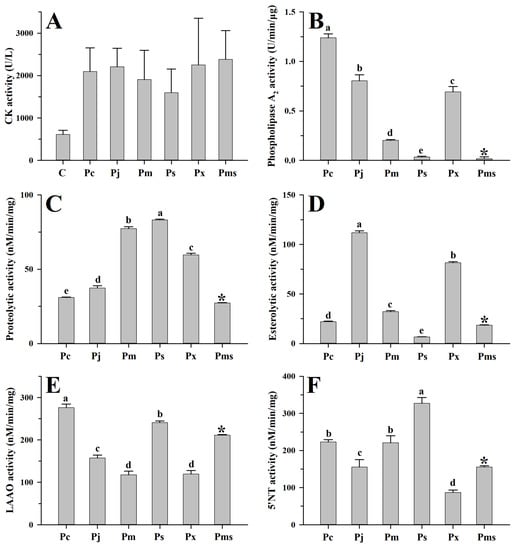
Figure 3.
Myotoxicity and enzymatic activities of venoms from habu snakes. C, saline; Pc, P. cornutus; Pj, P. jerdonii; Pm, P. mucrosquamatus; Ps, P. sieversorum; Px, P. xiangchengensis; Pms, P. mangshanensis. Data are expressed as mean value ± SD ((A): n = 4; (B–F): n = 3). The enzymatic activities of P. mangshanensis venom (indicated with “*” above the column) were excluded from the statistical analysis due to the venom having been collected from subadult specimens. The significance level is set at α = 0.05, a > b > c > d > e. A high-resolution image can be found in Figure S3.
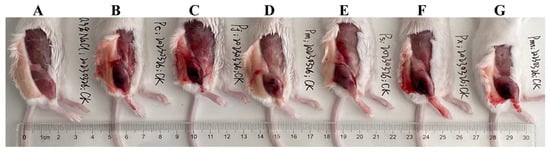
Figure 4.
Local symptoms in the gastrocnemius muscle of the mice after injection of (A) saline; (B) P. cornutus; (C) P. jerdonii; (D) P. mucrosquamatus; (E) P. sieversorum; (F) P. xiangchengensis; (G) P. mangshanensis venoms. High- resolution image can be found in Figure S4.
On the other hand, one-way ANOVA also revealed the interspecific variations in four other enzymatic activities of the venoms from five adult habu snakes (all p < 0.0001; Figure 3C–F). Specifically, the P. sieversorum venom expressed the highest capability (83.2 nM/min/mg) to degrade casein, and was nearly 2.7-fold more active than the P. cornutus venom, which expressed the lowest capability (30.9 nM/min/mg). Although SVMPs and SVSPs are generally deemed to be responsible for the proteolytic activity in viperid venoms [61,62], the relative abundance of these two components was weakly correlated with the capability of these habu snake venoms to degrade casein based on a moderate Spearman’s rank correlation coefficient (ρ = 0.60, p > 0.05). Among these five adult habu snake venoms, the P. cornutus and P. sieversorum venoms expressed the strongest LAAO (276.0 nM/min/mg) and 5′-NT (327.3 nM/min/mg) activities, respectively, and were about 2.3-fold and 3.8-fold more active than the P. mucrosquamatus (117.8 nM/min/mg) and P. xiangchengensis (87.1 nM/min/mg) venoms, which expressed the weakest activity in the corresponding enzymes. Except for the P. sieversorum venom (6.5 nM/min/mg), the four other adult habu snake venoms could apparently hydrolyze the synthetic substrate (BAPNA), with the P. jerdonii venom possessing the highest activity (111.9 nM/min/mg). However, nonparametric Spearman’s rank correlation analysis indicated that the variation in these enzymatic activities was not related to the differences in the relative abundance of the relevant venom components (all p > 0.05). This could be attributed to the potential bias in the estimation of the relative abundance of venom components, especially for those with trace or low abundances (e.g., LAAO and 5′-NT); in addition, it might also be related to the potential differences in the catalytic domains of the components from the same toxin family in these habu snake venoms, and the detailed mechanisms should be further explored.
Taken together, the interspecific differences in toxicological and enzymatic activities would potentially drive the variability between the local and systematic symptoms caused by these habu snake venoms, and therefore highlight the requirement of species-specific therapeutic schedules for the victims. Clinically, two heterologous monovalent antivenoms against Gloydius brevicaudus venom or Deinagkistron actus venom and a bivalent antivenom against venoms including P. mucrosquamatus venom were recommended to treat the victims envenomed by P. mucrosquamatus [58,63], and a species-specific monovalent antivenom against P. flavoviridis venom was available for the treatment of snakebites caused by P. flavoviridis [59], whereas the strict preclinical trials for these treatment are still insufficient [63], especially for those endemic to areas with few human inhabitants and recently classified habu snakes. Accordingly, preclinical evaluation of the immunological cross-reaction between these antivenoms and habu snake venoms should further be implemented using immunoassay methods, such as ELISA, Western blotting, and antivenomic strategy.
2.4. Estimation of the Phylogenetic Signal of the Interspecific Variations in Venom Traits
The proteomic profiles of venoms have been considered important complementary evidence in discriminating between related species with taxonomic controversy and in exploring the adaptive evolution trends in venom compositional patterns along with phylogenetic clues; thus, snake venom contains valuable information on the evolutionary history of venomous snakes [1,11,39,64,65,66]. The evaluation of the phylogeny-based evolutionary changes in venom traits (venom composition and function) of the habu snakes is shown in Table 1 (five species from the current study and four species from previous studies). Eight protein families (venom composition) with relative abundances higher than 1% in at least five of the nine taxa as well as an LD50 (venom function) were used in the analysis. Since the venom of P. mangshanensis was collected from subadult samples, the venom traits of this species were excluded from the analysis. Moreover, the major contribution of the variables that could not be well converged (less than 80%) on two vectors by principal component analysis, thus venom breadth was calculated as an alternatively integrative trait of venom composition for testing the potential phylogeny-related change. For venom composition, Blomberg’s analysis [67] revealed K values of eight venom protein families ranging from 0.324 (disintegrin) to 0.932 (SVSP) with a mean K value of 0.618 ± 0.194 (mean ± SD), and that of venom breadth was 0.472 (Table 3). The statistical result of 1000 times calculation on the trait values randomly assigned to the tree tips further indicated that, except for SVSP (p = 0.026), the other venom composition traits were not associated with phylogeny (all p ≥ 0.153, Table 3). Similar to the traits of venom composition, a moderate K value of 0.621 with a p-value of 0.311 for LD50 indicated no evidence for an association with phylogeny (Table 3).

Table 3.
Estimation of phylogenetic signal (K-value) for the variation in venom traits of the habu snakes excluding P. mangshanensis as shown in Table 1.
Overall, the resemblance of the relatives within the genus Protobothrops in most venom traits deviated from Brownian motion evolution, which has been similarly observed in the genera Agkistrodon and Sistrurus [10,12]. It has been proposed that snake venom is a relatively labile evolutionary trait, and a small number of taxa and different adaptation patterns to environmental factors might play important roles in the random evolution of venom traits [12]. Another possibility is that the similarity in venom traits might be more a reflection of similar selection pressures driven by high similarities in diets, rather than the phylogenetic similarity of the snakes [12]. It is undeniable that variable habitats can probably drive the habu snakes to develop different adaption patterns to the same environmental factor, especially for those with a narrow niche space, such as P. cornutus, P. xiangchengensis, and P. sieversorum. On the other hand, detailed information on diets of these habu snakes has not yet been reported, but it has been empirically recorded that some of them (including P. cornutus, P. jerdonii, P. mucrosquamatus, and P. xiangchengensis) mainly feed on birds and rodents [68]; thus, the habu snakes might possess a relatively narrow but highly similar diet niche, which would imply high similar selection pressures on the venoms of the habu snakes. Therefore, precise verification using quantifiable dietary data of these habu snakes is required in the future. Unlike the genera Agkistrodon and Sistrurus, at least one toxin family (SVSP) in venoms of the genus Protobothrops was found to be associated with phylogeny in the current study. A recent investigation on Bothrops atrox venoms claimed that the lethality patterns of snake venom can be variable depending on the prey [69]; thus, different conclusions on phylogeny-related venom functions might be obtained if the lethality of the habu snake venoms were evaluated in birds, which should be conducted in future research. In brief, the habu snake venoms might have evolved under a combination of adaptive and neutral mechanisms.
Moreover, based on a protein-by-protein comparison, the apparent mean K value of these habu snakes was larger than those of the genera Agkistrodon (0.50 ± 0.28) [12] and Sistrurus (0.11 ± 0.06) [10], which means that the intensity of the phylogenetic signal in venom composition variation across habu snakes would be much higher than that across the two other genera. Nonparametric comparisons (Wilcoxon signed rank test) further indicated that the genera Protobothrops and Agkistrodon showed no difference in phylogenetic signals (p = 0.68), despite both genera expressing higher phylogenetic signals than the genus Sistrurus (all p < 0.05). A reasonable explanation is that the degree of covariation between phylogeny and venom variation is evolutionarily labile and varies among different clades of closely-related venomous snakes [12].
3. Conclusions
We employed a combined proteomic strategy to decomplex the venom composition of pit vipers from a subset of Old World habu snakes (Protobothrops) and revealed remarkable variations in the venom proteomes of these habu snakes, both in the presence or absence and the relative abundance of venom protein families. Specifically, 14 protein families were identified in all chromatographic fractions and gel bands of these habu snake venoms, and 11 of them were shared among these venoms. Moreover, the venoms from adult habu snakes were overwhelmingly dominated by SVMP (32.56 ± 13.94%), PLA2 (22.93 ± 9.26%), and SVSP (16.27 ± 4.79%), with a total abundance of higher than 65% in the venom proteomes. These adult habu snakes were estimated to share relatively low similarities between their venom proteomes, with an average protein similarity coefficient (PSC) of 17.7% (which ranged from 7.6% to 39.2%). Compared with these adult snakes, the subadult P. mangshanensis venom contained an extremely low abundance of PLA2 (1.23%), but a high abundance of CTL (51.47%), followed by SVMP (22.06%) and SVSP (10.90%). An analysis integrated with the proteomic profiles of the habu snake venoms in the current and previous studies further indicated that the highly abundant protein families had much lower degrees of variation in the relative abundance than the trace families. Interspecific variation in lethality and enzymatic activities was also explored in the habu snake venoms in the current study, and it was found to potentially drive the variability in the local and systematic symptoms of snakebites and highlighted the requirement of species-specific therapeutic schedules for victims. Apparent variations in muscle necrosis were observed in the mice injected with these habu snake venoms, but there were no variations in the degree of myotoxicity. With regard to the venoms from the less clinically important habu snakes, such as P. cornutus, P. kelomohy, P. sieversorum and P. xiangchengensis, their toxicities were relatively strong among all the venoms; therefore, the envenomations by these snakes, as well as their clinical treatment, should be given more attention. Phylogeny-based comparisons of the variations in venom traits revealed that, except for SVSP, the resemblance of the relatives within the genus Protobothrops in venom traits deviated from Brownian motion evolution. Given the potential similarity of the selection pressures due to diet on the venoms of the habu snakes, it was inferred that these venoms might have evolved under a combination of adaptive and neutral mechanisms. The mean K value of the venom traits from this subset of habu snakes was larger than those of the genera Agkistrodon and Sistrurus, which can further validate the assumption that the degree of covariation between phylogeny and venom variation is evolutionarily labile and varies among different clades of closely related venomous snakes.
4. Materials and Methods
4.1. Animals, Snake Venom Extraction, and Ethics
Six adult P. jerdonii (snout-vent length (SVL), 52.9–77.8 cm) and nine adult P. mucrosquamatus (SVL, 61.2–78.2 cm) were collected from fields in Zhejiang and Guangxi, China, respectively, and then transferred to our laboratory and maintained in Reptile Pet Terrariums (60 × 45 × 45 cm, Reptizoo, Dongguan, China). Two adult specimens of each species of P. cornutus (SVL, 51.2–52.5 cm), P. sieversorum (SVL, 65.8–67.5 cm), and P. xiangchengensis (SVL, 61.8–62.5 cm), as well as two subadult specimens of P. mangshanensis (19-month-old; considering the potential injuries to the animals, the provider did not allow the measurement of the morphological characteristics of these specimens), were supplied by two anonymous reptile enthusiasts, and return to them after venom extraction. Fresh venom of each snake was extracted by biting on a parafilm-wrapped jar, centrifuged at 10,000× g 4 °C for 15 min to remove the impurities, then lyophilized, weighed, pooled (in equal amounts by species), and stored at −80 °C. The handling of all snakes collected for venom extraction and the ICR mice from the Laboratory Animal Center of Hangzhou Normal University for evaluating the toxicological activity of venoms and experimental procedures were supervised and approved by the Animal Research Ethics Committee of Hangzhou Normal University (AREC20140311).
4.2. Proteomic Analyses
4.2.1. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
A total of 5 mg crude venom powder was reconstituted in 0.1% TFA and centrifuged at 15,000× g at 4 °C for 10 min; the supernatant was then subjected to a Kromasil 300 RP-C18 column (250 × 4.6 mm, 5 μm; AkzoNobel) using a Waters E600 HPLC system (Waters, Milford, MA, USA). The venom proteins were separated at 1 mL/min using a linear gradient of the mobile phase A (0.1% TFA) and B (100% ACN) according to Gao et al. [62]: isocratically 10% B for 10 min, 10–15% B over 10 min, 15–45% B over 80 min, and 45–60% B over 50 min. The separation process was monitored at 215 nm. The eluted fractions were collected manually and concentrated in a CentriVap® Centrifugal Concentrator (Labconco, Kansas, MO, USA).
4.2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The lyophilized fractions were re-dissolved in ddH2O, and the concentration of protein in each fraction was quantified according to Bradford [70]. Then, the proteins of each fraction were mixed with loading buffer, loaded into the wells, and performed with 12% and 18% SDS-PAGE under reducing conditions. After the electrophoresis, the gels were stained with 0.1% CBB R-250, and the protein bands were scanned using a Umax2100 densitometer system (Novax Technologies, Taipei, China).
4.2.3. In-Gel and In-Solution Tryptic Digestion
The protein bands in the gels were excised and chopped into small pieces, sequentially incubated with 0.1 mM NH4HCO3 in 30% ACN and 0.1 mM NH4HCO3 at room temperature. The proteins in the gel pieces were reduced by incubation with 50 mM DTT in 0.1 M NH4HCO3, alkylated with 55 mM IAA in 0.1 M NH4HCO3, and then in-gel digested with trypsin (MS grade, Promega, Madison, WI, USA) for 20 h at 37 °C. Several chromatographic fractions with a low relative abundance were unsuitable for electrophoresis, and thus were re-dissolved, reduced, alkylated, and in-solution tryptic digested according to the procedure mentioned above. The peptide mixture in the supernatant was collected and lyophilized.
4.2.4. Mass Spectrometry (MS) Identification
For MALDI-TOF-MS/MS analysis, a 2 μL peptide mixture re-dissolved in 20% ACN was loaded onto a stainless steel plate and air-dried, then covered with 0.5 μL of 5 mg/mL α-cyano-4-hydroxycinnamic acid (ABI) in 0.1% TFA and 50% ACN and air-dried again. Subsequently, the peptide mixture was subjected to a 4800 Plus MALDI-TOF/TOF-MS mass spectrometer (AB SCIEX, Framingham, MA, USA) according to the instruction manual. For LC-MS/MS analysis, the peptide mixture was re-dissolved in 0.1% TFA, automatically subjected to an Acclaim™ PepMap™ 100 C18 column (Trap Cartridge; 5 × 0.3 mm, 5 μm; ThermoFisher, Waltham, MA, USA) using the Ultimate 3000 RSLCnano system (ThermoFisher, Waltham, MA, USA). Then, the mixture was separated using an Acclaim™ PepMap™ RSLC 100 C18 column (NanoViper; 75 μm × 15 cm, 2 μm; ThermoFisher, Waltham, MA, USA) with the mobile phase A (0.1% FA) and B (20% ACN in 0.1% FA) under a linear gradient according to Zheng et al. [39]: 4% B over 3 min, 4–50% B over 47 min, 50–99% B over 4 min, and 99% B over 6 min. The eluents were sequentially loaded onto a Q Exactive Orbitrap platform (ThermoFisher, Waltham, MA, USA) according to the operation manual. Moreover, the chromatographic fractions with a short retention time could not be effectively visualized in the SDS-PAGE, and thus they were re-dissolved and directly subjected to LC-MS/MS analysis.
The raw MS/MS spectra were interpreted using FlexAnalysis 3.4 (Bruker Daltonics, Brerica, MA, USA) or Xcalibur 2.2 (ThermoFisher, Waltham, MA, USA), and the assignment of sequence similarity was executed using PEAKS X (Bioinformatics Solutions Inc., Waterloo, ON, Canada) against the UniProt Serpentes database or an in-house database composed of toxin transcripts from P. jerdonii and P. mucrosquamatus venom-gland transcriptomes. The fragment mass tolerances were separately set at 0.4 Da and 0.1 Da for MALDI-TOF/TOF-MS and LC-MS/MS, carbamidomethyl (C) and oxidation (M) were set as fixed and variable modifications, respectively.
4.2.5. Protein Relative Abundance and Similarity Estimation
The relative abundance of each protein was evaluated according to our previous study [39]. Briefly, the relative abundance of each fraction was calculated using the integration of the chromatographic peaks in the RP-HPLC spectra by Empower software (Waters, Milford, MA, USA) and Origin 9.0 (OriginLab Corporation, Northampton, MA, USA), and that of each protein band was estimated using densitometry measurements of the SDS-PAGE gel by Gel-Pro Analyzer 4.0 software (Media Cybernetics, Rockville, MD, USA). If the fraction contains only one protein band in the SDS-PAGE gel, the proportion of the relevant chromatographic peak in the whole venom protein was directly recorded for the relative abundance; if the fraction contains more than one protein band in the SDS-PAGE gel, the proportion of each protein band was calculated by densitometry, and the chromatographic peak was proportionally assigned to each band for their relative abundance (relative abundance of each protein band in the fraction = (integration of the relevant chromatographic peak/accumulated integration of all chromatographic peaks × 100) × (optical density of each protein band/accumulated optical density of all protein bands in the relevant lane × 100)). Finally, the relative abundance of the bands belonging to the same protein family was accumulated to describe the proteomic profile of the venom. To estimate the similarity of venom proteins between each two (“a” and “b”) adult habu snakes, a protein similarity coefficient (PSC) was employed and calculated according to a previous study [1]: PSCab = (2 × (number of proteins shared between a and b)/(total number of distinct proteins in a + total number of distinct proteins in b)) × 100.
4.3. Toxicological and Enzymatic Activities of Venoms
4.3.1. Lethality
For determination of the median lethal dose (LD50), groups of four male ICR mice (22–26 g) received injections of various doses of venom dissolved in 100 μL physiological saline by the intraperitoneal route as previously described [62], and the control group only received an injection of the same volume of physiological saline. The number of deaths occurring during a period of 24 h were recorded, and the LD50 (μg/g) was estimated using the Spearman–Karber method.
4.3.2. Myotoxicity
For determination of the myotoxicity, each venom (10 μg in 20 μL physiological saline) was injected into the gastrocnemius muscle of the right hind limb in a group of four male ICR mice (22–26 g), with the controls only received an identical injection of physiological saline as previously described [12]. After 3 h, blood was collected from each mouse into a heparinized plastic tube, centrifugated at 3000× g at 4 °C for 10 min. The creatine kinase (CK) activity of the plasma was then determined using the CK Determination Kit (A032-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instruction manual, and expressed as U/L.
4.3.3. Phospholipase A2 Activity
The phospholipase A2 activity was evaluated according to Zheng et al. [39] with some modification. Specifically, each venom (0.8 μg in 1 μL 20 mM PBS) was quickly added into the substrate system (0.1 M NaCl, 10 mM CaCl2, 7 mM Triton X-100, 0.35% soybean lecithin, and 98.8 mM phenol red, pH 8.0). The absorbance was recorded at 558 nm at 28 °C for a 2.5 min interval. A change in absorbance of 0.3 OD value/min/μg venom was used to define one unit of phospholipase A2 activity.
4.3.4. Proteolytic Activity
The proteolytic activity of each venom was determined according to Gao et al. [62] and He et al. [71]. Briefly, a substrate system (0.5 mL 2% casein in 0.2 M Tris–HCl, pH 8.5) was incubated with the venom (40 μg in 100 μL 20 mM PBS) at 37 °C for 2 h. The reaction was stopped by incubation with 0.6 mL 0.44 M TCA at 37 °C for 30 min; the mixture was then centrifuged at 12,000× g at 4 °C for 15 min. Aliquots (0.8 mL) of the supernatant were sequentially mixed with 2.0 mL 0.4 M Na2CO3 and 0.4 mL folin reagent (1:2 dilution), and the absorbance was recorded at 660 nm. l-Tyrosine was used as the standard, and the proteolytic activity was defined as nmol of l-Tyrosine released/min/mg venom.
4.3.5. Esterolytic Activity
The esterolytic activity was tested according to the method from Tu et al. [72] with a slight modification. The substrate system (180 μL 1 mM BAPNA in 0.1 M Tris-HCl, pH 8.0) in the wells of a 96-well micro-plate was incubated with each venom (8 μg in 2 μL 20 mM PBS) at 37 °C for 30 min. Then, the reaction was stopped by adding 18 μL 30% acetic acid, and the absorbance of each well in the micro-plate was read at 405 nm. p-nitroaniline was used as the standard, and the esterolytic activity was defined as nmol of p-nitroaniline released/min/mg venom.
4.3.6. l-Amino Acid Oxidase Activity
The l-amino acid oxidase activity was determined following the method of Toyama et al. [73]. The substrate solution (90 μL 50 mM Tris-HCl, pH 8.0, containing 0.25 mM l-Leucine, 2 mM o-phenylenediamine, and 0.81 U/mL horseradish peroxidase) in the wells of a 96-well micro-plate was incubated with the venom (0.8 μg in 1 μL 20 mM PBS) at 37 °C for 1 h. Then, the reaction was stopped by adding 50 μL 2 M H2SO4, and the absorbance was read at 490 nm. H2O2 was used as the standard, and the activity was expressed as nmol of H2O2 degraded/min/mg venom.
4.3.7. 5′-Nucleotidase Activity
The 5′-nucleotidase activity was assayed following the method of Dhananjaya et al. [74]. The substrate system (90 μL 50 mM Tris–HCl, pH 7.4, containing 10 mM MgCl2, 50 mM NaCl, 10 mM KCl, and 10 mM 5′ AMP) was incubated with each venom (0.8 μg in 10 μL 20 mM PBS) at 37 °C for 30 min. Then, the reaction was stopped by adding 100 μL 0.42% ammonium molybdate in 1 M sulfuric acid and 10% ascorbic acid, and the absorbance was read at 660 nm. KH2PO4 was as the standard, and the activity was defined as nmol of inorganic phosphate released/min/mg venom.
4.3.8. Statistical Analyses
The LD50 with a 95% confidence interval was performed using the Trimmed Spearman–Karber 1.5 program. Student’s t-test, one-way ANOVA, and Tukey’s test were used to analyze the myotoxicity and enzymatic activities based on Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA); nonparametric Spearman’s rank correlation analysis and linear correlation analysis were further employed to assess the correlation between the enzymatic activities and the relative abundance of the relevant venom components. Descriptive statistics were defined as mean ± standard deviation (SD), and the significance level was set at α = 0.05.
4.4. Phylogeny-Based Comparative Analyses
The phylogeny-based comparative exploration of variations in venom traits of habu snakes in Table 1 was carried out following the methods advanced by Gibbs et al. [10] and Lomonte et al. [12]. For estimation of the phylogenetic relationships between these habu snakes, Azemiops feae and Gloydius brevicaudus were selected as outgroups; the venom proteomes of these two species were decomplexed in our previous studies [39,62,75] and can be used in the current study. Three mtDNA genes including 12S RNA, 16S RNA, and cytochrome b genes were used for constructing a Bayesian inference tree. These mtDNA genes were mainly screened from a recent study [76] and the NCBI database and were assigned individual samples based on the location from which the snake was collected; the accession numbers were listed in Table S7. Briefly, the sequences were aligned and merged using Geneious 4.8.3 (Biomatters Ltd., Auckland, New Zealand), and the best-fitting model was screened using PartitionFinder 1.1.1 [77] based on the BIC and greedy search. The phylogeny was then constructed using BEAST 2.2 [78], and the maximum clade credibility tree was obtained using TreeAnnotator 2.2. Then, the Bayesian phylogeny in Newick format was deposited in Data File S1. Venom traits for each taxon were represented by the relative abundance of eight protein families, the venom complexity, and the LD50. The venom complexity, defined as venom breadth, was calculated using the standardized Levin index [7,79].
The multiPhylosignal subroutine in the R-package Picante [80] was performed to test the phylogenetic signal of variation in the venom traits of the habu snakes. For each venom trait, Blomberg’s K analysis [67] based on the multiPhylosignal program generated a K value, which can vary from 0 (under random evolution, trait variation is independent of phylogeny) to 1 (under Brownian evolution, trait variation is correlated with the amount of phylogenetic divergence) to greater than 1 (trait is very conserved in evolution). Whether the observed K was greater than random expectation was analyzed by a statistical test of 1000 times random calculation based on the trait values randomly assigned to the tree tips.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxins15050350/s1. Table S1: Assignment of the reverse-phase chromatographic fractions from Protobothrops cornutus venom to protein families by nESI-MS/MS of selected peptide ions from in-gel digested protein bands separated by SDS-PAGE; Table S2: Assignment of the reverse-phase chromatographic fractions from Protobothrops jerdonii venom to protein families by MALDI-TOF-MS/MS and nESI-MS/MS of selected peptide ions from in-gel digested protein bands separated by SDS-PAGE; Table S3: Assignment of the reverse-phase chromatographic fractions from Protobothrops mangshanensis venom to protein families by nESI-MS/MS of selected peptide ions from in-gel digested protein bands separated by SDS-PAGE; Table S4: Assignment of the reverse-phase chromatographic fractions from Protobothrops mucrosquamatus venom to protein families by nESI-MS/MS of selected peptide ions from in-gel digested protein bands separated by SDS-PAGE; Table S5: Assignment of the reverse-phase chromatographic fractions from Protobothrops sieversorum venom to protein families by nESI-MS/MS of selected peptide ions from in-gel digested protein bands separated by SDS-PAGE; Table S6: Assignment of the reverse-phase chromatographic fractions from Protobothrops xiangchengensis venom to protein families by MALDI-TOF-MS/MS and nESI-MS/MS of selected peptide ions from in-gel digested protein bands separated by SDS-PAGE; SVMP, snake venom metalloproteinase; SVSP, snake venom serine proteinase; PLA2, phospholipase A2; CRISP, cysteine-rich secretory protein; BPP/CNP, bradykinin-potentiating and C-type natriuretic peptides; CTL, C-type lectin; LAAO, L-amino acid oxidase; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; PDE, phosphodiesterase; 5′NT, 5′-nucleotidase; PLB, phospholipase B; QC, glutaminyl-peptide cyclotransferase. Unknown: unidentified components; Table S7: GenBank accession numbers of the sequences for mitochondrial gene markers from snakes for phylogenetic relationship reconstruction; Data File S1: Bayesian phylogeny in Newick format for the snakes in Table S7; Figures S1–S4: High-resolution images for Figure 1, Figure 2, Figure 3 and Figure 4 in the text.
Author Contributions
Conceptualization, J.-F.G.; funding acquisition, J.-F.G. and H.-Y.Z.; investigation, H.-Y.Z., N.H., Y.S., Y.-C.W., H.-B.Z., H.-H.C., Y.-Q.Z. and J.-F.G.; resources, J.-F.G.; writing—original draft, J.-F.G., H.-Y.Z., N.H. and Y.S.; writing—review and editing, J.-F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of China (31770428, 31471995) and the Scientific Research Fund of Zhejiang Provincial Education Department (Y202250059).
Institutional Review Board Statement
The collection of all snakes, utilization of the ICR mice, and the experimental procedures were supervised and approved by the Animal Research Ethics Committee of Hangzhou Normal University (AREC20140311).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Lin Wen, Yu-Feng Miao, Shan-Shan Shen, Yin Yin, Jun-Tao Kang, and Xiang-Yu Li for technical assistance and animal collection, and we also thank the two anonymous reptile enthusiasts for providing the animals.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calvete, J.J.; Escolano, J.; Sanz, L. Snake venomics of Bitis Species reveals large intragenus venom toxin composition variation: Application to taxonomy of congeneric taxa. J. Proteom. Res. 2007, 6, 2732–2745. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Daltry, J.C.; Wuster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wüster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. B 2009, 276, 2443–2449. [Google Scholar] [CrossRef]
- Healy, K.; Carbone, C.; Jackson, A.L. Snake venom potency and yield are associated with prey-evolution, predator metabolism and habitat structure. Ecol. Lett. 2019, 22, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef]
- Siqueira-Silva, T.; de Lima, L.A.G.; Chaves-Silveira, J.; Amado, T.F.; Naipauer, J.; Riul, P.; Martinez, P.A.; Sheard, C. Ecological and biogeographic processes drive the proteome evolution of snake venom. Glob. Ecol. Biogeogr. 2021, 30, 1978–1989. [Google Scholar] [CrossRef]
- Sasa, M. Diet and snake venom evolution: Can local selection alone explain intraspecific venom variation? Toxicon 1999, 37, 249–252. [Google Scholar]
- Mebs, D. Toxicity in animals. Trends in evolution? Toxicon 2001, 39, 87–96. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Sanz, L.; Sovic, M.G.; Calvete, J.J. Phylogeny-based comparative analysis of venom proteome variation in a clade of rattlesnakes (Sistrurus sp.). PLoS ONE 2013, 8, e67220. [Google Scholar] [CrossRef]
- Lomonte, B.; Rey-Suárez, P.; Fernández, J.; Sasa, M.; Pla, D.; Vargas, N.; Bénard-Valle, M.; Sanz, L.; Corrêa-Netto, C.; Núñez, V.; et al. Venoms of Micrurus coral snakes: Evolutionary trends in compositional patterns emerging from proteomic analyses. Toxicon 2016, 122, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Tsai, W.C.; Ureña-Diaz, J.M.; Sanz, L.; Mora-Obando, D.; Sánchez, E.E.; Fry, B.G.; Gutiérrez, J.M.; Gibbs, H.L.; Sovic, M.G.; et al. Venomics of New World pit vipers: Genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteom. 2014, 96, 103–116. [Google Scholar] [CrossRef]
- Aird, S.D.; Arora, J.; Barua, A.; Qiu, L.; Terada, K.; Mikheyev, A.S. Population genomic analysis of a pitviper reveals microevolutionary forces underlying venom chemistry. Genome Biol. Evol. 2017, 9, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.-F. Evolution of the Mitochondrial Genome Structure in Snake, with the Biogeography Analysis of Protobothrops. Ph.D. Thesis, Anhui Uniersity, Hefei, China, 2018. [Google Scholar]
- Gumprecht, A.; Tillack, F.; Orlov, N.L.; Captain, A.; Ryabov, S. Asian Pitvipers; GeitjeBooks: Berlin, Germany, 2004. [Google Scholar]
- Guo, P.; Malhotra, A.; Li, P.-P.; Pook, C.E.; Creer, S. New evidence on the phylogenetic position of the poorly known Asian pitviper Protobothrops kaulbacki (Serpentes: Viperidae: Crotalinae) with a redescription of the species and a revision of the genus Protobothrops. Herpetol. J. 2007, 17, 237–246. [Google Scholar]
- Orlov, N.; Ryabov, S.A.; Nguyen, T.T. Two new species of genera Protobothrops Hoge et Romano-Hoge, 1983 and Viridovipera Malhotra et Thorpe, 2004 (Ophidia: Viperidae: Crotalinae) from karst region in Northeastern Vietnam. Part I. Description of a new species of Protobothrops. Russ. J. Herpetol. 2009, 16, 69–82. [Google Scholar]
- Yang, J.-H.; Orlov, N.; Wang, Y.-Y. A new species of pitviper of the genus Protobothrops (Squamata: Viperidae) from China. Zootaxa 2011, 2936, 59–68. [Google Scholar] [CrossRef]
- Huang, X.; Pan, T.; Han, D.; Zhang, L.; Hou, Y.; Yu, L.; Zheng, H.; Zhang, B. A new species of the genus Protobothrops (Squamata: Viperidae: Crotalinae) from the Dabie Mountains, Anhui, China. Asian Herpetol. Res. 2012, 3, 213–218. [Google Scholar]
- Sumontha, M.; Vasaruchapong, T.; Chomngam, N.; Suntrarachun, S.; Pawangkhanant, P.; Sompan, W.; Smits, T.; Kunya, K.; Chanhome, L. Protobothrops kelomohy sp. nov. (Squamata Viperidae), the second known species of lance-headed pit viper from Thailand. Trop. Nat. Hist. 2020, 20, 43–59. [Google Scholar]
- Guo, P.; Liu, Q.; Wen, T.; Xiao, R.; Fang, M.; Zhong, G.-H.; Truong, N.Q.; Zhu, F.; Jadin, R.C.; Li, G. Multilocus phylogeny of the Asian Lance-headed pitvipers (Squamata, Viperidae, Protobothrops). Zootaxa 2016, 4093, 382–390. [Google Scholar] [CrossRef]
- Longbottom, J.; Shearer, F.M.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.J.; Ray, S.E.; Ray, N.; Warrell, D.A.; Ruiz de Castañeda, R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the production, control and regulation of snake antivenom immunoglobulins. In WHO Expert Committee on Biological Standardization Sixty-Seventh Report Annex 5 (WHO Technical Report Series, No 964); World Health Organization of the United Nations: Geneva, Switzerland, 2018. [Google Scholar]
- Yasunaga, H.; Horiguchi, H.; Kuwabara, K.; Hashimoto, H.; Matsuda, S. Short report: Venomous snake bites in Japan. Am. J. Trop. Med. Hyg. 2011, 84, 135–136. [Google Scholar] [CrossRef]
- Qin, G.-P. China Poisonous Snake Research; Guangxi Science and Technology Press: Nanning, China, 1998. [Google Scholar]
- Hifumi, T.; Sakai, A.; Kondo, Y.; Yamamoto, A.; Morine, N.; Ato, M.; Shibayama, K.; Umezawa, K.; Kiriu, N.; Kato, H.; et al. Venomous snake bites: Clinical diagnosis and treatment. J. Intensive Care 2015, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-R.; Pan, J.-Q.; Hu, X.-J.; Zhang, S.-N.; Shen, S.-X. Epidemiological investigation of the poisonous snake bite around Nanchang area in recent two years. J. Snake 2020, 32, 19–22. [Google Scholar]
- Yang, K.; He, Y.-J.; Yang, S.-Q. Epidemiological investigation of 1167 cases of snake bite. J. Traum. Surg. 2021, 23, 539–541. [Google Scholar]
- Tomari, T.; Wakisaka, I.; Yanagihashi, T. Comparison of the epidemiological features of habu bites among Amamioshima, Tokunoshima and Okinawa. Jpn. J. Health Hum. Ecol. 1987, 53, 87–96. [Google Scholar] [CrossRef]
- Villalta, M.; Pla, D.; Yang, S.L.; Sanz, L.; Segura, A.; Vargas, M.; Chen, P.Y.; Herrera, M.; Estrada, R.; Cheng, Y.F.; et al. Snake venomics and antivenomics of Protobothrops mucrosquamatus and Viridovipera stejnegeri from Taiwan: Keys to understand the variable immune response in horses. J. Proteom. 2012, 75, 5628–5645. [Google Scholar] [CrossRef]
- Damm, M.; Hempel, B.F.; Nalbantsoy, A.; Süssmuth, R.D. Comprehensive snake venomics of the Okinawa habu pit viper, Protobothrops flavoviridis, by complementary mass spectrometry-guided approaches. Molecules 2018, 23, 1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Wu, C.-J.; Hsiao, Y.-C.; Yang, Y.-H.; Liu, K.-L.; Huang, G.-J.; Hsieh, C.-H.; Chen, C.-K.; Liaw, G.-W. Snake venom proteome of Protobothrops mucrosquamatus in Taiwan: Delaying venom-induced lethality in a rodent model by inhibition of phospholipase A2 activity with varespladib. J. Proteom. 2021, 234, 104084. [Google Scholar] [CrossRef]
- Aird, S.D.; Aggarwal, S.; Villar-Briones, A.; Tin, M.M.-Y.; Terada, K.; Mikheyev, A.S. Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly. BMC Genom. 2015, 16, 647. [Google Scholar] [CrossRef]
- Aird, S.D.; Watanabe, Y.; Villar-Briones, A.; Roy, M.C.; Terada, K.; Mikheyev, A.S. Quantitative high-throughput profiling of snake venom gland transcriptomes and proteomes (Ovophis okinavensis and Protobothrops flavoviridis). BMC Genom. 2013, 14, 790. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Lin, C.-C.; Hsiao, Y.-C.; Wang, P.-J.; Yu, J.-S. Proteomic characterization of six Taiwanese snake venoms: Identification of species-specific proteins and development of a SISCAPA-MRM assay for cobra venom factors. J. Proteom. 2018, 187, 59–68. [Google Scholar] [CrossRef]
- Chanhome, L.; Khow, O.; Reamtong, O.; Vasaruchapong, T.; Laoungbua, P.; Tawan, T.; Suntrarachun, S.; Sitprija, S.; Kumkate, S.; Chaiyabutr, N. Biochemical and proteomic analyses of venom from a new pit viper, Protobothrops kelomohy. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210080. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Wu, Z.; Chen, P.; Xiang, Y.; Nie, D.; Yi, J.; Yi, J.; Liu, Z. Electrophysiological and proteomic studies of Protobothrops mangshensis venom revealed its high bioactivities and toxicities. J. Integr. OMICS 2012, 2, 132–139. [Google Scholar]
- Calvete, J.J.; Lomonte, B.; Saviola, A.J.; Bonilla, F.; Sasa, M.; Williams, D.J.; Undheim, E.A.B.; Sunagar, K.; Jackson, T.N.W. Mutual enlightenment: A toolbox of concepts and methods for integrating evolutionary and clinical toxinology via snake venomics and the contextual stance. Toxicon X 2021, 9–10, 100070. [Google Scholar] [CrossRef]
- Zheng, S.-R.; Sun, Y.; Zhao, H.-Y.; Wen, L.; Ji, X.; Gao, J.-F. Differences between two groups of Burmese vipers (Viperidae: Azemiops) in the proteomic profiles, immunoreactivity and biochemical functions of their venoms. Toxins 2022, 14, 572. [Google Scholar] [CrossRef]
- Zhao, E.-M. Snakes of China; Anhui Science and Technology Publishing House: Hefei, China, 2006. [Google Scholar]
- Fry, B.G.; Wickramaratna, J.C.; Hodgson, W.C.; Alewood, P.F.; Kini, R.M.; Ho, H.; Wüster, W. Electrospray liquid chromatography/mass spectrometry fingerprinting of Acanthophis (death adder) venoms: Taxonomic and toxinological implications. Rapid Commun. Mass Spectrom. 2002, 16, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-F.; Qu, Y.-F.; Zhang, X.-Q.; Ji, X. Within-clutch variation in venoms from hatchlings of Deinagkistrodon acutus (Viperidae). Toxicon 2011, 57, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Angulo, Y.; Escolano, J.; Lomonte, B.; Gutiérrez, J.M.; Sanz, L.; Calvete, J.J. Snake venomics of central American pitvipers: Clues for rationalizing the distinct envenomation profiles of Atropoides nummifer and Atropoides picadoi. J. Proteom. Res. 2008, 7, 708–719. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern venomics-Current insights, novel methods, and future perspectives in biological and applied animal venom research. GigaScience 2022, 11, giac048. [Google Scholar]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Calvete, J.J.; Borges, A.; Segura, Á.; Flores-Díaz, M.; Alape-Girón, A.; Gutiérrez, J.M.; Diez, N.; De Sousa, L.; Kiriakos, D.; Sánchez, E.; et al. Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snakebite management. J. Proteom. 2009, 72, 227–240. [Google Scholar] [CrossRef]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating toxin diversity and abundance in snake venom proteomes. Front. Pharmacol. 2021, 12, 768015. [Google Scholar] [CrossRef]
- Mebs, D.; Kuch, U.; Coronas, F.I.; Batista, C.V.; Gumprecht, A.; Possani, L.D. Biochemical and biological activities of the venom of the Chinese pitviper Zhaoermia mangshanensis, with the complete amino acid sequence and phylogenetic analysis of a novel Arg49 phospholipase A2 myotoxin. Toxicon 2006, 47, 797–811. [Google Scholar] [CrossRef]
- Murakami, M.T.; Kuch, U.; Betzel, C.; Mebs, D.; Arni, R.K. Crystal structure of a novel myotoxic Arg49 phospholipase A2 homolog (zhaoermiatoxin) from Zhaoermia mangshanensis snake venom: Insights into Arg49 coordination and the role of Lys122 in the polarization of the C-terminus. Toxicon 2008, 51, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.-H.; Lan, H.; Chen, Y.-H.; Wang, Y.-M. Proteomic analyses on the venom of an individual Protobothrops mangshanesis. J. Snake 2010, 22, 325–331. [Google Scholar]
- Nie, D.-S.; Liu, Y.; Wu, Z.; Liu, L.-C.; Zhu, Z.-B. Analysis of two-dimensional electrophoresis of toxin from the Ermia mangshanensis. J. Hunan Inst. Sci. Technol. (Nat. Sci.) 2011, 24, 48–51. [Google Scholar]
- Margres, M.J.; Wray, K.P.; Seavy, M.; McGivern, J.J.; Herrera, N.D.; Rokyta, D.R. Expression differentiation is constrained to low-expression proteins over ecological timescales. Genetics 2016, 202, 273–283. [Google Scholar] [CrossRef]
- Yamakawa, M.; Nozaky, M.; Hokama, Z. Fractionation of sakishimahabu (Trimeresurus elegans) venom and lethal, hemorrhagic and edema-forming activities of the fractions. In Animal, Plant and Microbial Toxins; Ohsaka, A., Hayashi, K., Sawai, Y., Eds.; Plenum Press: New York, NY, USA, 1976; Volume 1, pp. 97–109. [Google Scholar]
- Huang, S.; Huang, J.-T. An anti-hemorrhagic factor in Zaocys dhumnades serum: A promising source for antivenom drug. Acta Zool. Sin. 2006, 52, 1113–1118. [Google Scholar]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef]
- Yu, P.-N.; Zhong, H.; Yu, Y.-Z.; Yu, Y.-Z. Zhongguo Sheshang Xue; Shanxi Science and Technology Publishing House: Taiyuan, China, 2009. [Google Scholar]
- Ogawa, T.; Tobishima, Y.; Kamata, S.; Matsuda, Y.; Muramoto, K.; Hidaka, M.; Futai, E.; Kuraishi, T.; Yokota, S.; Ohno, M.; et al. Focused proteomics analysis of habu snake (Protobothrops flavoviridis) venom using antivenom-based affinity chromatography reveals novel myonecrosis-enhancing activity of thrombin-like serine proteases. Front. Pharmacol. 2021, 12, 766406. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Chien, K.-Y.; Su, H.-Y.; Chen, Y.-C.; Mao, Y.-C.; Wu, W.-G. Comparison of protein variation in Protobothrops mucrosquamatus venom between Northern and Southeast Taiwan and association with human envenoming effects. Toxins 2022, 14, 643. [Google Scholar] [CrossRef]
- Serrano, S.M.T. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-F.; Wang, J.; He, Y.; Qu, Y.-F.; Lin, L.-H.; Ma, X.-M.; Ji, X. Proteomic and biochemical analyses of short-tailed pit viper (Gloydius brevicaudus) venom: Age-related variation and composition–activity correlation. J. Proteom. 2014, 105, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Shih, C.-P.; Wang, C.-C.; Ouyang, C.-H.; Liu, C.-C.; Yu, J.-S.; Lo, C.-H. The clinical usefulness of Taiwan bivalent freeze-dried hemorrhagic antivenom in Protobothrops mucrosquamatus- and Viridovipera stejnegeri-envenomed patients. Toxins 2022, 14, 794. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Fernández, J.; Sanz, L.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Venomous snakes of Costa Rica: Biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteom. 2014, 105, 323–339. [Google Scholar] [CrossRef]
- Souza, G.H.M.F.; Catharino, R.R.; Ifa, D.R.; Eberlin, M.N.; Hyslop, S. Peptide fingerprinting of snake venoms by direct infusion nano-electrospray ionization mass spectrometry: Potential use in venom identification and taxonomy. J. Mass Spectrom. 2008, 43, 594–599. [Google Scholar] [CrossRef]
- Zhao, E.-M.; Wu, G.-F.; Wu, X.-F.; Chen, Y.-C.; Jiang, M.-S.; Zhang, J.-K.; Hsu, K. A comparison of snake venom eletrophoretogram of Agkistrodon species in China and their value in snake taxonomy. Acta Zool. Sin. 1981, 27, 213–217+302. [Google Scholar]
- Blomberg, S.P.; Garland, T., Jr.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar]
- Guo, P.; Liu, Q.; Wu, Y.-Y.; Zhu, F.; Zhong, G.-H. Pitvipers of China; Science Press: Beijing, China, 2022. [Google Scholar]
- Sousa, L.F.; Holding, M.L.; Del-Rei, T.H.M.; Rocha, M.M.T.; Mourao, R.H.V.; Chalkidis, H.M.; Prezoto, B.; Gibbs, H.L.; Moura-da-Silva, A.M. Individual variability in Bothrops atrox snakes collected from different habitats in the Brazilian Amazon: New findings on venom composition and functionality. Toxins 2021, 13, 814. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, J.-F.; Lin, L.-H.; Ma, X.-M.; Ji, X. Age-related variation in snake venom: Evidence from two snakes (Naja atra and Deinagkistrodon acutus) in southeastern China. Asian Herpetol. Res. 2014, 5, 119–127. [Google Scholar]
- Tu, A.T.; James, G.P.; Chua, A. Some biochemical evidence in support of the classification of venomous snakes. Toxicon 1965, 3, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.H.; Toyama, D.d.O.; Passero, L.F.D.; Laurenti, M.D.; Corbett, C.E.; Tomokane, T.Y.; Fonseca, F.V.; Antunes, E.; Joazeiro, P.P.; Beriam, L.O.S.; et al. Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon 2006, 47, 47–57. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; Nataraju, A.; Rajesh, R.; Raghavendra Gowda, C.D.; Sharath, B.K.; Vishwanath, B.S.; D’Souza, C.J.M. Anticoagulant effect of Naja naja venom 5′nucleotidase: Demonstration through the use of novel specific inhibitor, vanillic acid. Toxicon 2006, 48, 411–421. [Google Scholar] [CrossRef]
- Wen, L. Interspecific Variations in Venom Proteomic and Venom-Gland Transcriptomic Profiles across the Genus Gloydius. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2020. [Google Scholar]
- Li, J.-N.; Liang, D.; Wang, Y.-Y.; Guo, P.; Huang, S.; Zhang, P. A large-scale systematic framework of Chinese snakes based on a unified multilocus marker system. Mol. Phylogenet. Evol. 2020, 148, 106807. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Pianka, E.R. Organization in natural assemblages of desert lizards and tropical fishes. Ecol. Monogr. 1990, 60, 27–55. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).