Retrospective Observational Study of Treatment Patterns and Efficacy of onabotulinumtoxinA Therapy in Patients with Refractory Overactive Bladder in Clinical Practice
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
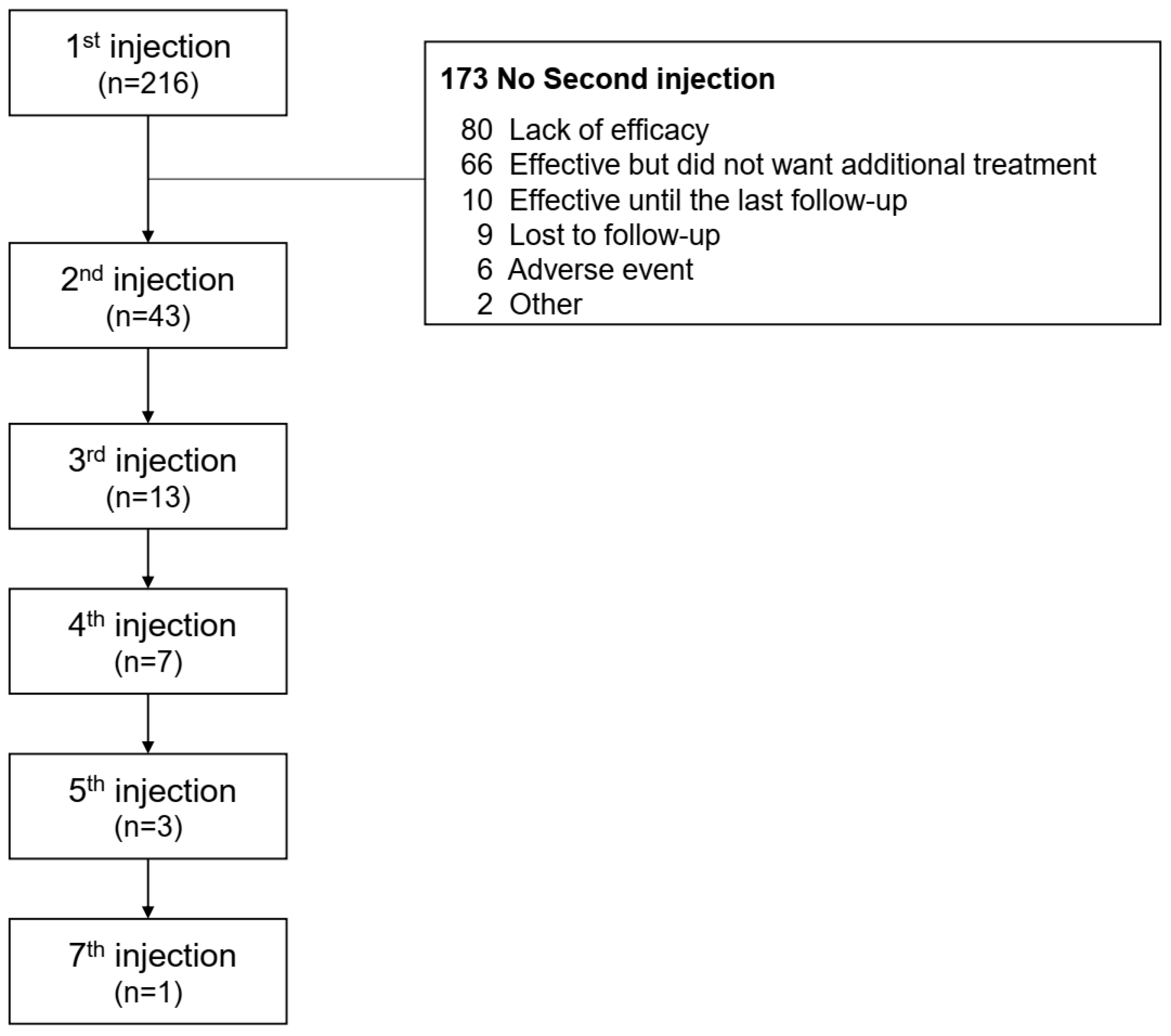
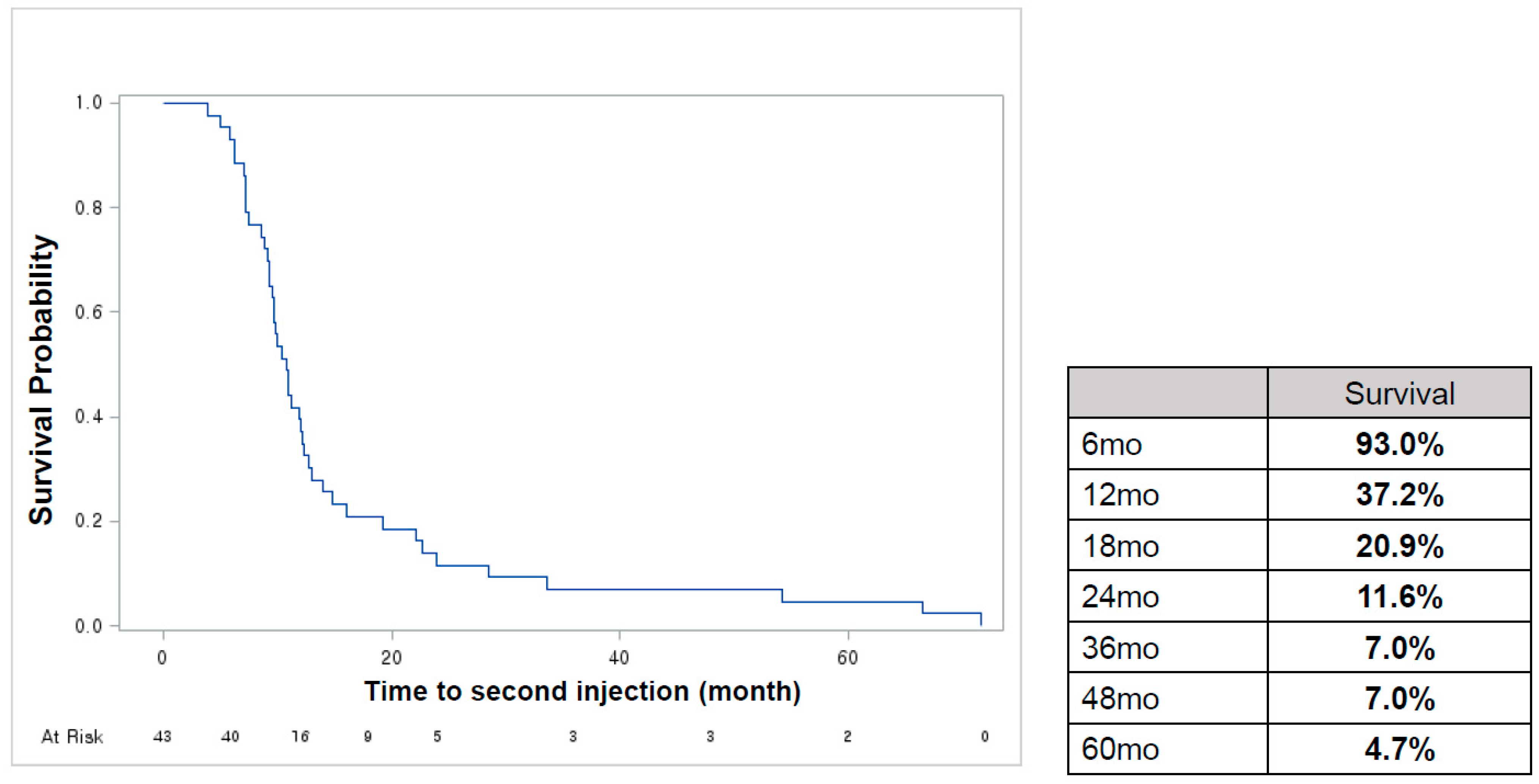
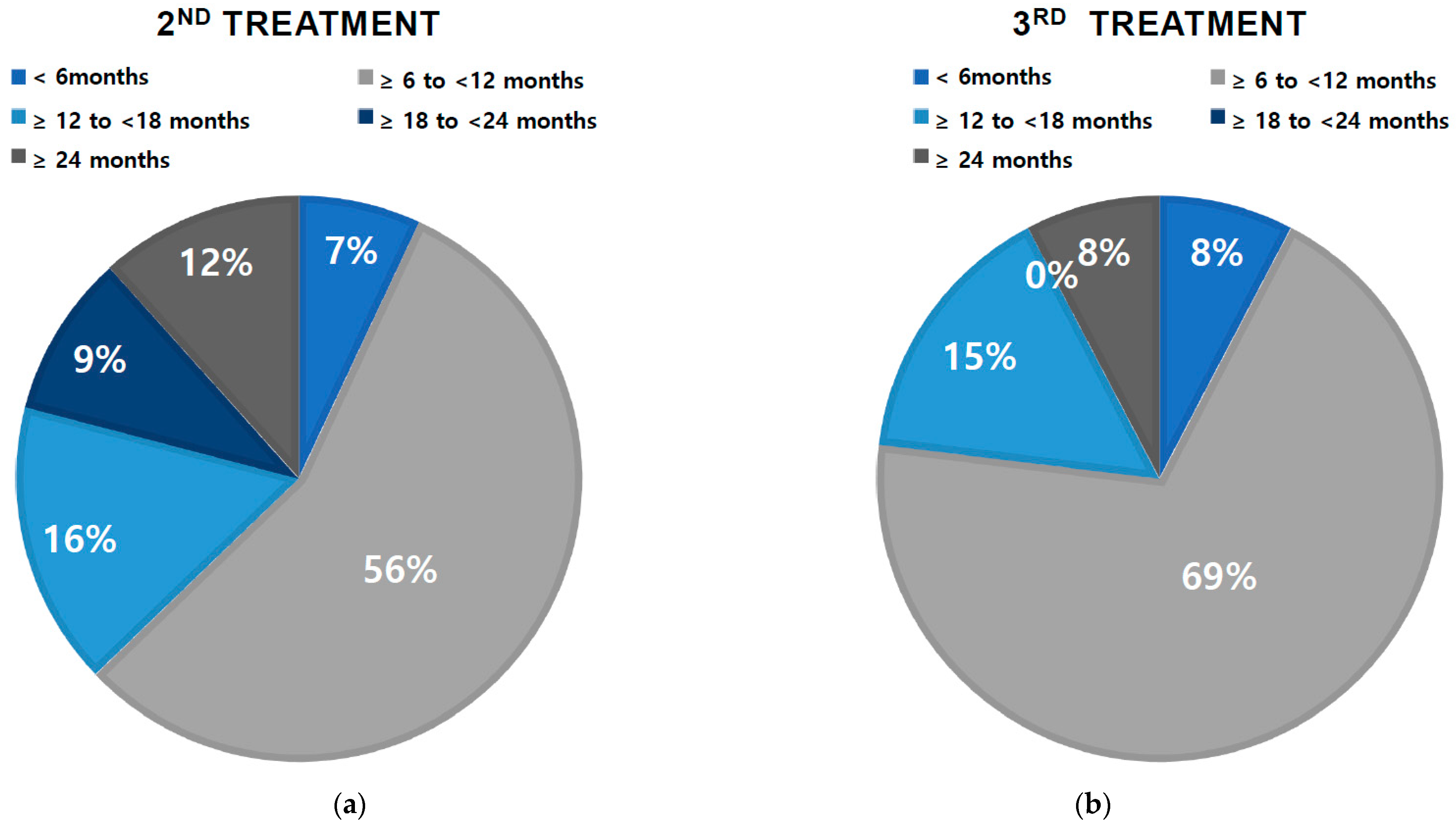
2.2. Treatment Pattern
2.3. Outcomes
2.4. Response Rate and Predictive Factors for Response
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peyronnet, B.; Mironska, E.; Chapple, C.; Cardozo, L.; Oelke, M.; Dmochowski, R.; Amarenco, G.; Gamé, X.; Kirby, R.; Van Der Aa, F.; et al. A Comprehensive Review of Overactive Bladder Pathophysiology: On the Way to Tailored Treatment. Eur. Urol. 2019, 75, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Chapple, C.R.; Jünemann, K.P.; Sharpe, S. Urinary urgency: A review of its assessment as the key symptom of the overactive bladder syndrome. World J. Urol. 2012, 30, 385–392. [Google Scholar] [CrossRef]
- Marcelissen, T.; Cornu, J.N.; Antunes-Lopes, T.; Geavlete, B.; Delongchamps, N.B.; Rashid, T.; Rieken, M.; Rahnama’i, M.S. Management of Idiopathic Overactive Bladder Syndrome: What is the Optimal Strategy After Failure of Conservative Treatment? Eur. Urol. Focus 2018, 4, 760–767. [Google Scholar] [CrossRef]
- Schwantes, U.; Grosse, J.; Wiedemann, A. Refractory overactive bladder: A common problem? Int. Urogynecol. J. 2015, 26, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Kuo, H.C. Pathophysiology of refractory overactive bladder. Low Urin. Tract. Symptoms 2019, 11, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Chermansky, C.; Schurch, B.; Rahnama’i, M.S.; Averbeck, M.A.; Malde, S.; Mancini, V.; Valentini, F.; Sahai, A. How can we better manage drug-resistant OAB/DO? ICI-RS 2018. Neurourol. Urodyn. 2019, 38 (Suppl. S5), S46–S55. [Google Scholar] [CrossRef]
- Duthie, J.B.; Vincent, M.; Herbison, G.P.; Wilson, D.I.; Wilson, D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst. Rev. 2011, 2011, Cd005493. [Google Scholar]
- Wang, M.; Jian, Z.; Ma, Y.; Jin, X.; Li, H.; Wang, K. Percutaneous tibial nerve stimulation for overactive bladder syndrome: A systematic review and meta-analysis. Int. Urogynecol. J. 2020, 31, 2457–2471. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.; Connelly, L.; Dickson, S.; Duncan, F.; Lawrence, M. The effectiveness of transcutaneous tibial nerve stimulation (TTNS) for adults with overactive bladder syndrome: A systematic review. Neurourol. Urodyn. 2018, 37, 528–541. [Google Scholar] [CrossRef]
- Tilborghs, S.; De Wachter, S. Sacral neuromodulation for the treatment of overactive bladder: Systematic review and future prospects. Expert. Rev. Med. Devices 2022, 19, 161–187. [Google Scholar] [CrossRef]
- Nitti, V.W.; Dmochowski, R.; Herschorn, S.; Sand, P.; Thompson, C.; Nardo, C.; Yan, X.; Haag-Molkenteller, C.; EMBARK Study Group. OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J. Urol. 2017, 197, S216–S223. [Google Scholar] [CrossRef]
- Chapple, C.; Sievert, K.D.; MacDiarmid, S.; Khullar, V.; Radziszewski, P.; Nardo, C.; Thompson, C.; Zhou, J.; Haag-Molkenteller, C. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: A randomised, double-blind, placebo-controlled trial. Eur. Urol. 2013, 64, 249–256. [Google Scholar] [CrossRef]
- Smith, C.P.; Franks, M.E.; McNeil, B.K.; Ghosh, R.; De Groat, W.C.; Chancellor, M.B.; Somogyi, G.T. Effect of botulinum toxin A on the autonomic nervous system of the rat lower urinary tract. J. Urol. 2003, 169, 1896–1900. [Google Scholar] [CrossRef]
- Hsieh, P.F.; Chiu, H.C.; Chen, K.C.; Chang, C.H.; Chou, E.C. Botulinum toxin A for the Treatment of Overactive Bladder. Toxins 2016, 8, 59. [Google Scholar] [CrossRef]
- Mohee, A.; Khan, A.; Harris, N.; Eardley, I. Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). BJU Int. 2013, 111, 106–113. [Google Scholar] [CrossRef]
- Hamid, R.; Lorenzo-Gomez, M.F.; Schulte-Baukloh, H.; Boroujerdi, A.; Patel, A.; Farrelly, E. OnabotulinumtoxinA is a well tolerated and effective treatment for refractory overactive bladder in real-world practice. Int. Urogynecol. J. 2021, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Faure Walker, N.A.; Syed, O.; Malde, S.; Taylor, C.; Sahai, A. Onabotulinum toxin A Injections in Men With Refractory Idiopathic Detrusor Overactivity. Urology 2019, 123, 242–246. [Google Scholar] [CrossRef]
- Wennberg, A.L.; Molander, U.; Fall, M.; Edlund, C.; Peeker, R.; Milsom, I. A longitudinal population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women. Eur. Urol. 2009, 55, 783–791. [Google Scholar] [CrossRef]
- Malmsten, U.G.; Molander, U.; Peeker, R.; Irwin, D.E.; Milsom, I. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: A longitudinal population-based survey in men aged 45–103 years. Eur. Urol. 2010, 58, 149–156. [Google Scholar] [CrossRef]
- Chuang, F.C.; Liu, H.T.; Wang, L.Y.; Kuo, H.C. Overactive bladder changes with time: A 5-year longitudinal followup of changes in overactive bladder symptoms, urodynamic studies and urinary nerve growth factor levels. J. Urol. 2014, 192, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sahai, A.; Khan, M.S.; Dasgupta, P. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: Results from a single center, randomized, double-blind, placebo controlled trial. J. Urol. 2007, 177, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Urodynamic evidence of effectiveness of botulinum A toxin injection in treatment of detrusor overactivity refractory to anticholinergic agents. Urology 2004, 63, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Clinical effects of suburothelial injection of botulinum A toxin on patients with nonneurogenic detrusor overactivity refractory to anticholinergics. Urology 2005, 66, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.M.; Lin, H.H.; Kuo, H.C. Factors Associated with Therapeutic Efficacy of Intravesical OnabotulinumtoxinA Injection for Overactive Bladder Syndrome. PLoS ONE 2016, 11, e0147137. [Google Scholar] [CrossRef]
- Rachaneni, S.; Latthe, P. Effectiveness of BTX-A and neuromodulation in treating OAB with or without detrusor overactivity: A systematic review. Int. Urogynecol. J. 2017, 28, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, J.C.; Lapitan, M.C.; Truzzi, N.C.; Iacovelli, V.; Averbeck, M.A. Botulinum toxin for treating overactive bladder in men: A systematic review. Neurourol. Urodyn. 2022, 41, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Mateu Arrom, L.; Mayordomo Ferrer, O.; Sabiote Rubio, L.; Gutierrez Ruiz, C.; Martínez Barea, V.; Palou Redorta, J.; Errando Smet, C. Treatment Response and Complications after Intradetrusor OnabotulinumtoxinA Injection in Male Patients with Idiopathic Overactive Bladder Syndrome. J. Urol. 2020, 203, 392–397. [Google Scholar] [CrossRef]
- Wang, C.C.; Lee, C.L.; Kuo, H.C. Efficacy and Safety of Intravesical OnabotulinumtoxinA Injection in Patients with Detrusor Hyperactivity and Impaired Contractility. Toxins 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Homma, Y.; Oh, S.J. Korean version of the overactive bladder symptom score questionnaire: Translation and linguistic validation. Int. Neurourol. J. 2011, 15, 135–142. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A.; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Homma, Y.; Yokoyama, O.; Nishizawa, O. Responsiveness and minimal clinically important change in overactive bladder symptom score. Urology 2011, 78, 768–773. [Google Scholar] [CrossRef] [PubMed]

| Age | 63.4 ± 15.5 |
| ≥65, n (%) | 126 (58.3%) |
| ≥75, n (%) | 54 (25.0%) |
| Sex | |
| Male, n (%) | 91 (42.1%) |
| Female, n (%) | 125 (57.9%) |
| HTN, n (%) | 80 (38.0%) |
| DM, n (%) | 48 (22.2%) |
| Previous OAB medication history | |
| Anticholinergics, n (%) | 165 (76.4%) |
| Mirabegron, n (%) | 164 (75.9%) |
| Previous sacral neurostimulation, n (%) | 6 (2.8%) |
| Overactive Bladder Symptom Score | |
| Total score, mean ± SD | 10.13 ± 3.17 |
| Q3, mean ± SD | 3.95 ± 1.3 |
| 3-day voiding diary | |
| Frequency, mean ± SD | 11.4 ± 6.8 |
| Nocturia, mean ± SD | 2.2 ± 2.0 |
| Urgency episodes, mean ± SD | 10.0 ± 9.2 |
| Urgency urinary incontinence *, mean ± SD | 2.3 ± 5.7 |
| Male | Female | |
|---|---|---|
| N = 65 | N = 79 | |
| Simple Uroflowmetry | ||
| Maximum flow rate (mL/s) | 12.8 ± 8.3 | 15.3 ± 7.8 |
| Post-voided residual (mL) | 46.2 ± 71.0 | 39.7 ± 56.8 |
| Storage Phase (cystometry) | ||
| First sensation of bladder filling (mL) | 143.6 ± 56.5 | 180.11 ± 92.9 |
| First desire to void (mL) | 181.1 ± 71.9 | 226.77 ± 99.3 |
| Strong desire to void (mL) | 260.5 ± 113.4 | 326.3 ± 153.6 |
| Maximal cystometric capacity (mL) | 267.6 ± 114.0 | 328.8 ± 126.9 |
| Compliance | 50.4 ± 41.2 | 70.3 ± 80.3 |
| Detrusor overactive, n (%) | 50 (76.9%) | 60 (75.9%) |
| Voiding Phase (pressure-flow study) | ||
| Detrusor underactive, n (%) | 17 (26.2%) | 13 (16.7%) |
| Bladder contractility index * | 89.6 ± 40.4 | - |
| Bladder outlet obstruction index * | 22.6 ± 17.2 | - |
| 1st Injection | 2nd Injection | |
|---|---|---|
| OABSS total score | ||
| 1 month | ||
| N | 124 | 34 |
| Mean change from baseline | −2.26 ± 3.38 | −1.91 ± 3.18 |
| p | <0.001 | 0.001 |
| 3 month | ||
| N | 90 | 16 |
| Mean change from baseline | −2.04 ± 3.49 | −1.19 ± 3.02 |
| p | <0.001 | 0.136 |
| 6 month | ||
| N | 57 | 14 |
| Mean change from baseline | −2.52 ± 3.33 | −1.29 ± 4.48 |
| p | <0.001 | 0.131 |
| OABSS Q3 | ||
| 1 month | ||
| N | 124 | 34 |
| Mean change from baseline | −0.94 ± 1.55 | −0.76 ± 1.46 |
| p | <0.001 | 0.003 |
| 3 month | ||
| N | 90 | 16 |
| Mean change from baseline | −0.97 ± 1.58 | −0.69 ± 1.4 |
| p | <0.001 | 0.069 |
| 6 month | ||
| N | 57 | 14 |
| Mean change from baseline | −0.95 ± 1.49 | −0.71 ± 1.86 |
| p | <0.001 | 0.174 |
| 3-day voiding diary parameters | ||
| Frequency | ||
| 1 month | ||
| N | 58 | 8 |
| Mean change from baseline | −0.57 ± 5.33 | −0.42 ± 4.19 |
| p | 0.865 | 0.852 |
| 3 month | ||
| N | 63 | 9 |
| Mean change from baseline | −3.54 ± 8.13 | −1.89 ± 2.37 |
| p | <0.001 | 0.044 |
| 6 month | ||
| N | 34 | 4 |
| Mean change from baseline | −1.93 ± 5.42 | −4.50 ± 6.29 |
| p | 0.010 | 0.248 |
| Nocturia | ||
| 1 month | ||
| N | 57 | 8 |
| Mean change from baseline | 0.08 ± 1.22 | −0.29 ± 1.25 |
| p | 0.940 | 0.531 |
| 3 month | ||
| N | 63 | 9 |
| Mean change from baseline | −0.53 ± 1.83 | −0.19 ± 1.03 |
| p | 0.010 | 0.604 |
| 6 month | ||
| N | 34 | 4 |
| Mean change from baseline | −0.04 ± 1.61 | −0.5 ± 0.64 |
| p | 0.580 | 0.215 |
| Urgency | ||
| 1 month | ||
| N | 45 | 6 |
| Mean change from baseline | −0.12 ± 6.45 | −1.17 ± 6.91 |
| p | 0.902 | 0.696 |
| 3 month | ||
| N | 52 | 9 |
| Mean change from baseline | −5.46 ± 11.97 | −3.59 ± 8.23 |
| p | <0.001 | 0.227 |
| 6 month | ||
| N | 27 | 4 |
| Mean change from baseline | −2.48 ± 7.92 | −3.92 ± 5.23 |
| p | 0.116 | 0.231 |
| Urgency urinary incontinence | ||
| 1 month | ||
| N | 43 | 6 |
| Mean change from baseline | −0.78 ± 2.65 | 0.42 ± 2.88 |
| p | 0.096 | 0.738 |
| 3 month | ||
| N | 51 | 9 |
| Mean change from baseline | −0.91 ± 5.11 | −1.83 ± 3.51 |
| p | 0.098 | 0.156 |
| 6 month | ||
| N | 26 | 4 |
| Mean change from baseline | −0.05 ± 5.22 | −0.33 ± 0.47 |
| p | 0.348 | 0.252 |
| Good Response | Poor Response | p | OR (95% CI) | |
|---|---|---|---|---|
| N | 49 | 75 | ||
| Age | 62.37 ± 15.61 | 61.81 ± 16.6 | 0.851 | 1.00 (0.95–1.03) |
| Sex | 0.195 | 1.62 (0.78–3.37) | ||
| Male | 33.3% | 66.7% | ||
| Female | 44.8% | 55.2% | ||
| Presence of DO | 0.023 | 3.66 (1.20–11.21) | ||
| No | 20.8% | 79.2% | ||
| Yes | 50.9% | 49.1% | ||
| BOOI | 35.57 ± 24.03 | 18.23 ± 12.41 | 0.041 | 1.06 (1.00–1.12) |
| BCI | 82.31 ± 33.09 | 91.75 ± 46.78 | 0.510 | 0.99 (0.98–1.01) |
| Compliance | 54.80 ± 47.09 | 65.04 ± 74.80 | 0.496 | 1.00 (0.99–1.01) |
| Refractory to SNM | 40.0% | 60.0% | 0.982 | 1.02 (0.16–6.35) |
| Male | Female | |||
|---|---|---|---|---|
| p | OR [95% CI] | p | OR [95% CI] | |
| Presence of DO | 0.843 | 0.79 [0.08, 7.92] | 0.015 | 23.65 [1.84, 304.40] |
| Age | 0.862 | 1.00 [0.93, 1.06] | 0.101 | 0.95 [0.894, 1.01] |
| BOOI * | 0.063 | 1.07 [1.00, 1.14] | - | - |
| BCI * | 0.833 | 1.00 [0.97, 1.04] | - | - |
| Compliance | 0.710 | 0.99 [0.96, 1.03] | 0.729 | 1.00 [0.99, 1.01] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.J.; Lee, K.-S. Retrospective Observational Study of Treatment Patterns and Efficacy of onabotulinumtoxinA Therapy in Patients with Refractory Overactive Bladder in Clinical Practice. Toxins 2023, 15, 338. https://doi.org/10.3390/toxins15050338
Ko KJ, Lee K-S. Retrospective Observational Study of Treatment Patterns and Efficacy of onabotulinumtoxinA Therapy in Patients with Refractory Overactive Bladder in Clinical Practice. Toxins. 2023; 15(5):338. https://doi.org/10.3390/toxins15050338
Chicago/Turabian StyleKo, Kwang Jin, and Kyu-Sung Lee. 2023. "Retrospective Observational Study of Treatment Patterns and Efficacy of onabotulinumtoxinA Therapy in Patients with Refractory Overactive Bladder in Clinical Practice" Toxins 15, no. 5: 338. https://doi.org/10.3390/toxins15050338
APA StyleKo, K. J., & Lee, K.-S. (2023). Retrospective Observational Study of Treatment Patterns and Efficacy of onabotulinumtoxinA Therapy in Patients with Refractory Overactive Bladder in Clinical Practice. Toxins, 15(5), 338. https://doi.org/10.3390/toxins15050338





